Abstract
Planktonic community structure was investigated during outbreak of harmful Phaeocystis globosa bloom in a subtropical bay, the Maowei Sea, South China Sea. The phytoplankton assemblage was numerically dominated by colonial P. globosa, with its abundance ranging from 1.23 × 108 to 11.12 × 108 cells m−3 and contributing nearly 90 % to the total abundance. Totally 66 mesozooplankton (>169 µm) and 19 ciliates species were recorded, with the densities ranged from 169 to 1633 ind m−3 and 74 to 1118 cells L−1, respectively. The dominant species for mesozooplankton were Copepoda (larvae), Bestiola sinicus, B. amoyensis, Macrura (larvae) and Acartia spinicauda, respectively. The ciliate assemblage was numerically dominated by Codonella rapa, Strombidium globosaneum and Mesodinium rubrum. During the bloom, P. globosa seemed to be negatively affected by the nutrient phosphate significantly (p < 0.05). However, no correlation between P. globosa and ciliate assemblage was detected, but P. globosa was negatively correlated with total biomass of mesozooplankton and abundance of B. sinicus (p < 0.05), suggesting that P. globosa was uncoupled from the grazing by both ciliates and mesozooplankton when appearing as colonies form. On the other hand, both positive and negative correlations among the dominant groups of mesozooplankton and ciliates were observed (p < 0.05) which possibly indicated that the predation of mesozooplankton upon ciliates might be strengthened during the Phaeocystis bloom and the complex effect also varied from species to species.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phaeocystis (Prymnesiophyta) which can form nearly monospecific dense blooms and constitute environmental nuisance in global marine waters, is recognized worldwide as harmful algae (Grattepanche et al. 2011). Members of this genus have special characteristics of physiology, biochemistry and ecology and play a key role as an intermediary in the transfer of both carbon and sulfur between ocean and atmosphere (Schoemann et al. 2005). Most importantly, they possess extraordinary heterogenic life cycle that involves in sexual reproduction between free-living cells (6–9 µm in diameter) and gelatinous colonies (up to 3.0 cm in diameter). These special life strategies are functionally interpreted as the survival adaptation of Phaeocystis in both oligotrophic and eutrophic waters (Schoemann et al. 2005). For example, single cells with longer stationary phase and lower death rate are typically observed in nutrient-poor environment, while solitary non-flagellate cells in colony with short lag phase and high growth rate usually dominate in rapidly developing blooms (Peperzak et al. 2000). The exact transition between these two different life forms, however, is still unclear and evolution of Phaeocystis life-history strategy is generally believed to be forced by zooplankton grazing pressure (Riegman and van Boekel 1996; Widdicombe et al. 2004). Due to the large size of the colonies, however, Phaeocystis are supposed to be unsuitable food sources for copepods directly (Nejstgaard et al. 2007), but nano-sized single cells are heavily grazed by protozoa and can support a suboptimal growth of ciliates (Hensen et al. 1993; Tang et al. 2001). Since ciliates are usually considered as a food source for metazoan zooplankton or fish larvae, they consequently improve the food quality for higher predators in Phaeocystis-dominated ecosystem and playing a pivotal role in transferring the materials and energy in marine microbial food webs (Pierce and Turner 1992; Clarke et al. 2006).
As for the Phaeocystis bloom, some researchers believe that grazing of zooplankton is a negligible loss factor if concerning bloom dynamics (Davies et al. 1992), while others argue that copepods switch to ciliates as food source might have a positive influence on Phaeocystis population and thus stimulate the development of a bloom (Nejstgaard et al. 2007). Therefore, knowledge of trophic responses of grazers to Phaeocystis is essential to understand the potential top-down control of Phaeocystis bloom development (Jakobsen and Tang 2002). Many studies have focused on simple interaction (e.g. one prey–one predator) or single cells-based treatment in the laboratory conditions (Tang et al. 2001), yet it is still unclear to what extent the zooplankton species feed on different life forms of Phaeocystis globosa, in particular in the field conditions. Moreover, little is known about the variations of ciliate community in most marine ecosystems, especially during the harmful algae blooms in coastal waters of the South China Sea (Liu et al. 2010).
