Abstract
The extensive use of pharmaceuticals has resulted in the intensive contamination of water bodies. Tetracycline is a type of antibiotic and its potential toxicity is causing environmental concern. The effects of developmental toxicity and the mechanisms of tetracycline on fish embryos are not well understood. Zebrafish embryos are used in this study to investigate the developmental toxicity of this compound. Four hour post-fertilization (hpf) zebrafish embryos are exposed to different concentrations of tetracycline until 96 hpf. The larvae display developmental delay phenotypes, including hatching delay, shorter body length, increased yolk sac area and uninflated swim bladder upon exposure to tetracycline. Delayed yolk sac absorption and swim bladder deficiency at 96 hpf are observed in the zebrafish larvae upon exposure to 20 μg/L of tetracycline. To test whether tetracycline causes oxidative damage and the resulting oxidative stress-induced apoptosis, the generation of reactive oxygen species (ROS), Acridine Orange staining and real time polymerase chain reaction have been performed in this study. The results indicate that tetracycline exposure results in significant increases in ROS production and cell apoptosis, mainly in the tail areas at 96 hpf. The gene expression pattern demonstrates that tetracycline induces ROS which causes apoptosis in the zebrafish larvae, and the results also indicate that caspase-dependent apoptotic pathways may greatly contribute to tetracycline-induced apoptosis in the early-life stages of the zebrafish. In addition, we have investigated the effects of tetracycline on marker genes related to resistance mechanisms and gene regulating drug biotransformation. The results of these gene expression studies indicate that tetracycline could induce zebrafish to resist pharmaceuticals and Cytochrome P450s that are involved in the biotransformation of tetracycline in zebrafish larvae. The overall results indicate that tetracycline can produce oxidative stress and induce apoptosis, which brings about significant developmental delay in zebrafish embryos.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pharmaceuticals have been widely used as medication for humans to treat or prevent diseases, and as veterinary drugs and husbandry growth promoters in agricultural areas (Halling-Sørensen et al. 1998). As a result of wider usage, increased environmental concentrations and highly potent toxicity, pharmaceuticals have been recently identified as one of the major emerging environmental pollutants. Pharmaceuticals have been detected in the natural environment across the world (Kolpin et al. 2002; Ramirez et al. 2009; Yan et al. 2013). Landfill, and animal, freshwater aquaculture, hospital and industrial wastes as well as domestic waste are the major sources of environmental pharmaceuticals (Eggen et al. 2012; Pal et al. 2010; Lapworth et al. 2012). Although reported concentrations of pharmaceuticals in the environment are generally low, due to continuous emission and their resistance to degradation, many of the commonly found pharmaceuticals are pseudo-persistent in the environment (Monteiro and Boxall 2009; Peck 2006). The prevalence of pharmaceuticals is ubiquitous in water resources. The continuous discharging of pharmaceuticals into the environment might lead to uncertain ecological effects because they may act in an unpredictable manner when mixed with other chemicals (Gilbert 2012; Li 2014). Previous studies have demonstrated that pharmaceuticals can induce negative non-targeting effects onto aquatic organisms at different trophic levels, such as algae, fish and mussels (Wollenberger et al. 2000; Mehinto et al. 2010; Liu et al. 2011; Vannini et al. 2011; Cuklev et al. 2011; Gilbert 2011; Brodin et al. 2013; Ledford 2013).
Antibiotics, as a pharmaceutical product, have been used in both humans and animals for treating diseases, and also in the animal industry as growth promoters (Sapkota et al. 2008). Antibiotics have become more commonly used in the commercial and aquaculture industries, especially in developing countries in recent decades. According to statistics, the consumption of antibiotics can be up to 100,000–200,000 tons every year around the world (Kümmerer 2009). China, as the world’s largest market to produce and consume pharmaceutical products (Richardson et al. 2005), consumes more than 25,000 tons of antibiotics each year (Xu et al. 2007). The wide use and increased production of antibiotics has resulted in the frequent appearance of antibiotics in sewage, surface waters, sediments and biota around the world, especially in China (Gulkowska et al. 2007; Jiang et al. 2008; Lin and Tsai 2009; Xu et al. 2009; Zuccato et al. 2010; Rosal et al. 2010; Zhou et al. 2011; Luo et al. 2011; Jiang et al. 2011; Ji et al. 2011; Zou et al. 2011; Shen et al. 2012; Yan et al. 2013). Recent studies have demonstrated that antibiotics are toxic to aquatic organisms (Carlsson et al. 2013; Madureira et al. 2011; Lin et al. 2013). Furthermore, continuous exposure to low concentrations of antibiotics in the environment can induce antibiotic resistance genes (Thomas and Foster 2004; Kim et al. 2007; Zhang and Zhang 2011; Zhu et al. 2013).
Tetracycline antibiotics are a class of broad spectrum antibiotics produced by Streptomyces. Tetracycline antibiotics usually bind to 30S ribosomal subunits and prevent ammonia acyl tRNA from combining with ribosome. In recent years, with the rapid development of offshore intensive animal husbandry and aquaculture, tetracycline antibiotics are applied as a veterinary drug and feed additive, and its usage has increased every year. The annual consumption amount of antibiotics in the European Union (EU) is 5,000 tons, and the annual consumption amount of tetracycline antibiotics is about 2,300 tons, which is about 46 % of the total (Wang and Wei 2013). China produces about 210,000 tons of antibiotics each year, and 97,000 tons of raw materials are used for animal husbandry and aquaculture, which account for 46.1 % of the total consumption (Chen 2013). Tetracycline antibiotics are mainly used in the livestock and poultry breeding industries in China and other parts of the world (Wei et al. 2004; Sarmah et al. 2006).
As most antibiotic drugs used on animals cannot be fully absorbed, they are expelled along with their waste into sewage treatment plants, or directly into the environment (Sarmah et al. 2006). In recent years, scholars have identified the presence of antibiotics in farm wastewater, surface water and groundwater in the Shanghai waters (Jiang et al. 2008, 2011; Ji et al. 2011; Shen et al. 2012; Yan et al. 2013). The flow of antibiotics and their metabolites into surface waters poses a potential risk to aquatic organisms. Several widely reported antibiotics are scored based on their environmental concentrations combined with their LC50 values in mice (Zhang et al. 2014). The results are summarized in Table S1, and it indicates that tetracycline may have higher toxicity in China surface waters as compared with other antibiotics. Tetracycline is hence chosen as the target for further examination in this study.
