Abstract
Round goby (Neogobius melanostomus) is a benthic freshwater fish native to the Ponto-Caspian region in Europe that was first recorded in the Laurentian Great Lakes basin in 1990 in the St. Clair River in Sarnia, Ontario. It has since become one of the major invasive species of the Great Lakes. The mechanisms through which round goby has become a successful invader are poorly understood. It has been hypothesized that phenotypic plasticity of species may influence their establishment, spread, and impact. If a species is phenotypically plastic, it could more easily adapt to a variety of environments. We examine whether phenotypic variation is present in round goby in the Laurentian Great Lakes and whether morphological variation in dorsal and lateral shape is related to habitat type and time since invasion. Morphological variation in preserved round goby specimens was analyzed for dorsal and lateral shape differences between lake, large river, and river habitats, waterbody of origin (Erie, Huron, Michigan, Ontario, Simcoe) and time since initial invasion (i.e., early, mid, recent) using a Procrustes ANOVA and visualized using a principal component analysis. There is significant variation in body shape among lake and river populations, which may be due to differences in water flows between these habitats. Lake specimens have an overall deeper body shape, whereas river specimens have an overall shallow body shape and large river specimens are intermediate in shape. The results of this study help better understand what morphological mechanisms facilitate invasions and provide valuable information for management decisions related to spread of round goby in the Great Lakes basin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Round goby (Neogobius melanostomus) is a benthic fish native to the Caspian, Black, and Azov seas in Europe (Balshine et al. 2005). It has become invasive in the Laurentian Great Lakes, one of several areas in which it has been introduced globally (Kornis et al. 2012). Round goby was first documented in the Great Lakes in 1990 in the St. Clair River in Sarnia, Ontario (Jude et al. 1992). It was likely introduced by ballast water released from transoceanic vessels (Kornis et al. 2012). Since its first introduction in the St. Clair River, through additional ballast-water movement, natural spread, and bait release, round goby has spread throughout the five Great Lakes and established in varying abundances (Kornis et al. 2012). It has also spread through the Great Lakes into wetland and tributary habitats directly by dispersal and inland lakes indirectly through human-aided movement through bait-bucket transfer and accidental release (Kornis et al. 2012).
Since its initial introduction and spread, round goby has been a highly successful invader in terms of establishment and spread. Its initial establishment was likely the result of several introduction events and high genetic variation that provided a larger number of founder individuals and lower probability of inbreeding depression, respectively (Kornis et al. 2012). Round goby establishment may be further facilitated by wide tolerance to salinity (Skόra et al. 1999; Cross and Rawding 2009; Kornis et al. 2012), low dissolved-oxygen levels (Kornis et al. 2012), its ability to spawn multiple times over a season (Jude et al. 1992; Charlesbois et al. 2001), broad diet and ability to adapt to new food sources in a different habitat (Kornis et al. 2012), and aggressive competitiveness (Balshine et al. 2005). It has been successful in establishing in areas where there are high numbers of native species, which is unusual for invasive species (Carman et al. 2006; Kornis et al. 2012). Round goby appears to exhibit phenotypic plasticity (Simonovic et al. 2001; Polacik et al. 2012; MacInnis and Corkum 2011; Brandner et al. 2013; Hôrková and Kováč 2013; Cerwenka et al. 2014; Hôrková and Kováč 2015), which could be an important factor for establishment as it would enable round goby to adapt to different, possibly changing, aquatic environments, thereby allowing round goby to live in a greater variety of habitats. In addition, round goby has exhibited life-history trait variation among individuals; Cerwenka et al. (2017) hypothesized that several trait trajectories within a species led to successful invasion.
