Abstract
The age, growth, and maturity of bonnetheads, Sphyrna tiburo, inhabiting estuarine and coastal waters of the U.S. Gulf of Mexico (GOM) were investigated. Based on results of a concurrent population genetics study, two populations were examined, the eastern GOM and western GOM. Vertebrae were collected and aged from 1081 females and 811 males ranging in size 261–1060 mm and 227–898 mm fork length (FL), respectively. The von Bertalanffy growth model provided the best fit to length-at-age data. Eastern GOM von Bertalanffy parameters (length parameters in mm FL) were L∞ = 844, k = 0.23, to = -1.99, and Lo = 310 for females and L∞ = 680, k = 0.39, to = -1.44, and Lo = 294 for males. Western GOM von Bertalanffy parameters were L∞ = 1005, k = 0.20, to = -1.81, and Lo = 298 for females and L∞ = 807, k = 0.30, to = -1.44, and Lo = 285 for males. Maximum observed age was similar between populations with an overall maximum of 17.1 years for females, and 12.1 years for males. Length and age at 50% maturity for the eastern GOM was 661.5 mm and 4.9 years for females, and 564.1 mm and 3.5 years for males and for the western GOM 772.7 mm and 5.3 years for females, and 644.9 mm and 4.4 years for males. Bonnetheads in the eastern GOM generally grow faster and to smaller asymptotic lengths than those from the western GOM; however, longevity is similar between the two populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The bonnethead, Sphyrna tiburo Linnaeus 1758, occurs in coastal waters of the western Atlantic Ocean from North Carolina to Brazil (Compagno 1984; Castro 2011). In U.S. waters, bonnetheads are found along the Atlantic Coast of the southern U.S. (hereafter Atlantic), the Gulf of Mexico (GOM), and the Caribbean Sea. The species has a short gestation period of four to five months (Parsons 1993b; Gonzales de Acevado et al. 2020), with parturition occurring in the late summer to early fall and mating occurring shortly thereafter; sperm is stored until late spring when ovulation and fertilization occur (Manire et al. 1995; Manire and Rasmussen 1997; Ulrich et al. 2007). Bonnetheads are thought to mature between 1 year (Lombardi-Carlson et al. 2003) and 7 years of age (Frazier et al. 2014) and females give birth to 2–14 (mean ~ 9) fully developed pups annually (Gonzales de Acevado et al. 2020). Bonnetheads in the Atlantic and GOM migrate seasonally and all life stages are commonly found in bays, estuaries, and nearshore waters from May to November (Cortés et al. 1996; Ulrich et al. 2007). Unlike many other coastal sharks, observed migratory behavior does not appear to be associated with the use of nursery areas (Heupel et al. 2007; Knip et al. 2010), but instead may be related to food availability for gestating females and access to potential mates for males (Driggers et al. 2014).
The coastal distribution of bonnetheads makes them susceptible to both commercial and recreational fisheries. Specifically, they have historically been a component of the directed gillnet fishery in the southeastern U.S., where they also are frequently caught by recreational anglers and are a common component of bycatch in Atlantic and GOM shrimp fisheries (Scott-Denton et al. 2012, 2020; Zhang et al. 2013). In the U.S., bonnetheads are managed as a component of the small coastal shark complex (SCS) which also includes finetooth sharks, Carcharhinus isodon Valenciennes 1839, and Atlantic sharpnose sharks, Rhizoprionodon terraenovae Richardson 1836 (SEDAR 2013). While Atlantic sharpnose sharks are the most commonly landed SCS, in some years bonnetheads make up ~ 50% of the total directed-commercial catch (Cortés 2002). The current stock status of bonnetheads is considered to be “unknown” with separate stocks in the Atlantic and GOM (SEDAR 2013). The most recent assessment found bonnetheads were not thought to be overfished, nor experiencing overfishing (SEDAR 2013); however, assessment results were rejected as populations in the Atlantic and GOM were assessed as a single stock despite preliminary genetic, life history, and tagging data indicating the occurrence of distinct stocks in the Atlantic and GOM. Furthermore, prior studies employing both genetic and life history analyses only involved samples from the eastern GOM, precluding the possibility of detecting other stocks elsewhere in the GOM. Finally, the assessment used combined life history parameters, even though these parameters differed significantly between bonnetheads in the Atlantic and those in the GOM. Averaging these life history parameters had the effect of increasing longevity as well as age and length at maturity, while decreasing growth rates and average brood sizes for GOM bonnetheads and led to projections of a more productive stock in the GOM (SEDAR 2013). Given the high mortality of bonnetheads in the GOM from shrimp trawling (post release mortality is assumed to be 100%, SEDAR 2013) and lower productivity indicated by life history parameters, previous assessments likely do not adequately represent the status of bonnethead stocks in the GOM.
Recent research suggests bonnetheads have fine-scale population structure in U.S. waters. Several studies have documented significant differences in life history across small geographic regions. Differences found between bonnetheads from the eastern GOM include size at age, growth rate, and size and age at maturity (Parsons 1993a, b; Carlson and Parsons 1997; Lombardi-Carlson et al. 2003). In addition, studies in both the Atlantic and eastern GOM have shown site fidelity by adult bonnetheads (both sexes) to specific estuaries or bays during the summer months, on inter- and intra-annual time scales (Heupel et al. 2006; Driggers et al. 2014), results supported by Portnoy et al. (2015) using genomic techniques. Further, results of tag-recapture and acoustic monitoring in the eastern GOM indicate that individuals in some locations may remain resident for large portions of the year (Heupel et al. 2006). Several published studies now exist on stock structure of bonnetheads in the western North Atlantic; however, no studies to date have sampled the entirety of the range of bonnetheads in U.S. waters. Two studies examined genetic structure using mtDNA, with the first study demonstrating genetic differences between the eastern GOM, Atlantic and southern GOM (Mexico) and the second showing genetic structure present within the GOM (Escatel-Luna et al. 2015; Fields et al. 2016). More recent work by Díaz-Jaimes et al. (2021) using microsatellites and single nucleotide polymorphisms confirmed well-defined structure in the Atlantic, eastern GOM and southern GOM (Mexico); however, samples were lacking for the north-western GOM. Life history work by Frazier et al. (2014) found large differences in life history parameters for bonnetheads in the Atlantic and the eastern GOM. For example, maximum longevity and age at 50% maturity were observed to be almost twice as large for bonnetheads in the Atlantic as compared to the GOM, and significant differences were found for length at 50% maturity, fecundity and key von Bertalanffy growth function parameter estimates (e.g., asymptotic average length and coefficient of growth). However, recent research using age-independent methods (mark and recapture) suggest that life history parameters from the eastern GOM are more similar to the Atlantic than the current literature suggests (Frazier et al. 2020).
To date, no studies have examined the life history of bonnetheads in the western GOM. Therefore, given the disparities among bonnethead life history parameters between the eastern GOM and Atlantic and the lack of life history data from the western GOM, the objectives of this study were to describe the age and growth, maturity, and fecundity of the bonnethead using samples collected throughout the U.S. GOM. A concurrent population genetics study, using paired samples, was used to define populations for life history analyses.