With regard to the potential ecotoxicity of antibiotic contamination to aquatic organisms, there has been much concern in recent years. The toxicity of tetracycline to aquatic organisms has been reported in several studies. The inhibition of protein synthesis and chloroplast formation is the primary negative effects of tetracycline on microalgae (Halling-Sørensen 2000; Ferreira et al. 2007; Xu et al. 2013). Tetracycline can inhibit plant growth via inhibiting chloroplast synthase (Bradel et al. 2000; Kasai et al. 2004). Tetracycline also inhibits the activity of several important enzymes (Lunden et al. 1998), and damage the DNA of exposed fish (Qu et al. 2004; Li et al. 2006). However, little is known about the potential developmental toxicity of tetracycline antibiotics to aquatic organisms.
Fish, as a vertebrate at the top of the food chain, plays a very important role in aquatic ecosystems. Fish is also one of the major food and nutrient sources for humans. Fish embryo assays are considered as pain-free in vivo tests and accepted as a replacement of animal experiments (Voelker et al. 2007). Zebrafish is one of the most widely adopted model species for investigating the developmental toxicity of chemical exposure during early life stages. Zebrafish embryo is small in size and transparent, and easy to maintain and handle, and the species has high fecundity, rapid embryogenesis and continuous reproduction. In addition, the zebrafish genome has been sequenced and genetic information is rapidly accumulating (http://zfin.org), which makes it feasible to find the genes and pathways involved in toxicant exposure.
In the present study, zebrafish embryos are employed to investigate the developmental toxicity of tetracycline. Different endpoints, such as survivorship, hatching rates, morphology of the larvae, as well as certain gene expression levels have been selected for this study. However, some endpoints employed in this study (e.g., hatching rates, phenotypes, larvae survival) may provide low sensitivity compared to effects detected during low-level exposure, and these endpoints generally do not provide any information on the mode of action that leads to toxic effects. Alternatively, approaches that use gene expression are likely to indicate long-term detrimental effects (Voelker et al. 2007). Therefore, genes that are involved in apoptosis (p53, AIF, Bax, Apaf-1, Bcl-2, Cyt c, and Caspases-3, 6, 7, 8 and 9), oxidative damage (CAT, SOD), multidrug resistance (ABCC1, ABCC2 and ABCC4) and genes that play critical roles in detoxification (CYP1A) are also investigated. Finally, apoptotic cell staining and reactive oxygen species (ROS) detection and total protein content are studied to elucidate the toxicity mechanism of tetracycline in zebrafish embryos.
Materials and methods
Chemicals and reagents
Tetracycline (purity: 90 %) was purchased from MERYER CO LTD. (Shanghai, China). Tricaine (MS-222) and RNAlater Storage Solution were obtained from Sigma-Aldrich Shanghai Trading Co. Ltd. (China). All other chemicals and reagents utilized in this study are analytical grade.
Data collection strategies
To provide an overall of view of antibiotics research in the aquatic environment in Shanghai waters, a systematic literature review was performed by carrying out electronic searches on the ISI Web of Knowledge, PubMed, Elsevier, Springer, and Google® Scholar. Literature published in Chinese was retrieved from the China Knowledge Resource Integrated Database and the WANFANG Data of E-Resources for China Studies. Given the large number of studies found in the literature, our study focused on those which were the most relevant to China aquatic environments. We collected the data that were considered to be useful, and calculated the median of the data to reflect the average level of tetracycline antibiotics in different water areas within these years stated in the studies.
Zebrafish maintenance, embryo collection and experimental setup
Wild-type (AB strain) zebrafish were maintained at 28 ± 0.5 °C in a 14:10 h light:dark cycle in a closed flow-through system with charcoal-filtered tap water. The fish were fed brine shrimp twice daily. The zebrafish embryos were obtained from spawning adults placed in groups of about 20 males and 10 females in tanks overnight. Spawning was induced in the morning when the light was turned on. At 4 h post-fertilization (hpf), the embryos were examined under a dissection microscope (Motic, Xiamen, China), and embryos that had developed normally and reached the blastula stage were selected for subsequent experiments. Twenty embryos were randomly placed into each well of 24 tissue culture plates which contained 2 mL of solution that comprised 2 mM CaCl2·2H2O, 0.5 mM MgSO4·7H2O, 0.75 mM NaHCO3 and 0.08 mM KCl (ISO 6341-1982). A series of tetracycline hydrochloride concentrations (0, 2, 10, 20, 200, 2000 and 20,000 μg/L) were applied for the acute exposure and gene expression studies. Four replicates for each concentration were used. The water was aerated for 24 h prior to the preparation of the treatment solutions. The solutions were changed once every 24 h. All of the embryos or larvae were kept at 28 ± 0.5 °C and subjected to a no light environment.
Measurement of the toxicity endpoints
The development of zebrafish embryos and larvae was monitored at specified time points. Endpoints used for assessing the developmental effects of tetracycline hydrochloride on the zebrafish included survival, hatching success, body length, and other morphological and functional malformations. The hatching was monitored from 48 to 72 hpf, and survival rates were recorded at 24, 48, 72 and 96 hpf. The body length (96 hpf) of each zebrafish was measured by using a dissection microscope (Leica, Wetzlar, Germany) through which embryos and larvae in each replicate were examined for malformation at 96 hpf. After the measurement, the embryos or larvae were returned to the well for subsequent experiments and the dead were discarded. The experimental temperature was maintained at 28 ± 0.5 °C.
Acridine orange staining
Acridine orange (AO) staining is a nucleic acid selective metachromatic stain technique that can identify cell apoptosis (Chan and Cheng 2003; Deng et al. 2009; Zeng et al. 2014; Yuan et al. 2014). After 96 h of exposure to a series of tetracycline hydrochloride concentrations (0, 5, 10, and 20 μg/L), five larvae from each replicate (n = 4) were washed twice in 30 % Danieau’s solution (58 mM of NaCl, 0.7 mM of KCl, 0.4 mM of MgSO4, 0.6 mM of Ca(NO3)2, and 5 mM of HEPES, pH 7.4), and then transferred into 5 μg/mL of AO dissolved in 30 % Danieau’s solution for 20 min at room temperature. The larvae were then washed with 30 % Danieau’s solution three times for 5 min each. Before examination, the embryos were anesthetized with 0.016 M tricaine for 3 min. The apoptotic cells were identified with a fluorescence microscope (Leica, Wetzlar, Germany). The apoptotic cells appeared as obvious bright spots.