During the last two decades, blooms of Phaeocystis have frequently occurred in coastal waters of Southern China, e.g. Shantou, Hong Kong, Zhanjiang, Hainan Island and Beihai, leading to a great impact on local fishery and environmental health (Huang et al. 1999; Li et al. 2012). These Chinese strains were characterized by extraordinary large size of the colony (up to 3.0 cm in diameter), high temperature requirements and hemolytic properties which make them special members of the P. globosa complex (Shen et al. 2011a). All these characteristics should be taken into account when trying to understand their role in the coastal ecosystem. In autumn of 2011, P. globosa bloom reoccurred in the Maowei Sea, SCS. As Phaeocystis blooms have a large ecological impact on ecosystem, the aims of present study are to explore the planktonic community structure during the bloom and the possible trophic interactions among the mesozooplankton, ciliates and phytoplankton assemblages; to assess whether ciliates play a potential top-down control on the P. globosa bloom development through acting as an intermediate prey between the mesozooplankton and the algae. Moreover, these investigations could also gain insight into the microbial food web structure in P. globosa-dominated ecosystem in the typical subtropical bay of the SCS.
Materials and methods
Study area, filed sampling and treatment
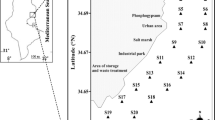
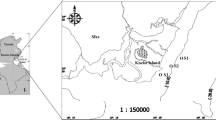
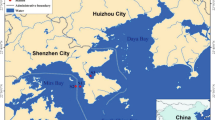
The Maowei Sea is a semi-enclosed shallow bay, with an area about 135 km2 and the water depth usually less than 10 m. It is located in the northwestern part of the South China Sea (SCS) and receives freshwater from the Qinjiang River and Maoling River. As in the subtropical area, it is significantly influenced by South-west monsoon in summer and North-east monsoon in winter, with nearly 80 % of the discharge occurred during summer and 20 % during winter. More recently, the rapid development around this area (mainly anthropogenic drainage and oyster aquiculture in the bay) has brought about environmental pressures on this bay ecosystem, resulting in frequent occurrence of eutrophication and harmful algae blooms (HABs). During the outbreak of P. globosa bloom, 16 sampling sites were arranged to cover both the bloom center and non-bloom area in the bay, from which water samples were collected on October 29, 2011 (Fig. 1).
Water samples were collected using a 5 L niskin bottle at surface layer and environmental factors (temperature and salinity) were measured by an YSI 6600 multi-parameter water quality monitor (Yellow Springs Instrument Co., USA) at each sampling station. After collection, the water samples for later inorganic nutrients and chlorophyll a determination were filtered through 0.45 µm cellulose filters and both the water and filters were preserved at 4 °C in dark before further processing in the laboratory. The nutrient (nitrate, nitrite, ammonia, phosphate and silicate) concentrations were analyzed by a nutrient-analyzer (Quickchem 8500, USA) according to the method described in Kirkwood et al. (1996). Chlorophyll a (Chl a) concentration was also measured using a Turner Design 10-AU fluorometer according to the method described in Parsons et al. (1984).
Planktonic community structure
Mesozooplankton was collected by vertical tows from 1.0 m above the sediment to the water surface with 169 µm mesh-size plankton net (0.5 m in mouth diameter and 1.45 m long), fitting with a Hydro-Bios flowmeter in the net mouth. Samples were fixed and preserved with formalin in 5 % final concentration, and identified to species level where possible. Mesozooplankton abundance was counted under an inverted microscope and the wet weight was measured according to the method of Li et al. (2006). Microphytoplankton (>55 μm) including P. globosa was collected using 55 μm mesh-size plankton net (0.37 m in mouth diameter and 1.3 m long, fitted a Hydro-Bios flowmeter) and preserved with 1 % Lugol’s iodine solution (no glacial acetic acid). Phytoplankton species were identified and enumerated under a microscope (Leica DM2000). The P. globosa free-living cells and colonies were counted separately, and the diameter of each observed colony was measured to estimate the cell number based on the regression relationship between colony diameter and cells number per colony (LogY = 1.34 × logX − 0.44, Y is the cell number in colony and X is the diameter of the colony, Huang et al. 2012).
One liter of water sample for ciliates analysis was fixed with Lugol’s solution and stored in darkness for later analyses. The water sample was concentrated to 20–30 mL using Utermöhl method (1958), and the ciliates were identified by referring to Kofoid and Campbell (1929, 1939), Carey (1992) for tintinnids and Maeda and Carey (1985), Lynn et al. (1991) for aloricate ciliates, respectively.