Materials and methods
Sample collection
Life history data from bonnetheads were collected by collaborators throughout the U.S. GOM with collections occurring from 2012 to 2019. Sharks were captured using fishery-dependent and -independent methods with gear types that included longline, rod and reel, gillnet, seine, and otter trawl. Following capture, specimens were euthanized if not already dead, measured to fork length (FL) to the nearest mm or 0.5 cm, and if present, umbilical scars of neonates were noted as “umbilical remains,” “fresh,” “partially healed,” “mostly healed,” or “well healed” following Pratt et al. (1998). Laboratory analysis consisted of assessment of the reproductive stage. Females were considered mature if they had developing pups. If they were not gravid, uterine scarring, vitellogenic oocytes (> 10 mm) and/or developed uteri and oviducal glands were used (Parsons 1993b). For gravid females, total brood size was counted, and embryos were sexed and measured. Males were considered mature if they had fully calcified, rotating claspers, functional siphon sacs, and functional rhipidions (Clarke and von Schmidt 1965). Outer clasper length (tip of the clasper to insertion of the pelvic fin) and degree of clasper calcification were recorded for all males. Finally, a section of 10–12 vertebrae were removed from the cervical region of the vertebral column (i.e., between the occiput and first dorsal fin), frozen and shipped on ice to the South Carolina Department of Natural Resources. Archived samples (1998–2001) from previous studies in the eastern GOM were also obtained and used for life history analyses.
Age estimation
To prepare vertebrae for analysis, excised samples were thawed and excess tissue removed from the vertebral column using a scalpel. Individual vertebra were then separated by severing connective tissues (e.g., intervertebral ligaments). Vertebrae were then soaked in 5% sodium hypochlorite for 3-15 min to remove remaining muscle tissue, rinsed under running tap water for five minutes, and stored in 95% ethanol. Cleaned vertebrae were then mounted to a glass slide using Crystalbond 509™ and a 0.4 mm sagittal section containing the focus was removed using a Buehler Isomet low speed saw. The resulting section was monitored while drying to ensure a preferred viewing state before being permanently mounted and preserved on a glass slide using Cytoseal™-XYL. This step was taken because some band pairs may become less visibly apparent if sections are allowed to fully dry, leading to underestimation of age (Frazier et al. 2014). Each mounted vertebra was examined using a Nikon SMT-2 T dissecting microscope at 20X magnification with a transmitted light source.
Vertebral slides were selected at random and the number of translucent bands on the corpus calcareum were counted independently by two readers, each without knowledge of the other’s reading, previous readings or of the sex, size or date of capture of the shark from which the sample was removed. Opaque bands representing summer growth and translucent bands representing winter growth were identified following the description and terminology of Cailliet and Goldman (2004). If there were discrepancies between readings, the section was re-read simultaneously by both readers to resolve the difference. If no agreement was reached, the sample was discarded from all analyses.
A birth date of September 1 was assigned to all individuals based on evidence that bonnethead parturition occurs over a period of several weeks from early August through September (Parsons 1993b; Gonzales de Acevado et al. 2020). Due to variability in presence of a translucent birthmark, the change in the angle of the corpus calcareum was counted as a birthmark/band for individuals without a discernible band (Goldman 2004). The second band representing winter growth was assumed to form five months later, and subsequent band pairs were assumed to form 12 months thereafter (Parsons 1993a; Frazier et al. 2014). Therefore, for all band counts of two and over, assigned age = (band pair count-1.5). In addition to assigned ages, fractional ages were calculated by setting the birth month as month zero and dividing the numeric capture month by 12.
Reader precision and bias
Multiple methods were used to examine reader bias and precision. Overall percent agreement (PA = [number agreed between readers/number read] X 100) and percent agreement \(\pm\) 1 year were calculated to evaluate precision. Percent agreement was also examined in 100 mm FL groups as recommended by Goldman (2004). Age agreement tables were generated and tested for symmetry using Bowker’s test of symmetry (Hoenig et al. 1995). Age bias plots (Campana et al. 1995) were used to evaluate bias between readers as well as between age estimates of this study and age estimates of vertebrae used in Lombardi-Carlson (2007). A subset of 100 randomly selected specimens was also re-read by Reader 1 to examine within-reader bias. The index of average percent error (\({I}_{APE}\); Beamish and Fournier 1981) was calculated to assess between-reader error:
where R = number of times each fish is aged, \({X}_{ij}\) = ith age estimation of jth shark, and \({X}_{j}\) = mean age estimate of the jth shark. While \({I}_{APE}\) assumes standard deviation of age estimates are proportional to the mean of the age estimates, Chang (1982) instead suggested that the coefficient of variation (CV) should be used to measure precision:
Tests of precision and bias were generated using the FSA (Ogle et al. 2021) package in R (R Core Team 2021).
Data analysis
Measured FL and age estimates were used to generate sex-specific von Bertalanffy (von Bertalanffy 1938), Gompertz (Ricker 1975), and logistic (Ricker 1979) growth models. The von Bertalanffy growth model, as adapted by Beverton (1954) and Beverton and Holt (1957), is:
where \({L}_{t}\) is length-at-age t and \({L}_{\infty }\) (asymptotic length), k (coefficient of growth) and \({t}_{o}\) (theoretical age at which length equals zero) are fitted parameters. The original von Bertalanffy growth model was also fit to data as recommended by Cailliet et al. (2006):
where \({L}_{o}\) (mean length at birth) is a fitted parameter. Mean length-at-birth was determined for males and females through measurements of free-swimming neonates with an umbilical stage of open, partly healed or mostly healed. The modified form of the Gompertz growth model was also generated (Ricker 1975; Mollet et al. 2002) using the equation:
where:
is a fitted parameter. Finally, a logistic growth model was generated using the equation (Ricker 1979):
where \({L}_{\infty }, k,\) and a (time at which the absolute rate of increase in weight begins to decrease or the inflection point of the curve, equivalent to \({t}_{o}\) in Ricker 1979) are fitted parameters. Confidence intervals for all model parameters were generated by bootstrapping (5,000 replicates). Models and confidence intervals were generated using the FSA (Ogle et al. 2021) package in R (R Core Team 2021). Model fit and selection were assessed by examination of residuals, Akaike information criterion (AIC, Akaike 1973) and residual sums of squares. Examination of residuals and residual sums of squares were used to assess relative strength of models fit to assigned or fractional age data sets.
Maximum likelihood ratio tests (Kimura 1980) generated using the Fishmethods (Nelson 2021) package in R (R Core Team 2021) were used to detect if there were significant differences between male and female parameter estimates, latitudinal groups, or differences between populations. Results from a concurrent populations genetics study using fin clips sampled along with vertebrae indicated that the U.S. GOM is differentiated into two populations (Portnoy and Fields, Unpublished Data) and specimens were assigned either to the eastern or western GOM population based on catch location, with the dividing line set at longitude -87.75° just east of Mobile Bay, AL. To examine previously described latitudinal variation (e.g., Parsons 1993a; Carlson and Parsons 1997; Lombardi-Carlson et al. 2003), populations were binned into low (< 26.5°), medium (26.5° – 29.0°) and high (> 29.0°) latitude groupings. Length-at-age data from Lombardi-Carlson (2007) were used to regenerate eastern GOM von Bertalanffy parameters for comparison. To facilitate comparisons with the current study, age and length data from Lombardi-Carlson (2007) were remodeled using FL at estimated age (total length at band count was originally used to model growth in that study) allowing direct comparison of growth between these studies. If FL was missing from an aged GOM individual, a GOM-specific total length (TL) to FL regression from Lombardi-Carlson (2007) was used to convert measurements.
Maturity and fecundity
To determine median FL (\({L}_{50})\) and age (\({A}_{50})\) at which 50% of the population was considered mature, a logistic model was fit to binomial maturity data using nonlinear least squares regression:
where 0 = immature, and 1 = mature. Median \({L}_{50}\) and \({A}_{50}\) was determined by –a/b (Mollet et al. 2002). Models and confidence intervals were generated using the FSA (Ogle et al. 2021) package in R (R Core Team 2021). Confidence intervals were generated by bootstrapping (5,000 replicates). The parameters generated by this study were compared to previous eastern GOM \({L}_{50}\) and \({A}_{50}\) generated by Frazier et al. (2014) using original raw FL and age data from Parsons (1993a) and Lombardi-Carlson (2007), as well as to Atlantic \({L}_{50}\) and \({A}_{50}\) from Frazier et al. (2014). Comparisons were considered significant if confidence intervals did not overlap. For each region, the mean and standard deviation of brood size was calculated. Regions were compared using Welch’s t-tests, and differences in brood size by latitude grouping were tested using analysis of variance. Region-specific relationships between maternal length and brood size were tested using general linear models.