ROS measurement
The generation of ROS in the embryos exposed to tetracycline hydrochloride (0, 2, 10 and 20 μg/L) at 96 hpf was measured by using dichloro-dihydro-fluorescein diacetate (DCFH-DA; Zeng et al. 2014) obtained from the Beyotime Institute of Biotechnology (Jiangsu, China). Twenty larvae were washed with cold 30 % Danieau’s solution three times and then incubated with 20 μM/L DCFH-DA solution for 1 h. After 1 h incubation, the larvae were washed with 30 % Danieau’s solution three times, and then homogenized in 200 μL cold 30 % Danieau’s solution. The homogenate was centrifuged at 8,000×g at 4 °C for 4 min, and the supernatant (200 μL) was transferred into a 96-well plate and incubated at room temperature for 5 min. The fluorescence intensity was measured by using a SpectraMax M5 multi-mode microplate reader (Molecular Device, USA) with excitation and emission at 485 and 530 nm, respectively.
Total protein content assay
Briefly, the embryos or larvae were treated from 4 to 72 hpf before collection for analysis. To evaluate the effect of the deyolking process, the yolk sacs were first ruptured with a fine needle and the deyolked embryos were washed twice with a phosphate buffer saline (PBS; pH 7.4) and then homogenized in an ice-cold protein extraction buffer to extract the proteins. The homogenates were centrifuged for 30 min at 12,000×g and the supernatants were collected. Protein concentrations were determined by using the Bradford method (Bradford 1976).
Total RNA extraction and RT-PCR
The total RNA was extracted from the studied zebrafish embryos or larvae by using the Qiagen kit (Qiagen China Co., Ltd, Shanghai, China). The total RNA contents were determined by measuring the absorbance at 260 nm and the quality was verified by measuring the 260/280 nm ratio. One percent agarose formaldehyde gel electrophoresis with ethidium bromide staining was used to further verify the quality of the total RNA. First-strand cDNA synthesis was performed by using a commercial kit (Takara, Kyoto, Japan) and following the manufacturer’s instructions. Real-time quantitative polymerase chain reaction (PCR) was carried out by using an ABI-7500 detection system (Applied Biosystems, Foster City, CA, USA).
In order to examine whether tetracycline hydrochloride has effects on the oxidative damage of the genes of zebrafish embryos, we conducted a preliminary study on the signaling pathways related to apoptosis. A total of 2 genes related to oxidative damage (CAT, SOD), 11 genes related to apoptosis (p53, AIF, Bax, Apaf-1, Bcl-2, Cyt c, Caspases-3, 6, 7, 8 and 9), 3 genes related to ABC transporter proteins (ABCC1, ABCC2 and ABCC4) and 1 gene related to drug metabolism (Cytochrome P450, CYP1A) were chosen in this study. β-actin was chosen as the internal control to normalize the data. The primers for these genes are listed in Table S2. Amplification was carried out in 96-well PCR plates (Applied Biosystems, Foster City, CA, USA) with a volume of 20 μL, which contained 10 μL of 2 × SYBER Green real time PCR master mix from Takara Biotechnology Co Ltd (Dalian), 0.4 μM of each primer and 2 μL of 10 × diluted cDNA samples. All of the reactions were carried out in triplicate. The reactions were carried out in the following order: temperature of 95 °C for 1 min (1 cycle) and 95 °C for 3 s, and 34 s at the annealing temperatures (60 °C for β-actin, p53, AIF, Bax, Apaf-1, Bcl-2, Cyt c, Caspases-3, 6, 7, 8 and 9, CAT, SOD, ABCC1, ABCC2, ABCC4 genes; 62 °C for CYP1A), 72 °C for 20 s (40 cycles), followed by a melting curve analysis to determine the specificity of the reaction. The ΔΔCT method was used to calculate the relative mRNA expression levels of genes with the β-actin gene.
Statistical analysis
All values were presented as the mean ± standard error (SE) from four separate experiments. The assumptions of homogeneity of variances were checked by Levene’s test. The main factor for analysis of variance was treatment. Significant differences between mean values were determined using one-way analysis of variance (ANOVA), and the Dunnett’s test was used to determine the significant difference (p < 0.05) between tetracycline-treated and control groups. Percentage data were arc-sin square-root-transformed before analysis. The ANOVA was performed using SPSS 17.0 (SPSS, Chicago, IL, USA) and the figures were generated by using Graphpad Prism 5.0. A value of p < 0.05 was used as the criterion for statistical significance.
Results
Tetracycline antibiotics in China water bodies
Four kinds of tetracycline antibiotics have been detected in different waters in recent years, as shown in Table 1. Taking both concentration and detection frequency into consideration, tetracycline and oxytetracycline are found to be the two major antibiotics among the tetracycline antibiotics that exist in the different waters of China. Besides that, between the two antibiotics, the concentration of tetracycline is lower than that of oxytetracycline in surface water. However, in the inflow of sewage plants, tetracycline is higher than oxytetracycline. Specific to tetracycline, livestock husbandry wastewater is the main water resource for the aquatic environment. Overall, compared with other waters, the concentrations of all of the tetracycline antibiotics in livestock husbandry wastewater are the highest. Antibiotics lead to high toxicity and environmental risk to aquatic organisms when wastewaters are discharged into natural water. The toxicity data of several major antibiotics in mice is collected, and Table S1 shows that tetracycline poses the greatest potential risk in the aquatic environment.
Effect of tetracycline on zebrafish embryos and larvae
The exposure study showed that tetracycline has significant effects on the growth and development of zebrafish embryos and larvae, including decreased body length, delayed hatching, increased yolk sac area and absence of a swim bladder.
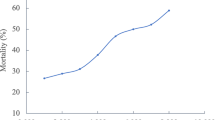
The fitting curve of the dose–response effect of tetracycline on the embryos/larvae is given in Fig. 1. The total effects of tetracycline on the zebrafish embryos/larvae increased in a dose–response manner. The fitting curve equation is shown in Fig. 1. The EC10 of tetracycline to zebrafish larvae after 96 h of exposure is 3.16 μg/L, which is close to its environmental concentrations (0.26–1 μg/L; Shen et al., 2012), while the concentration from wastewater that originated from breeding farms is even higher than the EC10 here (Ji et al., 2011). This result suggests that the tetracycline in the aquatic environment has potential ecotoxicological effects on aquatic organisms.
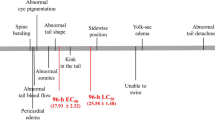
As shown in Fig. 2, compared with the control group (Fig. 2a), the group treated with 20 μg/L of tetracycline shows an increased yolk sac area, uninflated swim bladder and shorter body length. The percentage of major malformation phenotypes in the zebrafish embryos upon exposure to 20 μg/L tetracycline (Fig. 2c), and the correlation of different phenotype effects (Fig. 2d) are recorded at 96 hpf.