Statistical analysis
Species richness and diversity (Shannon–Wiener diversity index H′, based on log2) were calculated for each group. Multivariate analyses were used to detect any spatial differences in species composition and abundance of planktonic communities (after square root transformation based on plankton abundance data), and to assess which taxon mainly contributed to the spatial variability (Software PRIMER v6). Similarity matrices were constructed using Bray–Curtis similarity, and non-metric multidimensional scaling (nMDS) was also applied to the similarity matrixes to determine the similarity of sites with respect to plankton composition (Shen et al. 2012). Following the cluster analysis, the species having the greatest contribution to the division of samples into cluster were determined using the similarity percentage program (SIMPER) (Clarke and Gorley 2006). Pearson analysis was also performed to detect the relationships between the abundance of phytoplankton, mesozooplankton and ciliates populations with SPSS software. The spatial distribution of ciliates and their responses to biotic and/or abiotic factors were elucidated by means of canonical correspondence analysis (CCA). The CCA analysis was processed using statistical program CANOCO version 4.5 (Chen et al. 2011).
Results
Environmental parameters
Sea surface temperature (T) and salinity (S) of the MWS were apparently influenced by freshwater inputs from the Qinjiang River and the Maoling River. Temperature ranged from 22.8 to 25.4 °C and increased from the river mouth to the transition zone and the bay mouth. Salinity showed similar spatial changes along the gradient with the range between 11.3 and 22.9. To better understand the impacts of freshwater discharges, all stations were divided into saline water type (S > 17) and brackish water type (S < 17) (Table 1), respectively.
The MWS was typically nutrient-rich with dissolved inorganic nitrogen (DIN) ranging from 19.29 to 45.71 µmol L−1. The highest DIN concentration was found at station S14 which is located near the river mouth while the lowest at station S1 which is nearest to the bay mouth and connected with the open sea directly. Therefore, the mean DIN concentration was generally higher in brackish water (inner part of the bay) than in saline water (outer part of the bay). Concentration of phosphate was lower than 1 µmol L−1 in most area (Table 1) and similarly, the averaged concentration was higher in brackish water than in saline water. Chlorophyll a also showed spatial variation and its concentration fluctuated from 1.42 mg m−3 (S13) to 5.20 mg m−3 (S16), with a mean of 3.01 mg m−3 across the whole bay area. Although the maximum Chl a concentration recorded near the river mouth, the averaged value was generally lower in brackish water than in saline water (Table 1).
Planktonic community and its spatial distribution
Totally 89 phytoplankton species belonging to 51 genera were recorded in this study, with colonial P. globosa numerically dominated. The diameter of colonies ranged from 3.0 to 15.0 mm, and the colony density ranged from 0.12 × 104 to 0.68 × 104 ind m−3, with a mean of 0.37 × 104 ind m−3 throughout the bay. According to the regression relationship between colony diameter and cells number per colony, the total abundance of P. globosa was estimated ranging from 1.23 × 108 to 11.12 × 108 cells m−3, contributed nearly 90 % to the total abundance of phytoplankton. Spatially, high abundance was obviously found in the bay mouth which is connecting to the open sea and the mean value was usually higher in saline water than in brackish water.
A total of 66 mesozooplankton species were identified and copepods (26 species) were the most abundant group, followed by planktonic larvae (10 species). The dominant species were Copepoda larvae, Bestiola sinicus, B. amoyensis, Macrura larvae and Acartia spinicauda, respectively. The averaged species richness in saline water (34 species) was much higher than in brackish water (24 species), with the maximum occurred at station S1 (41 species) which is located in the bay mouth. Although the species diversity showed no significant difference among the stations, with most species diversity index higher than three except at station S7 which is located in the central bay, the dominant species at each station were various (Table 2). The spatial distribution of mesozooplankton was also apparent and its high abundance and biomass presented in the central bay, while low abundance occurred near the river mouth and the bay mouth. The abundance fluctuated between 169 and 1633 ind m−3 and biomass ranged from 58.33 to 133.33 mg m−3, respectively, with the maximum were both found in the central bay (station S7) (Fig. 2).
For ciliates, 19 species belonging to 13 genera were identified, among which 11 of them were tintinnids and the dominant species included Codonella rapa, Strombidium globosaneum and Mesodinium rubrum (Table 3). The species richness and diversity at each station ranged from 3 to 7 and 1.30 to 2.75, respectively, with high species number and diversity occurred near the bay mouth (e.g., S1, S3 and S5). The ciliate abundance fluctuated remarkably on a spatial scale, ranging from 74 to 1118 cells L−1 at each station. High abundance mainly occurred in estuarine area (e.g., S14 and S16), but the abundance was usually lower than 500 cells L−1 at most stations. Spatial variation was also found for the dominant species, for example, tintinnids, Strombidiida and M. rubrum appeared to abundant at stations S1–S7 (Bay mouth), S8–S11 (central Bay) and S14–S16 (river mouth), respectively.