Results
Sample collection
Vertebrae from 2,266 bonnetheads from the U.S. GOM were received from collaborators and historical archives, including specimens previously used by Lombardi-Carlson et al. (2003) for life history (Fig. 1). Of those, 1,902 had sufficient data (sex, lengths, and location) to generate life history models. Based on genetic results, specimens were assigned to the eastern or western GOM, delineated at longitude -87.75° just east of Mobile Bay, AL (A. Fields, unpublished). Results from genetic analyses also indicate low latitude FL Keys bonnetheads belong to the eastern GOM population, in agreement with NMFS management units. All three defined latitude groupings were present for the eastern GOM; only medium and high latitude groupings were available for the western GOM (Fig. 1). Sample size by sex, region and latitude grouping are reported in Table 1.
Map of the Gulf of Mexico with bonnethead, Sphyrna tiburo, life history specimen catch locations and abundance indicated by black circles. The vertical bar denotes the boundary between populations in the eastern and western Gulf of Mexico, and horizontal bars denote latitudinal groupings used to test for latitudinal variation in growth, no low latitude specimens were obtained for the western population
Age estimation, precision and bias
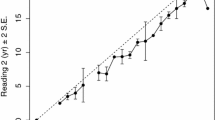
Of the 1,902 specimens aged, only ten specimens (< 1%) were discarded due to inability to reach a consensus age estimate. Readers agreed on age estimates of 55.1% of samples examined, and percent agreement was 91.8% ± 1 band. Percent agreements were higher between reader 1 and consensus age and reader 2 and consensus age (Table 2). Bowker’s test of symmetry results indicated no bias between reader 1 and reader 2, as well as between readers and consensus ages (Table 2). Results from Beamish’s IAPE, and Chang’s CV indicate estimated ages were relatively precise although CVs were above 5% between reader 1 and reader 2, indicating lower precision between readers (Table 2). Age bias plots for reader 1 and reader 2 revealed no systematic differences between readers among age classes (Supplementary Fig. 1). Significant differences were detected between age estimates from Lombardi-Carlson et al. (2003) and this study (Bowker’s test, \({\rm X}^{2}\) = 165, df 41, p < 0.001) with agreement from age 0 to 2 years, but thereafter age estimates in this study were significantly older (Supplementary Fig. 2).
Growth models
Significant differences in growth were detected between males and females for all aged bonnetheads (\({\rm X}^{2}\) = 227.1, df 3, p < 0.001); therefore, sex-specific growth curves were necessary. Sex- and region-specific growth models were generated for three growth models (von Bertalanffy, Gompertz, and Logistic). Each model was also generated separately using assigned ages and fractional ages. The AIC and residual sums of squares were lower using fractional ages; therefore, all results are presented using fractional ages. The von Bertalanffy growth model provided the best fit for all models except for females in the western GOM (Table 3); therefore, all growth comparisons were based on the von Bertalanffy model.
Previously reported differences in growth for the eastern GOM (Parsons 1993a; Carlson and Parsons 1997; Lombardi-Carlson et al. 2003) were confirmed with significant differences for females between low/medium latitude groupings (\({\rm X}^{2}\) = 52.8, df 3, p < 0.001), medium/high latitude groupings (\({\rm X}^{2}\) = 17.4, df 3, p = 0.001), and low/high latitude groupings (\({\rm X}^{2}\) = 102.8, df 3, p < 0.001). Differences in growth were driven by significant differences in L∞ among all groupings and significant differences in k between medium–high, and low–high groupings (Fig. 2 and Table 4). There were no significant differences in estimated Lo. For males in the eastern GOM, significant differences were found between low/medium (\({\rm X}^{2}\) = 20.2, df 3, p < 0.001) and low/high groupings (\({\rm X}^{2}\) = 52.0, df 3, p < 0.001). No significant differences were found between medium/high groupings (\({\rm X}^{2}\) = 7.4, df 3, p = 0.061). Significant differences in male groupings were driven by differences in L∞. No significant differences in k and Lo were found between groupings for eastern GOM males (Fig. 2).
von Bertalanffy growth models for female (a) and male (b) bonnetheads, Sphyrna tiburo, inhabiting the eastern Gulf of Mexico, fit to fork length (mm) at fractional age (years). Specimens were binned into low (< 26.5°), medium (26.5°–29.0°) and high (> 29.0°) latitude groupings to investigate latitudinal variation
Latitudinal variation was also investigated for the western GOM; however, only medium, and high latitude groupings were available. We were unable to sample enough low latitude bonnetheads as they occur in Mexican waters, which were not sampled for this study (Fig. 1). Significant differences were detected between medium/high latitude females in the western GOM (Fig. 3, \({\rm X}^{2}\) = 14.73, df 3, p = 0.002). Differences were driven by significant differences in k and Lo, likely due to sampling bias, as medium latitude female bonnetheads actually had a higher L∞ than high latitude bonnetheads; the opposite to what would be expected. No significant differences were found between medium/high latitude male bonnetheads for the western GOM (Fig. 3, \({\rm X}^{2}\) = 0.45, df = 3, p = 0.930).
Growth model results suggest bonnetheads in the western GOM grow to significantly larger asymptotic lengths, and at a slower rate than those in the eastern GOM for both females (Fig. 4, \({\rm X}^{2}\) = 177.7, df = 3, p < 0.001), and males (Fig. 4, \({\rm X}^{2}\) = 187.5, df = 3, p < 0.001). Parameters for length-at-birth were similar between regions and sexes (Table 3). Maximum estimated ages for eastern GOM bonnetheads were more than double previous estimated ages from Lombardi-Carlson et al. (2003) for both males (11.7 versus 5.5 + years, respectively), and females (16.9 versus 7.5 + years, respectively), and there were no differences in maximum observed longevity between regions (Table 5). Results from this study found significantly different growth parameters for the eastern GOM than previously published for both females (Fig. 5a, \({\rm X}^{2}\) = 18.0, df = 3, p < 0.001) and males (Fig. 5b, \({\rm X}^{2}\) = 28.5, df = 3, p < 0.001). For both sexes, estimates of parameters L∞, k, to, and Lo were all lower than previously published (Lombardi-Carlson et al. 2003), despite similar observed maximum lengths between the two studies. Model estimated lengths-at-birth for the current study were within the range of lengths of free swimming bonnetheads (215–297 mm) and were ~ 100 mm shorter than previous estimates from Lombardi-Carlson et al. (2003), which were well above observed lengths of free-swimming neonates (Table 5).
von Bertalanffy growth models for female (a) and male (b) bonnetheads, Sphyrna tiburo, inhabiting the eastern, and western Gulf of Mexico, fit to fork length (mm) at fractional age (years). Eastern and western populations were delineated just east of Mobile Bay, AL (longitude -87.75°). A growth model from the U.S. east coast (Atlantic, dashed line) from Frazier et al. 2014 was plotted to compare growth between populations
A comparison of von Bertalanffy growth curves fitted to fork length at assigned age for (a) male, and (b) female bonnetheads, Sphyrna tiburo from the present study and previous studies from the eastern Gulf of Mexico (parameter values are presented in Table 5)
Results from this study were also compared to growth models for bonnetheads in the Atlantic population. Growth models between the eastern GOM and the Atlantic were significantly different for both males (\({\rm X}^{2}\) = 83.4, df = 3, p < 0.001) and females (\({\rm X}^{2}\) = 151.1, df = 3, p < 0.001) with large differences in L∞ and k. Estimates of to and Lo were similar between regions. Growth models were also significantly different between the western GOM and Atlantic for females (Fig. 4a, \({\rm X}^{2}\) = 14.4, df = 3, p < 0.001) and males (Fig. 4b, \({\rm X}^{2}\) = 23.5, df = 3, p < 0.001). Growth models were much more similar between the western GOM and Atlantic as compared to the eastern GOM, despite the geographic distance between these populations. Generally, bonnetheads in the western GOM and Atlantic were found to grow more slowly, and to a larger average length than those in the eastern GOM, despite similar longevities among all three regions.