Representative control (a) and treated zebrafish embryos (20 μg/L, tetracycline) at 96 hpf. Exposure to tetracycline induces serious developmental delays in the treated zebrafish embryos, including uninflated swim bladder (red arrows in a, b), decreased body length and delayed absorption of the yolk sac. Percentage of major phenotypes in the zebrafish embryos upon exposure to 20 μg/L tetracycline at 96 hpf (c), and correlation of different phenotype effects (d). (n = 80) Scar bar = 300 μm (Color figure online)
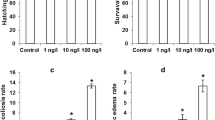
The growth in terms of the body length of the larvae was measured at 96 hpf and the results are shown in Fig. 3c. All of the concentrations of tetracycline do not have a significant effect on body length compared to the controls.
Over 98 % of the control zebrafish embryos hatched out of the chorion at 72 hpf (Fig. 3b). Even though there was not a significant dose-dependent decrease in the hatching rate of the tetracycline-treated groups compared with the control groups (p < 0.05), with hatching rates of 90.0 ± 0.2 % in 200 μg/L,tetracycline could significantly inhibit hatching at higher concentrations (>200 μg/L), and no embryo hatched above 500 mg/L (data not shown). At 96 hpf, the hatching rate restored in the treated groups (200 μg/L) (data not shown). However, no embryos treated with more than 500 mg/L of tetracycline successfully hatched out at 96 hpf.
In the most affected groups, the developmental abnormalities included increased yolk sac area and lack of a swim bladder (Fig. 3a, d). Among these, the yolk sac area in the control groups was significantly larger than the groups treated with 2, 20 and 200 μg/L of tetracycline, and also, the groups treated with 20 and 200 μg/L of tetracycline had a swim bladder deficiency.
AO staining
No obvious apoptotic cells were observed in the control embryos, whereas in the groups treated with 5, 10 and 20 μg/L of tetracycline, apoptotic cells appeared, mainly around the tail area, but in the groups treated with 20 μg/L of tetracycline, some of the apoptotic cells appeared around the heart area in a few of the larvae (Fig. 4).
Effect of tetracycline on production of ROS in zebrafish larvae
The fluorescence intensity of ROS in the zebrafish larvae at 96 hpf is shown in Fig. 5. No significant difference in the level of ROS production can be observed in the larvae treated with 2 μg/L of tetracycline when compared with the control. However, ROS levels in the embryos treated with higher concentrations of tetracycline (10 and 10 μg/L) are significantly higher than that of the control.
Total protein content assay
The total protein content of the zebrafish larvae at 72 hpf is shown in Fig. 6. The results show that there is no significant difference in the larvae treated with all of the concentrations (2, 20, and 200 μg/L) when compared with the control. However, this trend shows that higher tetracycline concentrations may inhibit the protein synthesis of zebrafish embryos or larvae.
Gene expression studies
Environmental pollutants are well-known inducers of ROS, and ROS can be reason for the depletion of antioxidant defenses (Livingstone 2001). The results demonstrated that tetracycline induced up-regulation of two ROS related genes (SOD and CAT) (Table 2) and ROS overproduction in zebrafish embryos at 96 hpf (Fig. 5). The mRNA expressions of p53, Apaf-1, Cyt c, and Caspases-3 and 9 genes from the zebrafish larvae were significantly up-regulated for the group treated with 10 μg/L of tetracycline (96 hpf) (Table 2), and the expression of Bcl-2 was significantly down-regulated for the same group (Table 2). However, the expression of AIF, Bax, and Caspases-3, 7 and 8 did not significantly change in the group treated with 10 μg/L of tetracycline (some data not shown). After 96 hpf exposure, the expression of the ABCC1, ABCC2 and ABCC4 genes significantly changed upon exposure to tetracycline (Table 2). The expression level of ABCC1 was significantly increased by 1.52, 1.83 and 1.64-fold in the groups treated with 10, 20 and 200 μg/L of tetracycline (Table 2). Moreover, the expressions of ABCC2 and ABCC4 were significantly up-regulated in the groups treated with 20 and 10 μg/L of tetracycline respectively (Table 2). The RT-PCR data demonstrated that CYP1A was significantly increased in the larvae treated with 20 and 200 μg/L of tetracycline compared with the control (Table 2).
Discussion
Zebrafish embryo serves as a widely adopted model for studies of mechanism-based developmental toxicity (Shi et al. 2008). Our results showed that tetracycline significantly affects embryo’s early development. The results shown in Figs. 1, 2, 3 indicate that tetracycline affects the development of embryos in a dose-dependent manner. Exposure to tetracycline has significant negative effects on the survival of the treated embryos and larvae. In this study, a significant increase in mortality was observed with exposure to 500 mg/L of tetracycline between 4 and 96 hpf (data not shown), and the LC10 and LC50 are 551.78 and 759.71 mg/L, respectively. The results indicate that the acute toxicity of tetracycline to zebrafish embryos is low. The growth inhibition in zebrafish embryos might be a secondary effect of tetracycline mediated toxicity caused by the malabsorption of nutrients required for normal development (Hill et al. 2004; Du et al. 2012).
Body length is an important indicator of embryo growth, and the loss of nutrients may induce a shorter body length. In the control group, the larvae were healthy and active with a body length of 3.8 ± 0.04 mm, had an inflated swim bladder, and could absorb nutrients from the yolk sac at 96 hpf. Zebrafish embryos exposed to lower concentrations (2, 20 and 200 μg/L) of tetracycline exhibited a significant (p < 0.05) reduction in the inflation of their swim bladder and delayed yolk sac absorption (Fig. 3).
Hatching is known to be a critical period of time for zebrafish embryogenesis. Delayed hatching may be due to retarded development or the inability of embryos to break the chorion (Osman et al. 2007). Hatching delay or hatching failure will induce the death of embryos. Hatching is often the result of a combination of biochemical (enzymatic), biophysical (mechanical) and osmotic mechanisms (Yamagami 1981), and so, the observed inhibition may have resulted from the effects of tetracycline on more than one or all of these mechanisms. The marked difference in the hatching rates of the embryos with higher concentrations of tetracycline compared to the control is likely to be a function of their slower development.