Spatial variation was also detected in the classification and ordination analysis (one-way ANOSIM, Global R = 0.77, p < 0.001), which separated the sampling stations, at 50 % of similarity, into two main groups (named inner group and outer group, which are in agreement with the brackish water and saline water types, respectively), reflecting high heterogeneity among the stations (Figs. 3, 4). The outer group includes S1–S5 and S9, dominated by homogeneous saline water and the plankton community structure being uniform. Other stations were assigned to the inner group, influenced by riverine runoffs and the planktonic community structure being diverse. Moreover, under each cluster, two sub-clades corresponding to the inner and outer sectors were defined (Figs. 3, 4). Other sites comprised higher similarities than the S9 within the outer group, and the same situation existed at S7 within the inner group, indicating that the plankton communities in the central bay were more variable because of the influence of diffusive mixing of freshwater and oceanic water (Figs. 3, 4). According to SIMPER results, the species contributing the greatest to the division of stations into different groups were M. rubrum, S. globosaneum, C. rapa, larvae of Copepoda, B. sinicus, S. conicum and Tintinnopsis tubulosa (SIMPER, 51 % cutoff).
The 2D non-multidimensional scaling ordination (nMDS) plots showing similarities between samples after square root transformation based on plankton abundance data. Contours represent the 50 % similarity level among sites. The size of the bubble indicates the abundance of each site (Color figure online)
The ciliates and abiotic parameters that determine the spatial variability of the assemblage are shown in the bi-plots of CCA analysis (Fig. 5). Cumulative percentage of explained variance and correlation coefficients of each selected factor for the two main axes are summarized in Table 4. The two main axes cumulatively explained nearly 86.5 % variability in ciliates abundance and spatial distribution. Three factors including temperature, salinity and DIN were the main determinants of axis 1 (Fig. 5). Temperature and salinity showed opposite character to the DIN, which was consistent to the spatial distribution of other environmental parameters. CCA analysis revealed that S. globosaneum and M. rubrum were closely related to low temperature and salinity.
Canonical correspondence analysis (CCA) of the spatial distribution of ciliates. Environment factors include temperature (T), salinity (S), DIN, phosphate (PO4) and Chl a. Ciliates were shown in the figure, including Amphorella quadrilineata (Amph qu), Amphorellopsis acuta (Amph ac), Codonellopsis morchella (Codo), C. rapa (Coll), Craterella retusa (Crat), Cyclidium sp.(Cycl), Favella companula (Fave), Leprotintinnus simple (Lepr), M. rubrum (Meso ru), S. conicum (Stro co), S. globosaneum (Stro gl), S. major (Stro ma), S. tintinnodes (Stro ti), Tintinnopsis radix (Tint ra), Tintinnopsis schotti (Tint sc), Tintinnopsis tocantinencis (Tint to), Tintinnopsis tubulosa (Tint tu), Vorticella sp. (Vort)
Pearson correlation analysis revealed that P. globosa abundance was negatively related to the total biomass of mesozooplankton and abundance of B. sinicus (p < 0.05, n = 15). However, correlations among mesozooplankton and ciliates were much more complex and the effects were species-specific. For example, ciliates C. rapa and S. globosaneum was positively related to the abundance of larvae of Macrura and Polychaeta (p < 0.05, n = 15), but negatively related to the abundance of A. spinicauda (p < 0.05, n = 15), respectively. M. rubrum was also positively related to larvae of Macrura and Polychaeta (p < 0.05, n = 15) and S. conicum positively related to B. sinicus (p < 0.05, n = 15).
Moreover, environmental factors also play important role in affecting the abundance and distribution of planktonic assemblages. Field data showed a negative relationship between nutrient phosphate and the abundance of P. globosa (p < 0.05, n = 15). Temperature and salinity have a negative effect on the abundance of larvae of Macrura and Polychaeta, S. globosaneum and M. rubrum, but a positive effect on the abundance of A. spinicauda (p < 0.05, n = 15). DIN appeared to be positively related to the abundance of ciliates, especially to M. rubrum, S. globosaneum and C. rapa (p < 0.05, n = 15), which was consistent with the results of CCA analysis.