Maturity models
Latitudinal variation in maturity parameters was investigated for the eastern and western GOM. In the eastern GOM, significant latitudinal differences were observed for female L50, but not A50 (Table 4 and Fig. 6a, b). Estimates of A50 were lower for the medium latitude grouping; however, low and high latitudes estimates of A50 were similar. For males in the eastern GOM, significant differences in L50 were found between low latitude bonnetheads, and medium/high latitude bonnetheads (Table 4 and Fig. 6c, d), with no significant differences between medium and high latitudes. No latitudinal variation in A50 was observed among eastern GOM male groupings, with slightly higher estimates of A50 for medium latitude bonnetheads. For the western GOM, there were significant difference in L50 between groupings for males; however, there were no significant differences for females, with medium latitude females having a slightly higher L50 (Table 4 and Fig. 7). Estimates of A50 were lower for medium latitude males (4.2 versus 4.8 years) and higher for females (5.6 versus 4.9 years) compared to the high latitude group.
Fork length (mm) and fractional age (years) at maturity ogives for female (a, b, respectively) and male (c, d, respectively) bonnetheads, Sphyrna tiburo, in the eastern Gulf of Mexico. Specimens were binned into low (< 26.5°), medium (26.5°–29.0°), and high (> 29.0°) latitude groupings to investigate latitudinal variation. The solid line is the expected proportion mature at a given fork length or age, symbols indicate observed data points
Fork length (mm) and fractional age (years) at maturity ogives for female (a, b, respectively) and male (c, d, respectively) bonnetheads, Sphyrna tiburo, in the western Gulf of Mexico. Specimens were binned into medium (26.5°–29.0°) and high (> 29.0°) latitude groupings to investigate latitudinal variation. The solid line is the expected proportion mature at a given fork length or age, symbols indicate observed data points
Significant differences in L50 were observed for both males and females between the eastern and western GOM (Table 5 and Fig. 8a, c). Similar to the growth models, bonnetheads in the eastern GOM matured at a significantly smaller length than those from the western GOM and Atlantic for both sexes. For females, L50 was largest in the Atlantic (Table 5 and Fig. 8a); for males, L50 was largest in the western GOM (Table 5 and Fig. 8c). Age-at-50% maturity was significantly younger (4.9 years) for the eastern versus western GOM (5.3 years) for female bonnetheads based on non-overlapping confidence intervals; however, estimates of A50 were significantly older (6.7 years) for the Atlantic (Table 5 and Fig. 8b). For male bonnetheads, A50 estimates were significantly different between the eastern (3.5 years) and western GOM (4.4 years) with estimates of A50 for the Atlantic (3.9 years) intermediate to the GOM estimates (Table 5 and Fig. 8d).
Fecundity
A total of 176 broods of bonnetheads were available to estimate fecundity in the GOM, comprised of 117 from the eastern GOM and 59 from the western GOM. The mean fecundity was 9.6 pups per brood (range 2–18, S.D. = 3.4) for the eastern GOM and 8.5 pups per brood (range 2–14, S.D. = 2.4) for the western GOM with significant differences between the regions (Welch’s t-test t = 2.63, df = 154.9, p = 0.009). There was no significant difference in brood size by latitude for the eastern GOM (ANOVA, p = 0.961, F = 0.04, df = 2). There was a significant relationship between maternal fork length and brood size for the eastern GOM; however, the R2 value was low indicating length is not a good predictor of brood size (Fig. 9a, p = 0.001, F = 12.7, R2 = 0.11). Brood size appeared to be slightly larger in larger females for the western GOM, but the relationship was not significant (Fig. 9b, p = 0.076, F = 3.3, R2 = 0.04). This likely resulted from the majority of samples being from the northern portion of the western GOM population. Surveys in TX did not encounter pregnant bonnetheads as they sampled in the spring and fall, prior to fertilization, and after parturition; therefore, samples were lacking from this area.
Discussion
This is the first study to examine growth and maturity of bonnetheads across the U.S. GOM. We detected significant differences between life histories for populations in the eastern and western GOM, with bonnetheads from the western GOM having life histories more similar to bonnetheads from the Atlantic rather than the adjacent population from the eastern GOM. Significant differences in growth were observed between previously published growth models for the eastern GOM and the current study, likely due to differences in estimated ages. Vertebrae from many of the specimens aged by Lombardi-Carlson et al. (2003) were provided for use in this study. Significant differences were found between the original estimated ages and the estimated ages from those vertebrae in this study. These differing age estimates could either be due to differences in preparation of vertebrae for age estimation or different interpretation of band pairs. Unfortunately, we did not have access to the slides used to estimate age in the original study; therefore, we cannot determine which may have occurred. However, similar results were noted in a study by Vinyard et al. (2019), which found significant differences in age estimates of finetooth sharks when using vertebrae from Drymon et al. (2006). Ages estimated from original slides were similar; however, ages from newly sectioned vertebrae were significantly older, leading the authors to determine that differences in estimated age were due to differences in processing, not interpretation of growth structures (Vinyard et al. 2019).
Annual band formation in bonnetheads was previously confirmed using marginal increment analysis as well as marking and recapturing bonnetheads injected with oxytetracycline (OTC) in the GOM (Parsons 1993a) and in the Atlantic (Frazier et al. 2014). Results from both studies indicate that band formation is annual; however, results from Frazier et al. (2014) found evidence of age underestimation as some OTC injected individuals failed to form annual bands. Despite the significant increase in age estimates in this study relative to previous studies, it is possible that ages of some specimens are still underestimated. Recent research has found age underestimation is likely more common than previously thought, as annual band formation may cease or become less visible once individuals reach asymptotic length (Harry 2018; Natanson et al. 2018). If age underestimation occurred in this study, it should have minimal effect on maturity estimates and growth parameters as most age underestimation occurs in individual approaching asymptotic length; however, maximum age estimates may be affected (Natanson et al. 2018).
Despite differences in age estimates between this study and previous studies, growth models confirmed previously documented latitudinal variation in growth along the eastern GOM (see Parsons 1993a; Carlson and Parsons 1997; Lombardi-Carlson et al. 2003 for discussion). However, sample sizes for low latitude bonnetheads in the eastern GOM were lower than other groupings leading to greater uncertainty to the extent of these differences. A clear pattern was not detected in the western GOM. However, it is important to note that we were unable to obtain samples from low latitudes in Mexico where differences would be most apparent, and therefore we cannot be certain whether latitudinal variation occurs in the western GOM. Recent research by Caña-Hernández et al. (2023) modeled growth of bonnetheads in the southwestern GOM (latitudes 21º to 18º); however, results from this study are not directly comparable as they used stretch total length at age to model growth. Morphometric conversions of the asymptotic average length of female (960 mm FL) and male (678 mm FL) bonnetheads in their study are inconclusive as males in the southwestern GOM study are significantly smaller on average than in the current study (non-overlapping 95% C.I.), but female asymptotic lengths are not significantly different in the two studies. Based on these results, if latitudinal variation does occur in the western GOM, it is likely not to the extent identified in the eastern GOM. Significant differences in growth exist between the eastern and western GOM, with bonnetheads in the western GOM having life histories more similar to the U.S. Atlantic than to the eastern GOM. Generally, bonnetheads in the western GOM grew slower, and reached larger average asymptotic lengths than bonnetheads in the eastern GOM; however, maximum age estimates were similar between the two regions. While there were no low latitude bonnetheads from the western GOM in this study to compare to the eastern GOM, the asymptotic average length of female bonnetheads in the recent Caña-Hernández et al. (2023) study was significantly larger (L∞ = 960) than bonnetheads from the eastern GOM regardless of latitude (Table 4), indicating these differences in growth between populations are likely maintained across all latitudes.