The yolk sac plays an important role during the early developmental stage, because it is the only source of nutrition for embryos, and its physical size will decrease along with the embryonic development. The swim bladder is an air-filled sac located dorsally in the abdominal cavity, which helps fish to balance hydrostatic pressure and reduce the energetic cost of swimming (Jönsson et al. 2012). No deformities were noticed in the control group. However, in the treated group, both delayed yolk sac absorption and uninflated swim bladder appeared. These results illustrate that tetracycline can significantly delay the development of zebrafish embryos.
Furthermore, tetracycline exposure results in the superfluous generation of ROS in zebrafish embryos. Studies have demonstrated that embryonic development is especially sensitive to ROS and the resulting oxidative stress-induced cell apoptosis is thought to contribute to abnormal embryonic development (Yamashita 2003; Shi and Zhou 2010). The results of the AO staining are consistent with the speculation.
Apoptosis, also called as programmed cell death (Ulukaya et al. 2011), is an important regulator of growth and development. In the present study, apoptotic cells are observed in the tail of the zebrafish larvae as revealed by AO staining (Fig. 4). Generally, the p53 and caspase pathways are the two main pathways involved in contaminant-triggered apoptosis. Typically, the p53 pathway induces apoptosis by up-regulating the transcription of pro-apoptotic genes, such as p53 and Bax, and down-regulating anti-apoptotic genes, including the Bcl-2. Up-regulation of p53 can lead to apoptosis by the up-regulating expression of Bax and down-regulating expression of Bcl-2, which was observed in some studies (Li et al. 2011; Zeng et al. 2014). In this study, in the group exposed to 10 μg/L of tetracycline, up-regulation of p53 and down-regulation of Bcl-2 were detected, but did not include the up-regulation of Bax. So, the p53-Bax-Bcl-2 pathway is not the pathway for tetracycline-induced apoptosis.
The caspase pathway is also an important pathway involved in contaminant-triggered apoptosis (Xiong et al. 2009). Caspase-3 is confirmed as a key executor to be activated down-stream in apoptosis pathways (Liu et al. 2007; Deng et al. 2009). Generally, two pathways of caspase activation related to caspase pathways are involved in apoptosis. The first one is cytochrome c, which is released from the mitochondria, promotes caspase-3 activation through formation of the cytochrome c/Apaf-1/caspase-9-containing apoptosome coplex which then leads to apoptosis (Gao et al. 2013). Typically, the reduction of Bcl-2 factor expression has relation with activation of caspases 3 and 9 (Morales-Cano et al. 2013). In the latter, the stimulation of Fas, a tumour necrosis factor receptor (TNFR) or TNF-related apoptosis-inducing ligand receptor (TRAILR), results in the activation of the initiator caspase-8 (Fulda 2009). In the present study, the gene transcription of Apaf-1, Cyt c, and caspases-3 and -9 are greatly increased for the 10 μg/L of tetracycline treated groups, and the gene transcription of Bcl-2 is down-regulated for the groups treated with 10 μg/L of tetracycline. Taken together, these results indicate that caspase-dependent apoptotic pathways may greatly contribute to tetracycline-induced apoptosis in the early-life stages of zebrafish. The tetracycline-induced apoptosis pathway is shown in Fig. 7.
As an antibiotic, tetracycline can kill bacteria but also induce resistance. In tumor cells, an ATP-dependent decrease in cellular drug accumulation is often attributed to overexpression of certain ABC transporter proteins (Keppler et al. 1999). In addition to their roles in resisting anti-tumor drugs, these efflux pumps have important functions in the transport of a wide variety of compounds across biological membranes (Leslie et al. 2005; Takahashi et al. 2005). In aquatic organisms, the presence of ABC transporters which are involved in multixenobiotic resistance (MXR) has been demonstrated (Kingtong et al. 2007). It has been demonstrated that heavy metals can induce the overexpression of ABCC genes during the early development stage of zebrafish (2–120 hpf) and ABC transporters are involved in the efflux of heavy metals (Long et al. 2011a, b, c, d). In this study, we have investigated the transcriptional response of ABCC1, ABCC2, ABCC3 and ABCC4 cDNA from zebrafish larvae upon exposure to tetracycline. The significant changes of ABCC1, ABCC2 and ABCC4 mRNA expression upon exposure to a certain concentration of tetracycline suggest that zebrafish larvae may transfer tetracycline or degradation products out via multidrug resistance-associated proteins.
In zebrafish, Cytochrome P450s (CYPs) are important xenobiotic metabolizing proteins. CYPs are involved in the biotransformation of many endogenous and exogenous compounds, and have been characterized in eukaryotics (Otyepka et al. 2007; Seliskar and Rozman 2007, Smith and Wilson 2010), and the majority of zebrafish CYP genes are expressed in embryos (Goldstone et al. 2010). In this study, CYPs are involved in the biotransformation of tetracycline in zebrafish larvae.
In summary, the present study demonstrates that tetracycline has developmental toxicity on zebrafish embryos/larvae, and can significantly affect embryo development. The developmental toxicity may be related to the generation of ROS and the consequent triggering of apoptosis. In addition, gene expression studies shows that caspase-dependent apoptotic pathways may play important roles in tetracycline-induced apoptosis in the treated zebrafish embryos. However, the exact mechanisms still require further study.