Discussion
Regulation of planktonic community structure
Planktonic community structures are complicated and regulated by both abiotic factors and biotic interactions. In eutrophic transitional zones, such as estuaries and bays, physic-chemical variables (e.g. temperature, salinity, light and nutrients) are supposed to be the driving forces to regulate community structure (Muylaert et al. 2000; Giberto et al. 2007; Shen et al. 2011b; Chen et al. 2011). Many studies including laboratory and filed observations have demonstrated the strongly influence of the environmental factors upon the species composition, distribution and abundance of biological communities (Kchaou et al. 2009). In present study, dominant species also showed great spatial variations in the bay along the environmental gradients, e.g. tintinnids dominated the ciliate populations near the bay mouth where exchanged with oceanic water from outside the bay. The abundance of larvae of Macrura and Polychaeta, S. globosaneum, A. spinicauda and M. rubrum were closely associated with temperature and salinity, which were consistent to others’ results that the key drivers of zooplankton dynamics were abiotic factors in many rivers and bays ecosystem (Dickerson et al. 2010; Johnson et al. 2013). As a semi-closed shallow bay, high nutrients-load due to anthropogenic input has lead to the development of massive algal blooms in the MWS, including P. globosa and M. rubrum as well (Lan 2012). DIN appeared to be significantly related to the abundance of ciliates, especially the pigmented ciliate M. rubrum, reflecting its high demand of inorganic nitrogen. For P. globosa, although a negative effect of phosphorous upon this harmful alga was detected from the field data, this result should be considered with caution as it was derived from field observation during the increasing phase of bloom when rapid uptake by the colonial algae and consequently P-depletion were supposed to be happened simultaneously. Nonetheless, these bottom-up processes, involving the ambient physic-chemical factors such as temperature, salinity, light and nutrients, played a most important role in coastal and particular estuarine and bay ecosystems (Steen 2004; Cohen and Fong 2004; Worm and Lotze 2006). This is a complex research area that needs to be addressed with more detailed studies in the future.
Trophic relationships in Phaeocystis-dominated ecosystem
Phaeocystis globosa can form nearly monospecific dense blooms of gelatinous colonies in global marine waters and the complexity of trophic relationships in Phaeocystis-dominated ecosystem was previously pointed out (Tang et al. 2001). It is still unclear to which extent their success is due to their high competitiveness for the niches (light and nutrients) or to their resistance to predation (Schoemann et al. 2005 and references therein). Knowledge of the trophic responses of grazers to Phaeocystis is essential to understand the potential top-down control of Phaeocystis bloom development (Jakobsen and Tang 2002). Although ciliates are assumed to act as an intermediate prey between mesozooplankton and P. globosa, no significant correlation between P. globosa and ciliates was detected during the bloom, but negative impact of P. globosa upon the total biomass of mesozooplankton and B. sinicus abundance was significant, indicating that P. globosa was uncoupled from the grazing by both ciliates and mesozooplankton when appearing as colonies form. This result consistents with other study that grazing on Phaeocystis cells differ among protozoans and ciliate Mesodinium pulex may exploit Phaeocystis indirectly by preying on intermediate grazers (Tang et al. 2001). On the one hand, it can be attributed to the Phaeocystis multiple life stages, occurring as colonies during bloom which are large-sized and protected by a thin but tough skin, deterring mainly small, fast-growing pathogens and predators (Hamm et al. 1999; Hamm 2000). On the other hand, the production of acrylic acid and toxins in this harmful alga may also help to defend against predators, competitors and parasites (Verity and Smetacek 1996; Peng et al. 2005). Moreover, adverse inhibition effect on the abundance and biomass of mesozooplankton was also observed, indicating that the higher predators were not expected to obtain a better nutrition through the Phaeocystis-based food chains. Thus, potential grazers may switch to other suitable food sources, such as diatoms. It has been demonstrated that diatoms could not only compete for nutrients, but are also preyed by mesozooplankton (Verity and Smayda 1989). As a consequence, grazing on diatoms are likely to favor the formation of nearly monospecific dense blooms and have a positive influence on the bloom dynamics of P. globosa (Nejstgaard et al. 2007).