There was no significant difference in brood size by latitude in the eastern GOM, despite observed differences in growth. It has been hypothesized that maternal body size may limit fecundity (Holden 1973); however, it appears that bonnetheads are equally productive at lower latitudes by producing similar brood sizes, albeit with smaller lengths-at-birth, compared to conspecifics at higher latitudes (Parsons 1993b). Among sharks in the families Carcharhinidae and Sphyrnidae, bonnetheads are in a small group that have relatively large brood sizes and small size at birth, in relation to body size (Cortés 2000). This strategy has tradeoffs, as mortality rates likely increase with decreasing pup size (Branstetter 1990; Cortés 2000). It is important to note that bonnetheads have a short, highly synchronized reproductive cycle, with parturition thought to occur over a discrete period (Parsons 1993a; Gonzales de Acevado 2020; Frazier et al. 2014). In captivity, Parsons (1993a) noted birth occurred over a period of a few days. The exact timing and location of parturition in the wild is less clear. In South Carolina, parturition appears to occur in the second week of September with no postpartum individuals observed the preceding week and no pregnant bonnetheads captured after the third week of September (Frazier et al. 2014; Gonzales de Acevado et al. 2020); However, where parturition occurs is unknown. It is possible that survival across all latitudes is maximized through predator swamping (e.g., Estes 1976; Ims 1990; Santos et al. 2016; Sweeney and Vannote 1982), allowing bonnetheads at lower latitudes to produce similar relatively large brood sizes rather than smaller broods with larger pups, a strategy seen in other coastal sharks, e.g. finetooth sharks and blacknose sharks Carcharhinus acronotus Poey 1860 (Brown et al. 2020; Driggers et al. 2004). If parturition is synchronous among bonnetheads in an area, survival of pups may temporarily increase as predators can only consume a limited number of neonates. Despite being one of the more abundant sharks found in the southeastern U.S. (Ulrich et al. 2007), little to nothing is known regarding the location of parturition and habitats used by neonate bonnetheads. While not in the bounds of this study, future research should investigate the timing and synchrony as well as location of parturition and neonate habitat use for bonnetheads.
Length- and age-at-maturity were also significantly different among regions, with bonnetheads in the western GOM taking an average of 0.6 years longer and 170 mm greater FL to reach maturity than those in the eastern GOM; however, brood size was significantly smaller in the western GOM with an average of 1.2 fewer pups per brood. Driggers et al. (2020) found significant differences in brood size between Atlantic sharpnose sharks in the GOM, with western GOM sharks having larger brood sizes than their eastern GOM counterparts. These differences in brood sizes were attributed to preferred prey availability, with higher abundances of Atlantic croaker, Micropogonias undulatus Linnaeus 1766, in the western GOM. Unfortunately, the majority of bonnethead broods from the western GOM population were sampled east of Mobile Bay, Alabama, geographically adjacent to the eastern GOM population. The western most sampling areas in this study (i.e., Texas) were only sampled in spring and fall when pregnant bonnetheads were not present. Future research should focus on sampling pregnant bonnetheads off the coasts of Texas and Mexico to further examine spatial variability in brood sizes in the GOM.
Bonnethead movement ecology could explain observed life history differences between regions. In the Atlantic, Driggers et al. (2014) found bonnetheads demonstrated inter and intra-annual site fidelity to two specific estuaries in South Carolina, with seasonal coastal migrations to warmer waters off of Florida. In the eastern GOM (Heupel et al. 2006) and off of Dauphin Island, AL (Kroetz et al. 2015), studies found acoustically tagged bonnetheads displayed seasonal site fidelity to the estuaries where they were tagged, with little movements outside of the tagging area. However, data were lacking for winter months as acoustic receiver coverage was poor outside of study areas, and receivers were removed during winter months in the Heupel et al. (2006) study. Similarly, capture data suggest eastern GOM bonnethead migration may be limited as the species is found year-round in Saint George Sound, FL (high latitude group, Peterson and Grubbs 2023). If the western GOM population undergoes a temperature driven migration similar to the Atlantic population (> 500 km) and eastern GOM bonnetheads do not make an extensive migration, this could explain observed life history differences. Populations in the western GOM and Atlantic may put more energy into somatic growth, growing to larger sizes prior to maturity to provide the energy stores needed for long migrations, while those in the eastern GOM may put more energy towards maximizing reproductive output as they do not need to reserve energy for migration. While we are unaware of species-specific data showing differences in size between migratory and non-migratory populations of elasmobranchs, in general, species that undergo large migrations attain larger maximum sizes than those with limited migrations (Speed et al. 2010). To further investigate this concept, future research should investigate movement ecology of bonnetheads in both the eastern and western GOM.
Differential fishing mortality and clinal variation linked to differences in water temperature (e.g., Parsons 1993b; Yamaguchi et al. 2000) often explain life history differences within and between populations. Water temperature, combined with food availability, may explain the latitudinal variation in life history characteristics of bonnetheads in the eastern GOM; however, there was no evidence of latitudinal variation in the western GOM. Comparisons of growth between bonnetheads captured at the same latitudes in the eastern and western GOM (where water temperatures are similar) were significantly different; therefore, observed differences in life history cannot be explained by water temperature alone. Walker (2007) found differences in length-at-maturity of gummy sharks, Mustelus antarticus Günther 1870 that were attributed to length-selective fishing mortality with fished populations having a smaller length-at-maturity. Carlson and Baremore (2003) found evidence for density-dependent growth and maturity in Atlantic sharpnose sharks by sampling GOM populations at two time periods 20 years apart, with the later time period sample having smaller average maximum length and length at maturity, and faster growth rates. Differences were attributed to a compensatory response to fishing mortality. The observed trends in this study are not likely due to density-dependent compensation. The primary source of fishing mortality for bonnetheads in the GOM is through bycatch in shrimp trawls. Effort in this fishery, and consequently bonnethead mortality, is orders of magnitude higher in the western GOM than in the eastern GOM, and there are no major otter trawl gear differences between the regions that would lead to differential bycatch of bonnetheads (Scott-Denton et al. 2012). Therefore, observed life history differences are unlikely to be due to differential fishing mortality and density-dependent growth between regions.