References
Bradel BG, Preil W, Jeske H (2000) Remission of the free-branching pattern of Euphorbia pulcherrima by tetracycline treatment. J Phytopathol 148(11–12):587–590
Bradford MM (1976) A rapid and sensitive for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1):248–254
Brodin T, Fick J, Jonsson M, Klaminder J (2013) Dilute concentrations of a psychiatric drug alter behavior of fish from natural populations. Science 339(6121):814–815
Carlsson G, Patring J, Kreuger J, Norrgren L, Oskarsson A (2013) Toxicity of 15 veterinary pharmaceuticals in zebrafish (Danio rerio) embryos. Aquat Toxicol 126:30–41
Chan PK, Cheng SH (2003) Cadmium-induced ectopic apoptosis in zebrafish embryos. Arch Toxicol 77(2):69–79
Chen DM (2013) The present situation of Antibiotics industry development in China. Shanghai Industries Intelligence Services Web. http://www.hyqb.sh.cn/tabid/1164/InfoID/10337/frtid/1156/settingmoduleid/3139/Default.aspx
Cuklev F, Kristiansson E, Fick J, Asker N, Förlin L, Larsson DG (2011) Diclofenac in fish: blood plasma levels similar to human therapeutic levels affect global hepatic gene expression. Environ Toxicol Chem 30(9):2126–2134
Deng J, Yu LQ, Liu CS, Yu K, Shi XJ, Yeung LWY, Lam PKS, Wu RSS, Zhou BS (2009) Hexabromocyclododecane-induced developmental toxicity and apoptosis in zebrafish embryos. Aquat Toxicol 93(1):29–36
Du MM, Zhang DD, Yan CZ, Zhang X (2012) Developmental toxicity evaluation of three hexabromocyclododecane diastereoisomers on zebrafish embryos. Aquat Toxicol 112–113:1–10
Eggen T, Moeder M, Arukwe A (2012) Municipal landfill leachates: a significant source for new and emerging pollutants. Sci Total Environ 408(21):5147–5157
Ferreira CSG, Nunes BA, Henriques-Almeida JMM, Guilhermino L (2007) Acute toxicity of oxytetracycline and florfenicol to the microalgae Tetraselmis chuii and to the crustacean Artemia parthenogenetica. Ecotoxicol Environ Saf 67(3):452–458
Fulda S (2009) Caspase-8 in cancer biology and therapy. Cancer Lett 281(2):128–133
Gao D, Xu Z, Zhang X, Zhu C, Wang Y, Min W (2013) Cadmium triggers kidney cell apoptosis of purse red common carp (Cyprinus carpio) without caspase-8 activation. Dev Comp Immunol 41(4):728–737
Gilbert N (2011) Drug waste harms fish. Nature 476:265. doi:10.1038/476265a
Gilbert N (2012) Drug-pollution law all washed up. Nature 491:503–504. doi:10.1038/491503a
Goldstone JV, McArthur AG, Kubota A, Zanette J, Parente T, Jonsson ME, Nelson DR, Stegeman JJ (2010) Identification and developmental expression of the full complement of Cytochrome P450 genes in Zebrafish. BMC Genom 11(1):643
Gulkowska A, He Y, So MK, Yeung LWY, Leung HW, Giesy JP, Lam PKS, Martin M, Richardson BJ (2007) The occurrence of selected antibiotics in Hong Kong coastal waters. Mar Pollut Bull 54(8):1287–1306
Guo B, Yao LX, Liu ZZ, He ZH, Zhou CM, Li GL, Yang BM, Huang LX (2011) Environmental residues of veterinary antibiotics in Guangzhou city, China. J Agro-Environ Sci 30(5):938–945 (in Chinese)
Halling-Sørensen B (2000) Algal toxicity of antibacterial agents used in intensive farming. Chemosphere 40(7):731–739
Halling-Sørensen B, Nors Nielsen S, Lanzky PF, Ingerslev F, Lutzhoft HCH, Jorgensen SE (1998) Occurrence, fate and effects of pharmaceutical substances in the environment—a review. Chemosphere 36(2):357–394
Hill A, Howard V, Cossins A (2004) Characterization of TCDD-induced craniofacial malformations and retardation of zebrafish growth. J Fish Biol 64(4):911–922
Ji XL, Liu F, Shen QH, Liu Y (2011) Quantitative detection of sulfonamides and tetracycline antibiotics and resistance genes in sewage farms. Ecol Environ Sci 20(5):927–933 (In Chinese)
Jiang L, Chen SY, Yang R, Ren ZY, Yin DQ (2008) Occurrence of antibiotics in the aquatic environment of the Changjiang delta, China. Environ Chem 27(3):371–374 (in Chinese)
Jiang L, Hu XL, Yin DQ, Zhang HC, Yu ZY (2011) Occurrence, distribution and seasonal variation of antibiotics in the Huangpu River, Shanghai, China. Chemosphere 82(6):822–828
Jönsson ME, Kubota A, Timme-Laragy AR, Woodin B, Stegeman JJ (2012) Ahr2-dependence of PCB126 effects on the swim bladder in relation to expression of CYP1 and cox genes in developing zebrafish. Toxicol Appl Pharmacol 265(2):166–174
Kasai K, Kanno T, Endo Y, Wakasa K, Tozawa Y (2004) Guanosine tetra and pentaphosphate synthase activity in chloroplasts of a higher plant: association with 70S ribosomes and inhibition by tetracycline. Nucleic Acids Res 32(19):5732–5741
Keppler D, Cui Y, Konig J, Leier I, Nies A (1999) Export pumps for anionic conjugates encoded by MRP genes. Adv Enzyme Regul 39(1):237–246
Kim Y, Choi K, Jung J, Park S, Kim PG, Park J (2007) Aquatic toxicity of acetaminophen, carbamazepine, cimetidine, diltiazem and six major sulfonamides, and their potential ecological risks in Korea. Environ Int 33(3):275–370
Kingtong S, Chitramvong Y, Janvilisri T (2007) ATP-binding cassette multidrug transporters in Indian-rock oyster Saccostrea forskali and their role in the export of an environmental organic pollutant tributyltin. Aquat Toxicol 85(2):124–132
Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, Buxton HT (2002) Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999–2000: a national reconnaissance. Environ Sci Technol 36(6):1202–1211
Kümmerer K (2009) Antibiotics in the aquatic environment a review—part I. Chemosphere 75(4):417–434
Lapworth DJ, Baran N, Stuart ME, Ward RS (2012) Emerging organic contaminants in groundwater: a review of sources, fate and occurrence. Environ Pollut 163:287–303
Ledford H (2013) Anti-anxiety drug found in rivers makes fish more aggressive. Nature. doi:10.1038/nature.2013.12434
Leslie EM, Deeley RG, Cole SP (2005) Multidrug resistance proteins: role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol Appl Pharmacol 204(3):216–237
Leung HW, Minh BT, Murphy MB, Lam JCW, So MK, Martin M, Lam PKS, Richardson BJ (2012) Distribution, fate and risk assessment of antibiotics in sewage treatment plants in Hong Kong, South China. Environ Pollut 42:1–9