A small body of literature has examined the intraspecific phenotypic variation of freshwater fishes among populations or individuals occupying different habitats. Regions such as the Laurentian Great Lakes are poorly represented in the literature; however, there is considerable information about phenotypic variation in salmonids (Samways et al. 2015) and other fishes occupying different habitats. Some studies have found morphological differences between separate populations of the same species inhabiting different habitats (Robinson and Wilson 1994; Robinson and Parsons 2002), including topmouth gudgeon (Pseudorasbora parva) (Záhorská et al. 2009), and black bullhead (Ameiurus melas) (Novomeská et al. 2013). Robinson et al. (1993) explored such variation in pumpkinseed (Lepomis gibbosus) when another sunfish species, bluegill (Lepomis macrochirus), was absent in a lake habitat. Robinson et al. (1993) found that when bluegill is historically absent in a lake, pumpkinseed assumed two ecological niches. Typically, in the presence of bluegill, pumpkinseed is found in the littoral zone and consumes hard-bodied food (e.g., snails). However, when bluegill was absent, a second morphotype of pumpkinseed is found in open water and consumes zooplankton, typically consumed by bluegill. Phenotypic plasticity in pumpkinseed has been shown to account for 53% of total shape variation while genetic heritability accounts for 14% of total shape variation (Robinson et al. 2000). Numerous studies have shown ontogenetic changes in the body shape of pumpkinseed that are dependent upon the environment (Tomeček et al. 2005, 2007). Phenotypic plasticity may be the underlying mechanism for the different phenotypes found between pelagic and littoral habitats, and between lake and river habitats, due the expression of different phenotypes in different water velocities (fast- versus slow-moving water) and for different maneuverability and swimming needs (Brinsmead and Fox 2002; Imre et al. 2002; Yavno and Fox 2014; Binning and Roche 2015; Istead et al. 2015; Gaston and Lauer 2014; Samways et al. 2015).
It has been hypothesized that phenotypic plasticity may influence the ability of an introduced species to successfully establish, dominate, and spread within broad geographic areas (Baker, 1965). Having plasticity, manifested as changes in morphological forms, physiology, life-history traits, or behavior, increases ecological niche breadth by allowing individuals to express advantageous phenotypes over a broad range of environmental conditions (Pohlman et al. 2005). Successful non-native species that have specialized forms in their native range may acquire more generalized forms in a novel environment to cope with unknown and/or changing biotic and abiotic factors; invasion potential increases when the difference between the generalized form and specialized form is at its maximal, as predicted by the theory of alternative ontogenies and invasive potential (Kováč 2010; Záhorská et al. 2013; Hôrková and Kováč 2015). Morphology has been used as a proxy for a species’ ecological role within a community (Azzurro et al. 2014), and the success of round goby as an invasive species within the Laurentian Great Lakes may be due to a wide range of variation and plasticity in its morphological form, increasing the species’ ability to access resources and maximize its fitness.
Round goby exhibits differing morphology between its native and non-native ranges that could represent phenotypic plasticity or could be due to a founder effect of establishing a new population (Polacik et al. 2012). Brandner et al. (2013) and Cerwenka et al. (2014) provided strong evidence of phenotypic plasticity in round goby invasion in the Danube River. Brandner et al. (2013, 2018) found that the pioneering population differed compared to more recently established populations in morphology, body size, feeding behavior, sex ratio, and parasitic load. Cerwenka et al. (2014) found that body shape was associated with substrate type and geographic location of the round goby population. These changes in body shape may be a way of adapting to a new environment (Cerwenka et al. 2014). Furthermore, variation in size-at-age has been documented between the Danube River and the Upper Detroit River (Simonovic et al. 2001; MacInnis and Corkum 2000a, b; L’avrinčíková and Kováč 2007; Polacik et al. 2012). Pettitt-Wade et al. (2015) has shown niche plasticity in the round goby within the Laurentian Great Lakes, whereby niche plasticity and body size were larger in the invasive round goby compared to noninvasive tubenose goby (Proterorhinus semilunaris). However, there has been no research on phenotypic plasticity in round goby shape within the Laurentian Great Lakes basin with respect to habitat type and time since invasion. Such research would provide important information about how it adapts and expands its range in lake and river habitats. Ren and Zhang (2008) described an invasion mechanism whereby an organism can rapidly evolve by adaptation to the physical environment. This mechanism would be supported if round goby was able to adapt to novel environmental conditions, such as varying water velocities in lake and river habitats.
Our objective is to determine whether phenotypic variation, represented by variation in body shape related to swimming morphology, is present in round goby populations in lake and river habitats of the Laurentian Great Lakes basin. Using museum specimens, morphological variation was compared among several populations of round goby in three different habitats in the Laurentian Great Lakes basin over time: small river (hereafter referred to as river), large river, and lake. We propose three hypotheses that could explain potential morphological variation. First, round goby in river habitats will have a more streamlined body shape than those collected from lakes to allow for maintaining position in flowing waters. Second, round goby collected from lakes will be more similar in shape to round goby collected from large rivers than from rivers because the larger rivers generally have slower flows. Third, round goby collected from later stages in the invasion will be most diverged in shape from those from initial invasion as they undergo selection when they spread to new habitats.