In the contrast, correlations of mesozooplankton to ciliates were much more complex and both positive and negative effects among the dominant groups of mesozooplankton and ciliates were observed significantly which possibly indicated that the predation of mesozooplankton upon ciliates might be strengthened during the Phaeocystis bloom, but the effects were species-specific. It’s also consistent with others finding (Schoemann et al. 2005 and references therein). For example, ciliates C. rapa and S. globosaneum was positively related to the abundance of Macrura and Polychaeta, but had an adverse impact on the abundance of A. spinicauda. M. rubrum was also positively related to Macrura and Polychaeta and S. conicum associated positively with B. sinicus, indicating that different ciliates were preferred by selected mesozooplankton species.
Result from fatty acid analysis indicated that dominating crustacean zooplankton might derive a major part of their diet from P. globosa in lower latitudes, suggesting that P. globosa did enter the food web (Hamm and Rousseau 2003). Teixeira et al. (2012) indicated that higher predators may obtain a better nutrition from Phaeocystis blooms by feeding on intermediate prey, for example, ciliates and heterotrophic dinoflagellates which could consume P. globosa single cells, thus considerably reduced the overall grazing pressure and enhanced the Phaeocystis blooming (Hensen et al. 1993, 1997). In present study, although the coupling between Phaeocystis and ciliates was not detected as expected, obvious correlations among the ciliates and mesozooplankton assemblages still reflected some cues on the trophic relationships in Phaeocystis-dominated ecosystem in the nature conditions, implying that trophic efficiency was dependent on the complex food-chain structures.
Of course, due to the complexity of bottom-up and top-down controls of planktonic community structure and the difficulty in interpreting the existing data on the unique Phaeocystis-dominated ecosystem, the trophic relationships cannot be fully understood by simple correlation due to its multiple life stages and sizes (Schoemann et al. 2005). Therefore, to better understand the trophic relationships in Phaeocystis-dominated ecosystem, a most important process is to clarify the mechanisms controlling transition between the free-living cells and colonies of Phaeocystis both in laboratory and field study in the future.
Conclusion
In this descriptive study we have investigated the diversity and variation of the planktonic community in a Phaeocystis-dominated ecosystem using a variety of analysis methods. As a semi-closed shallow subtropical bay, environmental factors played an important role in affecting the abundance and distribution of the planktonic assemblages, in particular the temperature and salinity. No significant correlation between P. globosa and ciliates assemblage was detected, but negative impact upon the biomass of mesozooplankton and B. sinicus abundance was significant, suggesting that P. globosa was uncoupled from the grazing by both ciliates and mesozooplankton when appearing as colonies form. As consequence, mesozooplankton might switch to ciliates as food source and the predation upon ciliates was strengthened during the Phaeocystis bloom, but the effects were species-specific. In summary, trophic efficiency was not only dependent on the complex food-chain structures within the food web, but also on the bottom-up processes, involving the ambient physic-chemical factors, particular in coastal systems.
References
Carey PG (1992) Marine interstitial ciliates. Chapman and Hall, London, pp 14–324
Chen MR, Chen BZ, Harrison P, Liu HB (2011) Dynamics of mesozooplankton assemblages in subtropical coastal waters of Hong Kong: a comparative study between an eutrophic estuarine and a mesotrophic coastal site. Cont Shelf Res 31:1075–1086
Clarke KR, Gorley RN (2006) PRIMER v6. User Manual/Tutorial. PRIMER-E, Plymouth
Clarke AL, Weckstrom K, Conley DJ, Anderson NJ, Adser F, deJonge VN, Ellegaard M, Juggins S, Korhola A, Reuss N, Telford RJ, Vaalgamaa S (2006) Long-term trends in eutrophication and nutrients in the coastal zone. Limnol Oceanogr 51:385–397