Li WC (2014) Occurrence, sources, and fate of pharmaceuticals in aquatic environment and soil. Environ Pollut 187:193–201
Li ZL, Chen HG, Xu Y, Kong ZM (2006) Toxicological effects of three veterinary drugs and feed additives on fish. J Ecol Rural Environ 22(1):84–86
Li G, Chen J, Xie P, Jiang Y, Wu L, Zhang X (2011) Protein expression profiling in the zebrafish (Danio rerio) embryos exposed to the microcystin-LR. Proteomics 11(10):2003–2018
Lin AYC, Tsai YT (2009) Occurrence of pharmaceuticals in Taiwan’s surface waters: impact of waste streams from hospitals and pharmaceutical production facilities. Sci Total Environ 407(12):3793–3802
Lin T, Chen YQ, Chen W (2013) Impact of toxicological properties of sulfonamides on the growth of zebrafish embryos in the water. Environ Toxicol Pharmacol 36(3):1068–1076
Liu CS, Yu K, Shi XJ, Wang JX, Lam PKS, Wu RSS, Zhou BS (2007) Induction of oxidative stress and apoptosis by PFOS and PFOA in primary cultured hepatocytes of freshwater tilapia (Oreochromis niloticus). Aquat Toxicol 82(2):135–143
Liu H, Zhang GP, Liu CQ, Li L, Xiang M (2009) Characteristics of Chloramphenicol and Tetracyclines in municipal sewage and Nanming river of Guiyang city, hina. Environ Sci 30(3):687–692 (in Chinese)
Liu BY, Nie XP, Liu WQ, Snoeijs P, Guan C, Tsui MT (2011) Toxic effects of erythromycin, ciprofloxacin and sulfamethoxazole on photosynthetic apparatus in Selenastrum capricornutum. Ecotoxicol Environ Safety 74(4):1027–1035
Livingstone DR (2001) Contaminant-stimulated reactive oxygen species production and oxidative damage in aquatic organisms. Mar Pollut Bull 42(8):656–666
Long Y, Li Q, Wang YH, Cui ZB (2011a) MRP proteins as potential mediators of heavy metal resistance in zebrafish cells. Comp Biochem Physiol C 153(3):310–317
Long Y, Li Q, Cui ZB (2011b) Molecular analysis and heavy metal detoxification of ABCC1/MRP1 in zebrafish. Mol Biol Reports 38(3):1703–1711
Long Y, Li Q, Zhong S, Wang YH, Cui ZB (2011c) Molecular characterization and functions of zebrafish ABCC2 in cellular efflux of heavy metals. Comp Biochem Physiol C 153(4):381–391
Long Y, Li Q, Li J, Cui ZB (2011d) Molecular analysis, developmental function and heavy metal-induced expression of ABCC5 in zebrafish. Comp Biochem Physiol Part B 158(1):46–55
Lunden T, Miettinen S, Lonnstroml LG, Lilius EM, Bylund G (1998) Influence of oxytetracycline and oxolinic acid on the immune response of rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol 8(3):217–230
Luo Y, Xu L, Rysz M, Wang YQ, Zhang H, Alvarez PJJ (2011) Occurrence and transport of tetracycline, sulfonamide, quinolone, and macrolide antibiotics in the Haihe River Basin, China. Environ Sci Technol 45(5):1827–1833
Madureira TV, Cruzeiro C, Rocha MJ, Rocha E (2011) The toxicity potential of pharmaceuticals found in the Douro River estuary (Portugal)—experimental assessment using a zebrafish embryo test. Environ Toxicol Pharmacol 32(2):212–217
Mehinto AC, Hill EM, Tyler CR (2010) Uptake and biological effects of environmentally relevant concentrations of the nonsteroidal anti-inflammatory pharmaceutical diclofenac in rainbow Trout (Oncorhynchus mykiss). Environ Sci Technol 44(6):2176–2182
Monteiro SC, Boxall ABA (2009) Factors affecting the degradation of pharmaceuticals in agricultural soils. Environ Toxicol Chem 28(12):2546–2554
Morales-Cano D, Calviño E, Rubio V, Herráez A, Sancho P, Tejedor MC, Diez JC (2013) Apoptosis induced by paclitaxel via Bcl-2, Bax and caspases 3 and 9 activation in NB4 human leukaemia cells is not modulated by ERK inhibition. Exp Toxicol Pathol 65(7–8):1101–1108
Osman AGM, Wuertz S, Mekkawy IA, Exner HJ, Kirschbaum F (2007) Lead induced malformations in embryos of the African catfish Clarias gariepinus (Burchell, 1822). Environ Toxicol 22(7):375–389
Otyepka M, Skopalík J, Anzenbacherová E, Anzenbacher P (2007) What common structural features and variations of mammalian P450s are known to date? Biochim Biophys Acta 1770(3):376–389
Pal A, Gin KY, Lin AY, Reinhard M (2010) Impacts of emerging organic contaminants on freshwater resources: review of recent occurrences, sources, fate and effects. Sci Total Environ 408(24):6062–6069
Peck A (2006) Analytical methods for the determination of persistent ingredients of personal care products in environmental matrices. Anal Bioanal Chem 386(4):907–939
Qu MM, Sun LW, Chen J, Li YQ, Chen YG, Kong ZM (2004) Toxicological characters of arsanilic acid and oxytetracycline. J Agro-Environ Sci 23(2):240–242 (in Chinese)
Ramirez AJ, Brain RA, Usenko S, Mottaleb MA, O’Donnell JG, Stahl LL, Wathen JB, Snyder BD, Pitt JL, Perez-Hurtado P, Dobbins LL, Brooks BW, Chambliss CK (2009) Occurrence of pharmaceuticals and personal care products in fish: results of a national pilot study in the United States. Environ Toxicol Chem 28(12):2587–2597
Richardson BJ, Lam PKS, Martin M (2005) Emerging chemicals of concern: pharmaceuticals and personal care products (PPCPs) in Asia, with particular reference to Southern China. Mar Pollut Bull 50(9):913–920
Rosal R, Rodríguez A, Perdigón-Melón JA, Petre A, GarcíaCalvo E, Gómez MJ, Agüera A, FernándezAlba AR (2010) Occurrence of emerging pollutants in urban wastewater and their removal through biological treatment followed by ozonation. Water Res 44(2):578–588
Sapkota A, Sapkota AR, Kucharski M, Burke J, McKenzie S, Walker P, Lawrence R (2008) Aquaculture practices and potential human health risks: current knowledge and future priorities. Environ Int 34(8):1215–1226
Sarmah AK, Meyer MT, Boxall A (2006) A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 65(5):725–759
Seliskar M, Rozman D (2007) Mammalian cytochromes P450-importance of tissue specificity. Biochim Biophys Acta 1770(3):458–466
Shen QH, Ji XL, Fu SJ, Liu YY, Li L (2012) Preliminary studies on the pollution levels of antibiotic and antibiotic resistance genes in Huangpu River, China. Ecol Environ Sci 21(10):1717–1723 (in Chinese)