The primary prey of bonnetheads throughout their range is portunid crabs, specifically blue crabs, Callinectes sapidus M.J. Rathburn 1896, (Cortés et al. 1996; Lessa and Almeida 1998; Plumlee and Wells 2016; Branham et al. 2022). Blue crabs are known to have differing migration patterns between the western and eastern GOM. In the western GOM, blue crabs undergo limited movements (2–30 km), with seasonal inshore/offshore spawning migrations by females (More 1969; Perry 1975). In the Atlantic, blue crabs have a similar limited migration pattern to those in the western GOM, with little, to no movement outside of the estuary of tagging (Fischler and Walburg 1962). Conversely, female blue crabs in the eastern GOM display an alongshore migration, making large (up to 800 km) migrations to spawn in estuaries north of their tagging location, with Apalachicola Bay serving as a primary spawning area (Oesterling and Evink 1977; Steele 1991). Since male blue crabs in the GOM are typically found in brackish waters during summer months, juvenile and mature female blue crabs likely represent a larger component of bonnethead’s diet in the region. The predictable abundance of the bonnethead’s primary prey in the western GOM and Atlantic may give these bonnetheads an energetic advantage over bonnetheads in the eastern GOM which may face periods of low abundance of large female blue crabs, resulting in these sharks expending greater energy to find their preferred prey. A study of bonnethead diet in the GOM and Atlantic found the highest consumption of seagrass occurred in the eastern GOM, almost twice the relative importance of any other location (Branham et al. 2022). It is possible that bonnetheads in the eastern GOM spend more time and energy to find and consume crabs (and other prey) hiding in seagrass beds, thereby reducing energy stores available for somatic growth. These differences in migration patterns and/or seasonal crab abundance could, in part, explain latitudinal differences in growth in the eastern GOM first hypothesized by Parsons (1993b), as well as life history differences between the eastern GOM and western GOM and Atlantic. Future research should focus on refining diet studies to examine seasonal differences in diet as well as sex and maturity of crabs to determine if diet may explain observed differences in life history characteristics.
The results of these life history analyses coupled with concurrent and historic population genetic studies (A. Fields unpublished; Díaz-Jaimes et al. 2021) provide strong support for three populations of bonnetheads along the southeast U.S. coast: an Atlantic population, (U.S. East Coast), an eastern GOM population (Florida panhandle south to the Florida Keys), and a western GOM population (Texas through Alabama). Bonnetheads in the western GOM have slower growth rates, larger maximum length, and larger size- and higher age-at-sexual maturity as well as smaller brood sizes compared to those in the eastern GOM. This in turn may limit the reproductive potential of the western GOM population and increase the vulnerability of the populations to fishing mortality. Given the large bycatch of bonnetheads in the GOM shrimp trawl fishery (assumed 100% mortality), the species may be more susceptible to overfishing in the GOM than previously thought. Based on these life history differences and regional differences in fisheries interactions, future management and stock assessments should strongly consider genetically based population groupings when assessing the status of bonnetheads in U.S. waters of the western North Atlantic Ocean.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Akaike H (1973) Information theory as an extension of the maximum likelihood principle. In: Petrov BN, Csaki F (eds) Proceedings of the Second International Symposium on Information Theory. Akademiai Kiado, Budapest, pp 267–281
Beamish RJ, Fournier DA (1981) A method for comparing the precision of a set of age determinations. Can J Fish Aquat Sci 38:982–983. https://doi.org/10.1139/f81-132
Beverton RJH (1954) Notes on the use of theoretical models in the study of the dynamics of exploited fish populations. United States Fishery Laboratory, Beaufort. Miscellaneous Contribution (2), 159 pp
Beverton, RJH, Holt SJ (1957) On the dynamics of exploited fish populations. Fish and Fisheries Series 11, United Kingdom Ministry of Agriculture and Fisheries, United Kingdom. https://doi.org/10.1007/978-94-011-2106-4
Branham CC, Frazier BS, Strange JB, Galloway AS, Adams DH, Drymon JM, Grubbs RD, Portnoy DS, Wells RJD, Sancho G (2022) Diet of the bonnethead Sphyrna tiburo along the northern Gulf of Mexico and southeastern Atlantic coast of the United States. Anim Biodivers Conserv 45(2):257–267. https://doi.org/10.32800/abc.2022.45.0257
Branstetter S (1990) Early life history implications of selected carcharhinoid and lamnoid sharks of the Northwest Atlantic, vol 90. NOAA Tech Rep NMFS, VIMS Books and Book Chapters 40, Gloucester Point. https://scholarworks.wm.edu/vimsbooks/40pp
Brown AN, Frazier BS, Gelsleichter J (2020) Re-evaluation of reproductive cycle and fecundity of finetooth sharks Carcharhinus isodon (Valenciennes 1839) from the Northwest Atlantic Ocean, with new observations on ovarian cycle and reproductive endocrinology of biennially reproducing sharks. J Fish Biol 97:1780–1793. https://doi.org/10.1111/jfb.14542
Cailliet GM, Goldman KJ (2004) Age determination and validation in chondrichthyan fishes. In: Carrier J, Musick JA, Heithaus MR (eds) Biology of sharks and their relatives. CRC Press LLC, Boca Raton, pp 399–447
Cailliet GM, Smith WD, Mollet HF, Goldman KJ (2006) Age and growth of chondrichthyan fishes: the need for consistency in terminology, verification, validation, and growth function fitting. Environ Biol Fish 77:211–228. https://doi.org/10.1007/s10641-006-9105-5
Caña-Hernández S, Lara-Mendoza RE, Mendoza-Carranza M, Pérez-Jiménez JC (2023) Using a multimodel approach to determine the age and growth of an endangered small shark species in the southern Gulf of Mexico. Reg Stud Mar Sci 60:102836. https://doi.org/10.1016/j.rsma.2023.102836
Carlson JK, Parsons GR (1997) Age and growth of the bonnethead, Sphyrna tiburo, from northwest Florida, with comments on clinal variation. Environ Biol Fish 50:331–341. https://doi.org/10.1023/A:1007342203214
Carlson JK, Baremore IE (2003) Changes in biological parameters of Atlantic sharpnose Rhizoprionodon terraenovae in the Gulf of Mexico: evidence for density-dependent growth and maturity. Mar Fresh Res 54:227–234. https://doi.org/10.1071/MF02153
Campana SE, Annand MC, McMillan JI (1995) Graphical and statistical methods for determining the consistency of age determinations. Trans Am Fish Soc 124:131–138. https://doi.org/10.1577/1548-8659(1995)124%3C0131:GASMFD%3E2.3.CO;2
Castro JI (2011) The sharks of North America. Oxford University Press Inc, New York
Chang WYB (1982) A statistical method for evaluating the reproducibility of age determination. Can J Fish Aquat Sci 39:1208–1210. https://doi.org/10.1139/f82-158
Clarke E, Von Schmidt K (1965) Sharks of the central gulf coast of Florida. Bull Mar Sci 15:13–83