Cohen RA, Fong P (2004) Responses of bloom-forming green macroalga to short-term change in salinity, nutrients, and light help explain its ecological success. Estuaries 27:209–216
Davies AG, de Madariaga I, Bautista B, Fernandez E, Harbour DS, Serret P, Tranter PRG (1992) The ecology of a coastal Phaeocystis bloom in the northwestern English Channel in 1990. J Mar Biol Assoc UK 72:691–708
Dickerson KD, Medley KA, Havel JE (2010) Spatial variation in zooplankton community structure is related to hydrologic flow units in the Missouri River, USA. River Res Appl 26:605–618
Giberto DA, Bremec CS, Cortelezzi A, Rodrigues A, Capitulo A, Brazeiro A (2007) Ecological boundaries in estuaries: macrobenthic β-diversity in the Río de la Plata system (34–36°S). J Mar Biol Assoc UK 87:5012–5016
Grattepanche JD, Vincent D, Breton E, Christaki U (2011) Microzooplankton herbivory during the diatom–Phaeocystis spring succession in the eastern English Channel. J Exp Mar Biol Ecol 404:87–97
Hamm CE (2000) Architecture, ecology and biogeochemistry of Phaeocystis colonies. J Sea Res 43:307–315
Hamm CE, Rousseau V (2003) Composition, assimilation and degradation of Phaeocystis globosa-derived fatty acids in the North Sea. J Sea Res 50:271–283
Hamm CE, Simson DA, Merkel R, Smetacek V (1999) Colonies of Phaeocystis globosa are protected by a thin but tough skin. Mar Ecol Prog Ser 187:101–111
Hensen FC, Reckermann M, Klein Breteler WCM, Riegman R (1993) Phaeocystis blooming enhanced by copepod predation on protozoa: evidence from incubation experiments. Mar Ecol Prog Ser 102:51–57
Hensen PJ, Bjørnsen PK, Hansen BW (1997) Zooplankton grazing and growth: scaling within the 2–2,000 μm body size range. Limnol Oceanogr 42:687–704
Huang CJ, Dong QX, Zheng L (1999) Taxonomic and ecological studies on a large scale Phaeocystis pouchetii bloom in the southeast cast of China during late 1997. Oceanologia et Limnologia Sinica 30:581–589 (In Chinese with English abstract)
Huang TW, Wang XD, Wang Y (2012) Growth, architecture and cell distribution in Phaeocystis globosa colonies. Chin Bull Bot 47(5):508–514
Jakobsen HH, Tang KW (2002) Effects of protozoan grazing on colony formation in Phaeocystis globosa (Prymnesiophyceae) and the potential costs and benefits. Aquat Microb Ecol 27:261–273
Johnson MD, Stoecker DK, Marshall HG (2013) Seasonal dynamics of Mesodinium rubrum in Chesapeake Bay. J Plankton Res 35(4):877–893
Kchaou N, Elloumi J, Drira Z, Hamza A, Ayadi H, Bouain A, Aleya L (2009) Distribution of ciliates in relation to environmental factors along the coastline of the Gulf of Gabes, Tunisia. Estuar Coast Shelf Sci 83:414–424
Kirkwood DS, Aminot A, Carlberg SR (1996) The 1994 quasimeme laboratory performances study: nutrients in seawater and standard solutions. Mar Pollut Bull 32:640–645
Kofoid CA, Campbell AS (1929) A conspectus of the marine and freshwater Ciliata belonging to the Suborder Tintinoinea, with descriptions of new species principally from the Agassiz Expedition to the Eastern Tropical Pacific 1904–1905. University of California Press, Los Angeles, pp 1–403
Kofoid CA, Campbell AS (1939) The Ciliata: the Tintinnoinea. Museum of Comparative Zoology, Boston, pp 1–473
Lan WL (2012) Study on reasons and prevention measures of eutrophication during 2006–2010 in Maowei Sea. Environ Sci Manag 37(8):39–44 (In Chinese with English abstract)
Li KZ, Yin JQ, Huang LM, Tan YH (2006) Spatial and temporal variations of mesozooplankton in the Pearl River estuary, China. Estuar Coast Shelf S 67:543–552
Li YN, Shen PP, Huang LM, Qi YZ (2012) Taxonomy and phylogenetics of the genus Phaeocystis: research progress. Chin J Ecol 31(3):745–754 (In Chinese with English abstract)
Liu JS, Marion VR, Yang WD, Peng XC, Lu SH, Wang Y, Chen JF, Wang ZH, Qi YZ (2010) Negative effects of Phaeocystis globosa on microalgae. Chin J Oceanol Limnol 28:911–916 (In Chinese with English abstract)
Lynn DH, Roff JC, Hopcroft RR (1991) Annual abundance and biomass of aloricate ciliates in tropical neritic waters off Kingston. Jam Mar Biol 110:437–448
Maeda M, Carey PG (1985) An illustrated guide to the species of the family Strombidiidae (Oligotrichida, Ciliophora), free swimming protozoa common in the marine environment. Bull Ocean Res Inst Univ Tokyo 19:1–68