Shi XJ, Zhou BS (2010) The role of Nrf2 and MAPK pathways in PFOS-induced oxidative stress in zebrafish embryos. Toxicol Sci 115(2):391–400
Shi XJ, Du YB, Lam PKS, Wu RSS, Zhou BS (2008) Developmental toxicity and alteration of gene expression in zebrafish embryos exposed to PFOS. Toxicol Appl Pharmacol 230(1):23–32
Smith EM, Wilson JY (2010) Assessment of cytochrome P450 fluorometric substrates with rainbow trout and killifish exposed to dexamethasone, pregnenolone-16α-carbonitrile, rifampicin, and β-naphthoflavone. Aquat Toxicol 97(4):324–333
Takahashi K, Kimura Y, Nagata K, Yamamoto A, Matsuo M, Ueda K (2005) ABC proteins: key molecules for lipid homeostasis. Med Mol Morphol 38(1):2–12
Thomas PM, Foster GD (2004) Determination of nonsteroidal anti-inflammatory drugs, caffeine, and triclosan in wastewater by gas chromatography-mass spectrometry. J Environ Sci Health Part A 39(8):1969–1978
Ulukaya E, Acilan C, Yilmaz Y (2011) Apoptosis: why and how does it occur in biology? Cell Biochem Funct 29(6):468–480
Vannini C, Domingo G, Marsoni M, De Mattia F, Labra M, Castiglioni S, Bracale M (2011) Effects of a complex mixture of therapeutic drugs on unicellular algae Pseudokirchneriella subcapitata. Aquat Toxicol 101(2):459–465
Voelker D, Vess C, Tillmann M, Nagel R, Otto GW, Geisler R, Schirmer K, Scholz S (2007) Differential gene expression as a toxicant sensitive endpoint in zebrafish embryos and larvae. Aquat Toxicol 81(4):355–364
Wang R, Wei YS (2013) Pollution and control of tetracyclines and heavy metals residues in animal manure. J Agro-Environ Sci 32(9):1705–1719 (in Chinese)
Wei JY, Zhang R, Ding S, Zhang XJ, Luo AX (2004) The usage of antibiotic feed additive in the livestock husbandry. Inner Mongolia Agric Sci Technol 200(4):52–53 (In Chinese)
Wei RC, Ge F, Huang SY, Chen M, Wang R (2011) Occurrence of veterinary antibiotics in animal wastewater and surface water around farms in Jiangsu Province, China. Chemosphere 82:1408–1414
Wollenberger L, Halling-Sorensen B, Kusk KO (2000) Acute and chronic toxicity of veterinary antibiotics to Daphnia magna. Chemosphere 40(7):723–730
Xiong Q, Xie P, Li HY, Hao L, Li GY, Qiu T, Liu Y (2009) Involvement of Fas/FasL system in apoptotic signaling in testicular germ cells of male Wistar rats injected iv with microcystins. Toxicon 54(1):1–7
Xu WH, Zhang G, Zou SC, Li XD, Liu YC (2007) Determination of selected antibiotics in the Victoria Harbour and the Pearl River, South China using high-performance liquid chromatography-electrospray ionization tandem mass spectrometry. Environ Pollut 145(3):672–679
Xu WH, Zhang G, Zou SC, Ling ZH, Wang GL, Yan W (2009) A preliminary investigation on the occurrence and distribution of antibiotics in the Yellow River and its tributaries, China. Water Environ Res 81(3):248–254
Xu DM, Wang YH, Rao GW (2013) Cellular response of freshwater green algae to the toxicity of Tetracycline antibiotics. Environ Sci 34(9):3386–3390 (in Chinese)
Yamagami K (1981) Mechanisms of hatching in fish: secretion of hatching enzyme and enzymatic choriolysis. Am Zool 21(2):459–471
Yamashita M (2003) Apoptosis in zebrafish development. Comp Biochem Physiol B 136(4):731–742
Yan CX, Yang Y, Zhou JL, Liu M, Nie MH, Shi H, Gu LJ (2013) Antibiotics in the surface water of the Yangtze Estuary: occurrence, distribution and risk assessment. Environ Pollut 175:22–29
Yuan GG, Wang YM, Yuan XY, Zhang TF, Zhao J, Huang LY, Peng SQ (2014) T-2 toxin induces developmental toxicity and apoptosis in zebrafish embryos. J Environ Sci 26(4):917–925
Zeng C, Sun H, Xie P, Wang JH, Zhang GR, Chen N, Yan W, Li GY (2014) The role of apoptosis in MCLR-induced developmental toxicity in zebrafish embryos. Aquat Toxicol 149:25–32
Zhang X, Zhang T (2011) Occurrence, abundance, and diversity of tetracycline resistance genes in 15 sewage treatment plants across China and other global locations. Environ Sci Technol 45(7):2598–2604
Zhang DD, Lin LF, Luo ZX, Yan CZ, Zhang X (2011) Occurrence of selected antibiotics in Jiulongjiang River in various seasons, South China. J Environ Monit 13(7):1953–1960
Zhang Q, Xin Q, Zhu JM, Cheng JP (2014) The antibiotic contaminations in the main water bodies in China and the associated environmental and human health impacts. Environ Chem 33(7):1075–1083 (in Chinese)
Zhou LJ, Ying GG, Zhao JF, Yang JF, Wang L, Yang B, Liu S (2011) Trends in the occurrence of human and veterinary antibiotics in the sediments of the Yellow River, Hai River and Liao River in northern China. Environ Pollut 159(7):1877–1885
Zhu YG, Johnson TA, Su JQ, Qiao M, Guo GX, Stedtfeld RD, Hashsham SA, Tiedje JM (2013) Diverse and abundant antibiotic resistance genes in Chinese swine farms. Proc Natl Acad Sci 110(9):3435–3440
Zou SC, Xu WH, Zhang RJ, Tang JH, Chen YJ, Zhang G (2011) Occurrence and distribution of antibiotics in coastal water of the Bohai Bay, China: impacts of river discharge and aquaculture activities. Environ Pollut 159(10):2913–2920
Zuccato E, Castiglioni S, Bagnati R, Melis M, Fanelli R (2010) Source, occurrence and fate of antibiotics in the Italian aquatic environment. J Hazard Mater 179(1):1042–1048
Acknowledgments
This work was financially supported by grants from the National Science Foundation of China (41101489), the Natural Science Foundation of Guangdong Province, China (s2012010010847), the Program for New Century Excellent Talents in University from Ministry of Education of China (NECT-12-0181), and the State Key Lab in Estuarine and Coastal Research (2012RCDW01).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, Q., Cheng, J. & Xin, Q. Effects of tetracycline on developmental toxicity and molecular responses in zebrafish (Danio rerio) embryos. Ecotoxicology 24, 707–719 (2015). https://doi.org/10.1007/s10646-015-1417-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-015-1417-9