Compagno LGV (1984) FAO species catalogue. Vol 4. Sharks of the world. An annotated and illustrated catalogue of shark species known to date. Part 2 - Carcharhiniformes. FAO Fish Synopsis 125, Rome 251–655
Cortés E, Manire CA, Hueter RE (1996) Diet, feeding habits and diel feeding chronology of the bonnethead shark, Sphyrna tiburo, in southwest Florida. Bull Mar Sci 58:353–367
Cortés E (2000) Life history patterns and correlations in sharks. Rev Fish Sci 8:299–344. https://doi.org/10.1080/10408340308951115
Cortés E (2002) Stock assessment of small coastal sharks in the US Atlantic and Gulf of Mexico. National Marine Fisheries Service, Southeast Fisheries Science Center, Panama City, p 133. Sustainable Fisheries Division Contribution SFD-01802-152
Díaz-Jaimes P, Bayona-Vásquez NJ, Escatel-Luna E, Uribe-Alcocer M, Pecoraro C, Adams DH, Frazier BS, Glenn TC, Babbucci M (2021) Population genetic divergence of bonnethead sharks Sphyrna tiburo in the western North Atlantic: Implications for conservation. Aquat Conserv 31:83–98. https://doi.org/10.1002/aqc.3434
Driggers WB III, Oakley DA, Ulrich G, Carlson JK, Cullum BJ, Dean JM (2004) Reproductive biology of Carcharhinus acronotus in the coastal waters of South Carolina. J Fish Biol 64:1540–1551. https://doi.org/10.1111/j.0022-1112.2004.00408.x
Driggers WB III, Frazier BS, Adams DH, Ulrich GF, Jones CM, Hoffmayer ER, Campbell MD (2014) Site fidelity of migratory bonnethead sharks Sphyrna tiburo (L. 1758) to specific estuaries in South Carolina, USA. J Exp Mar Biol Ecol 459:61–69. https://doi.org/10.1016/j.jembe.2014.05.006
Driggers WB III, Hoffmayer ER, Campbell MD, Jones CM, Hannan KM, Sulikowski JA (2020) Spatial variability in the fecundity of Atlantic sharpnose sharks (Rhizoprionodon terraenovae) in the northern Gulf of Mexico. Fish Bull 118:51–62. https://doi.org/10.7755/FB.118.1.5
Drymon JM, Driggers WB III, Oakley D, Ulrich GF (2006) Investigating life history differences between finetooth sharks Carcharhinus isodon in the northern Gulf of Mexico and the western North Atlantic Ocean. Gulf Mex Sci 24:2–10. https://doi.org/10.18785/goms.2401.02
Escatel-Luna E, Adams DH, Uribe-Alcocer M, Islas-Villanueva V, Díaz-Jaimes P (2015) Population genetic structure of the bonnethead shark, Sphyrna tiburo, from the western North Atlantic Ocean based on mtDNA sequences. J Hered 106:355–365. https://doi.org/10.1093/jhered/esv030
Estes RD (1976) The significance of breeding synchrony in the wildebeest. East Afr Wildl J 14:135–152. https://doi.org/10.1111/j.1365-2028.1976.tb00158.x
Fields AT, Feldheim KA, Gelsleichter J, Pfoertner C, Chapman DD (2016) Population structure and cryptic speciation in bonnethead sharks Sphyrna tiburo in the south-eastern USA and Caribbean. J Fish Biol 89:2219–2233. https://doi.org/10.1111/jfb.13025
Fischler KJ, Walburg CH (1962) Blue crab movements in coastal South Carolina, 1958–1959. Trans Am Fish Soc 91:275–278. https://doi.org/10.1577/1548-8659(1962)91[275:BCMICS]2.0.CO;2
Frazier BS, Driggers WB III, Adams DH, Jones CM, Loefer JK (2014) Validated age, growth and maturity of the bonnethead Sphyrna tiburo in the western North Atlantic Ocean. J Fish Bio 85:688–712. https://doi.org/10.1111/jfb.12450
Frazier BS, Bethea DM, Hueter RE, McCandless CT, Tyminski JP, Driggers WB III (2020) Growth rates of bonnetheads (Sphyrna tiburo) estimated from tag-recapture data. Fish Bull 118(4):329–355. https://doi.org/10.7755/FB.118.4.3
Goldman KJ (2004) Age and growth of elasmobranchs fishes. In: Musick JA, Bonfil R (eds) Elasmobranch fisheries management techniques. Asia Pacific Economic Cooperation, Singapore, pp 97–132
Gonzales de Acevado M, Frazier BS, Belcher C, Gelsleichter J (2020) Reproductive cycle and fecundity of the bonnethead Sphyrna tiburo L. from the northwest Atlantic Ocean. J Fish Biol 97:1733–1747. https://doi.org/10.1111/jfb.14537
Harry AV (2018) Evidence for systemic age underestimation in shark and ray ageing studies. Fish Fish 19:185–200. https://doi.org/10.1111/faf.12243
Hoenig JM, Morgan MJ, Brown CA (1995) Analyzing differences between two age determination methods by tests of symmetry. Can J Fish Aquat Sci 52:364–368. https://doi.org/10.1139/f95-038
Holden MJ (1973) Are long-term sustainable fisheries for elasmobranchs possible? Rapp P-V Reun Cons Perm Int Explor Mer 164:360–367
Heupel MR, Simpfendorfer CA, Collins AB, Tyminski JP (2006) Residency and movement patterns of bonnethead sharks, Sphyrna tiburo, in a large Florida estuary. Environ Biol Fish 76:47–67. https://doi.org/10.1007/s10641-006-9007-6
Heupel MR, Carlson JK, Simpfendorfer CA (2007) Shark nursery areas: concepts, definition, characterization and assumptions. Mar Ecol Prog Ser 337:287–297. https://doi.org/10.3354/meps337287
Ims RA (1990) The ecology and evolution of reproductive synchrony. Trends Ecol Evol 5:135–140. https://doi.org/10.1016/0169-5347(90)90218-3
Kimura DK (1980) Likelihood methods for the von Bertalanffy growth curve. Fish Bull 77:765–776
Knip DM, Heupel MR, Simpfendorfer CA (2010) Sharks in nearshore environments: Models, importance, and consequences. Mar Ecol Prog Ser 402:1–11. https://doi.org/10.3354/meps08498
Kroetz AM, Powers SP, Drymon JM, Park K (2015) Anthropogenic modifications to a barrier island influence Bonnethead (Sphyrna tiburo) movements in the northern Gulf of Mexico. Anim Biotelem 3:38. https://doi.org/10.1186/s40317-015-0067-2
Lessa RP, Almeida Z (1998) Feeding habits of the Bonnethead shark, Sphyrna tiburo, from northern Brazil. Cybium 22(4):383–394
Lombardi-Carlson LA, Cortes E, Parsons GR, Manire CA (2003) Latitudinal variation in life history traits of bonnethead sharks, Sphyrna tiburo, (Carcharhiniformes, Sphyrnidae), from the eastern Gulf of Mexico. Mar Fresh Res 54:875–883. https://doi.org/10.1071/MF03023
Lombardi-Carlson LA (2007) Life history traits of bonnethead sharks, Sphyrna tiburo, from the eastern Gulf of Mexico. SEDAR (SouthEast Data, Assessment, and Review) 13-DW-24-V3, SEDAR, North Charleston
Manire CA, Rasmussen EL, Hess DL, Hueter RE (1995) Serum steroid hormones and the cycle of the female bonnethead shark, Sphyrna tiburo. Gen Comp Endocrinol 97:366–376. https://doi.org/10.1006/gcen.1995.1036
Manire CA, Rasmussen EL (1997) Serum concentrations of steroid hormones in mature male bonnethead shark, Sphyrna tiburo. Gen Comp Endocrinol 107:414–420. https://doi.org/10.1006/gcen.1997.6937
Mollet HF, Ezcurra JM, O’Sullivan JB (2002) Captive biology of the pelagic stingray, Dasyatis violacea (Bonaparte, 1832). Mar Fresh Res 53:531–541. https://doi.org/10.1071/MF01074
More WR (1969) A contribution to the biology of the blue crab (Callinectes sapidus Rathbun) in Texas, with a description of the fishery. Texas Parks and Wildife Department, Technical Series, Austin
Natanson LJ, Skomal GB, Hoffmann SL, Porter ME, Goldman KJ, Serra D (2018) Age and growth of sharks: do vertebral band pairs record age? Mar Fresh Res 69:1440–1452. https://doi.org/10.1071/MF17279
Nelson GA (2021) fishmethods: Fisheries methods and models in R. R package version 1.11-2. https://CRAN.R-project.org/package=fishmethods. Accessed June 2021