Muylaert K, Sabbe K, Vyverman W (2000) Spatial and temporal dynamics of phytoplankton communities in a freshwater tidal estuary (Schelde, Belgium). Estuar Coast Shelf Sci 50:673–687
Nejstgaard JC, Tang KW, Steinke M, Dutz J, Koski M, Antajan E, Long JD (2007) Zooplankton grazing on Phaeocystis: a quantitative review and future challenges. Biogeochemistry 83:147–172
Parsons TR, Maita Y, Lalli CM (1984) A manual of chemical and biological methods for seawater analyses. Pergamon Press, Oxford, p 173
Peng XC, Yang WD, Liu JS, Peng ZY, Lv SH, Ding WZ (2005) Characterization of the hemolytic properties of an extract from Phaeocystis globosa Scherffel. J Integr Plant Biol 47:165–171
Peperzak L, Duin RNM, Colijn F, Gieskes WWC (2000) Growth and mortality of flagellates and non-flagellate cells of Phaeocystis globosa (Prymnesiophyceae). J Plankton Res 22(1):107–120
Pierce RW, Turner JT (1992) Ecology of planktonic ciliates in marine food webs. Rev Aquat Sci 6:139–181
Riegman R, van Boekel W (1996) The ecophysiology of Phaeocystis globosa: a review. J Sea Res 35:235–242
Schoemann V, Becquevort S, Stefels J, Rousseau V, Lancelot C (2005) Phaeocystis blooms in the global ocean and their controlling mechanisms: a review. J Sea Res 53:43–66
Shen PP, Qi YZ, Wang Y, Huang LM (2011a) Phaeocystis globosa Scherffel, a harmful microalga, and its production of dimethylsulfoniopropionate. Chin J Oceanol Limnol 29(4):869–873
Shen PP, Li G, Huang LM, Zhang JL, Tan YH (2011b) Spatio-temporal variability of phytoplankton assemblages in the Pearl River estuary, with special reference to the influence of turbidity and temperature. Cont Shelf Res 31:1672–1681
Shen PP, Li YN, Qi YZ, Zhang LP, Tan YH, Huang LM (2012) Morphology and bloom dynamics of Cochlodinium geminatum (Schütt) Schütt in the Pearl River Estuary, South China Sea. Harmful Algae 13:10–19
Steen H (2004) Interspecific competition between Enteromorpha (Ulvales: Chlorophyceae) and Fucus (Fucales: Phaeophyceae) germlings: effects of nutrient concentration, temperature, and settlement density. Mar Ecol Prog Ser 278:89–101
Tang KW, Jakobsen HH, Visser AW (2001) Phaeocystis globosa (Prymnesiophyceae) and the planktonic food web: feeding, growth, and trophic interactions among grazers. Limnol Oceanogr 46:1860–1870
Teixeira IG, Crespo BG, Nielsen TG (2012) Role of microzooplankton during a Phaeocystis sp. bloom in the Oosterschelde (SW Netherlands). J Mar Syst 94:97–106
Utermöhl H (1958) Zurvervolkommungder quantitativen phytoplankton Methodik. Mitteilungen der Internationale Vereinigung fur Theoretische und Angewandte. Limnologie 9:1–38
Verity PG, Smayda TJ (1989) Nutritional value of Phaeocystis pouchetii (Prymnesiophyceae) and other phytoplankton for Acartia spp. (Copepoda): ingestion, egg production, and growth of nauplii. Mar Biol 100:161–171
Verity PG, Smetacek V (1996) Organism life cycles, predation, and the structure of marine pelaic ecosystems. Mar Ecol Prog Ser 130:277–293
Widdicombe CES, Archer SD, Burkill PH, Stefels J (2004) Microzooplankton grazing in Phaeocystis and diatom-dominated waters in the southern North Sea in spring. J Sea Res 51:37–51
Worm B, Lotze HK (2006) Effects of eutrophication, grazing, and algal blooms on rocky shores. Limnol Oceanogr 51:569–579
Acknowledgments
We thank Dr. Gang Li for his help in revision of the manuscript. This research was supported by the financial fund of the ministry of agriculture (NFZX2013), National Basic Research Program of China (973 Program, 2015CB452903), Special Fund for Agro-scientific Research in the Public Interest (201403008), Guangdong Natural Science Foundation (S2013040016424, 1015030101000002), Shantou Science and Technology Project (No. 99) and Open Project of the Key Laboratory of Marine Bio-resources and Ecology (LMB131004).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, HX., Huang, HH., Xu, SN. et al. Planktonic community structure during a harmful bloom of Phaeocystis globosa in a subtropical bay, with special reference to the ciliate assemblages. Ecotoxicology 24, 1419–1429 (2015). https://doi.org/10.1007/s10646-015-1464-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-015-1464-2