Oesterling MJ, Evink GL (1977) Relationship between Florida's blue crab population and Apalachicola Bay. In: Livingston RJ, Joyce EA (eds) Proceedings conference on the apalachicola drainage system, vol 26. Florida Marine Research Publication, Gainsville. pp 101–121
Ogle DH, Doll JC, Wheeler P, Dinno A (2021) FSA: Fisheries stock Analysis. R package version 0.9.1. https://github.com/droglenc/FSA. Accessed June 2021
Parsons GR (1993a) Age determination and growth of the bonnethead shark, Sphyrna tiburo: a comparison of two populations. Mar Biol 117:23–31. https://doi.org/10.1007/BF00346422
Parsons GR (1993b) Geographic variation in reproduction between two populations of the bonnethead shark, Sphyrna tiburo. Environ Biol Fish 38:25–35. https://doi.org/10.1007/978-94-017-3450-9_3
Perry HM (1975) The blue crab fishery in Mississippi. Gulf Res Rep 5(1):39–57. https://doi.org/10.18785/grr.0501.05
Peterson CT, Grubbs RD (2023) Temporal community structure and seasonal climatic migration of coastal sharks and large teleost fishes in the northeast Gulf of Mexico. Can J Fish Aquat Sci. https://doi.org/10.1139/cjfas-2022-0124
Plumlee JD, Wells RJD (2016) Feeding ecology of three coastal shark species in the northwest Gulf of Mexico. Mar Ecol Prog Ser 550:163–174. https://doi.org/10.3354/meps11723
Portnoy DS, Puritz JB, Hollenbeck CM, Gelsleichter J, Chapman D, Gold JR (2015) Selection and sex-biased dispersal in a coastal shark: the influence of philopatry on adaptive variation. Mol Ecol 24:5877–5885. https://doi.org/10.1111/mec.13441
Pratt HL, McCandless C, Kohler N, Jensen C, Ulrich GF, Gilligan M (1998) Report of the 1998 Apex predators program cooperative shark pupping and nursery (COASTSPAN) survey. Apex predators program. USDOC, NOAA, NMFS Narragansett Lab, Narragansett
R Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/. Accessed June 2021
Ricker WE (1975) Computation and interpretation of biological statistics of fish populations. Bull Fish Res Board Can 191:1–382
Ricker WE (1979) Growth rates and models. In: Hoar WS, Randall DJ, Brett JR (eds) Fish Physiology vol. VIII: Bioenergetics and growth. Academic Press, New York, pp 677–743
Santos RG, Pinheiro HT, Martins AG, Riul P, Bruno SC, Janzen FJ, Ioannou CC (2016) The anti-predator role of within-nest emergence synchrony in sea turtle hatchlings. Proc R Soc B 283. https://doi.org/10.1098/rspb.2016.0697
Scott-Denton E, Cryer PF, Duffy MR, Gocke JP, Harrelson MR, Kinsella DL, Nance JM, Pulver JR, Smith RC, Williams JA (2012) Characterization of the U.S. Gulf of Mexico and South Atlantic penaeid and rock shrimp fisheries based on observer data. Mar Fish Rev 74:1–27
Scott-Denton E, Cryer PF, Duffin BV, Duffy MR, Gocke JP, Harrelson MR, Whatley AJ, Williams JA (2020) Characterization of the U.S. gulf of Mexico and South Atlantic Penaeidae and rock shrimp (Sicyoniidae) fisheries through mandatory observer coverage, from 2011 to 2016. Mar Fish Rev 82:1–27
SEDAR (SouthEast Data, Assessment, and Review) (2013) SEDAR 34 stock assessment report: HMS bonnethead shark. SEDAR, North Charleston
Speed CW, Field IC, Meekan MG, Bradshaw CJA (2010) Complexities of coastal shark movements and their implications for management. Mar Ecol Prog Ser 408:275–293. https://doi.org/10.3354/meps08581
Steele P (1991) Population dynamics and migration of the blue crab Callinectes sapidus Rathbun, in the eastern Gulf of Mexico. Proc Fortieth Ann Gulf Caribb Fish Inst 40:241–244. https://doi.org/10.18785/goms.1501.04
Sweeney BW, Vannote RL (1982) Population synchrony in mayflies: a predator satiation hypothesis. Evolution 36:810–821. https://doi.org/10.2307/2407894
Ulrich GF, Jones CM, Driggers WB III, Drymon JM, Oakley D, Riley C (2007) Habitat utilization, relative abundance, and seasonality of sharks in the estuarine and nearshore waters of South Carolina. In: McCandless CT, Kohler NE, Pratt Jr HL (eds) Shark nursery grounds of the Gulf of Mexico and East Coast waters of the United States. American Fisheries Society Symposium 50, Bethesda, pp 125–139
Vinyard EA, Frazier BS, Drymon JM, Gelsleichter JJ, Bubley WJ (2019) Age, growth, and maturation of the finetooth shark, Carcharhinus isodon, in the Western North Atlantic Ocean. Environ Biol Fish 102:1499–1517. https://doi.org/10.1007/s10641-019-00929-9
Von Bertalanffy L (1938) A quantitative theory of organic growth (inquiries on growth laws. II). Hum Biol 10:181–213. https://www.jstor.org/stable/41447359
Walker TI (2007) Spatial and temporal variation in the reproductive biology of the gummy shark Mustelus antarticus (Chondrichthyes: Triakidae) harvested off of southern Australia. Mar Fresh Res 58:67–97. https://doi.org/10.1071/MF06074
Yamaguchi A, Taniuchi T, Shimizu M (2000) Geographic variations in reproductive parameters of the starspotted dogfish, Mustelus manazo, from five localities in Japan and Taiwan. Environ Biol Fish 57:221–233. https://doi.org/10.1023/A:1007558324902
Zhang X, Linton B, Cortes E, Courtney D (2013) Shrimp fishery bycatch estimates for Atlantic sharpnose and bonnethead sharks in the Gulf of Mexico, 1972–2011. SEDAR34-WP-18, SEDAR, North Charleston
Acknowledgements
We are especially grateful to the many folks that helped make this study possible. A. Galloway, N. Weber. M. Young, J. Cashour, E. Seubert, C. Manire, C. Peterson, J. Higgs, and Mote Marine Laboratory and TAMUCC Marine Genomics lab members helped with field work, specimen collection, workup, and vertebrae prepping. We would also like to thank the “Trawl and Plankton Branch” at the National Marine Fisheries Service, Southeast Fisheries Science Center, Mississippi Laboratories for specimen collection on the Southeast Area Monitoring and Assessment Program (SEAMAP) trawl surveys. Archived samples were provided by W. Bubley, L. Lombardi, and J. Carlson. Funding for this study was provided by Saltonstall-Kennedy Grant No. NA16NMF4270225. We appreciate the guidance of the editors and two anonymous reviewers who greatly improved the manuscript. This is contribution 865 of the South Carolina Marine Resources Center.
Author information
Authors and Affiliations
Contributions
Bryan Frazier and David Portnoy were responsible for study conception, design, and funding acquisition. All authors were responsible for data collection. Analyses and lab work was performed by Bryan Frazier and Elizabeth Vinyard. The first draft of the manuscript was written by Bryan Frazier, David Portnoy and William Driggers III and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
All field collections were carried out under necessary state, and university specific protocols. Collection of specimens was performed under SCDNR Scientific Permit #2212, under Florida Fish and Wildlife Conservation Commission Special Activities License SAL-1666-SRP, SAL-1092, and under protocols approved by New College of Florida (IS00001126 and IS00004541) and University of Southern Mississippi (11092217, 13101704, 15101509, 18121301, 18010501).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Frazier, B.S., Vinyard, E.A., Fields, A.T. et al. Age, Growth and Maturity of the Bonnethead Sphyrna tiburo in the U.S. Gulf of Mexico. Environ Biol Fish 106, 1597–1617 (2023). https://doi.org/10.1007/s10641-023-01439-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-023-01439-5