Methods
To examine body shape variation in round goby, we conducted a geometric morphometric analysis based on photographs of preserved museum specimens collected in three different habitats over time (1993–2015).
Specimens
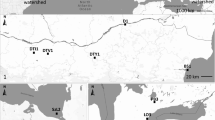
Round goby specimens were obtained from the fish collection of the Royal Ontario Museum. Only specimens first fixed in formalin and then preserved in ethanol were used to minimize the potential effects of different preservation methods. As most museum specimens were collected during the protracted spawning period of the goby, and body shape may vary between spawning and non-spawning seasons (Sisneros et al. 2009), only specimens collected during spawning season were included in this analysis. Additionally, due to preservation, we were unable to sex majority individual specimens and therefore cannot account for sexual dimorphism. A total of 182 specimens were included in the dorsal head shape analysis and 152 specimens were included in the lateral shape analysis. In total 30 catalogued lots were used, representing varying habitat types: fast-flowing small rivers (i.e., river); slow-flowing large rivers (i.e., large river); and, lakes with little or no flow. Catalogued lots also represented various times since initial invasion of the Great Lakes: early, 1993–1999; mid, 2000–2009; and, recent, 2010 to present (Fig. 1; Tables 1 and 2). These time frames were chosen for the natural breaks in the sampling dates of collections available while maintaining periods of relatively equal length that would encompass several generations consistent with the ability of round goby to rapidly adapt to new habitats (Kornis et al. 2012). Each catalogued lot had between one and 100 individuals. In smaller lots (less than 20), we photographed all individuals; if the sample lots were large (more than 20), we selected individuals based on how well the individual was preserved (e.g., minimal deformation) and to represent the full range of adult sizes. Each individual was photographed both dorsally and laterally using a Nikon Coolpix L330 digital camera on a tripod with two umbrella lamps.
Distribution of round goby specimens used in this study. Numbers correspond to localities in Table 1
Landmarking
Using TPSUtil64 (Rohlf 2016) and TPSDig2 (Rohlf 2017), we landmarked each photograph with fixed and semi-landmarks (Bookstein, 1991). Fixed landmarks are distinct anatomical points on the body present on each specimen; for example, the anterior insertion of dorsal fin or tip of snout. Semi-landmarks are points that are not fixed, but “slide” based on the specimen and can capture variation in curves. Six fixed landmarks (1–6) were placed in the dorsal head shape images (Fig. 2). Twenty-two fixed landmarks (number 1–22) and 38 semi-landmarks (23–60) were placed in the lateral images (Fig. 3). After landmarking, “unbend” was used in TPSUtil64 to adjust curvature in deformed specimens due to preservation effects (Rohlf 2016). A total of five points were used to fit the quadratic curve that adjusted for the curvature in the specimens. For each specimen, these points were placed along the midline between landmarks 13 and 7. Once the “unbend” procedure adjusted the curvature in the specimens, the additional points were removed for statistical analyses.
Morphological landmarks (n = 6) for dorsal head shape images of round goby. All landmarks (1–6) for dorsal head shape image analysis are fixed landmarks. Fixed landmarks in order are as follows: (1) dorsal point of right orbital, (2) dorsal point of left orbital, (3) widest point of right lateral side of head, (4) widest point of left lateral side of head, (5) dorsal insertion of right pectoral fin, and (6) dorsal insertion of left pectoral fin
Morphological landmarks (n = 60) for lateral images of round goby. Landmarks 1–22 (in red) are fixed landmarks and 23–60 (in white) are sliding landmarks. Fixed landmarks in order are as follows: (1) tip of snout, (2) dorsal edge of fish above landmark 14, (3) anterior-most end of scaled nape, (4) anterior insertion of dorsal fin, (5) posterior insertion of dorsal fin, (6) dorsal insertion of caudal fin, (7) posterior-most point of body midline/medial insertion of caudal fin, (8) ventral insertion of caudal fin, (9) posterior insertion of anal fin, (10) anterior insertion of anal fin, (11) anterior insertion of pelvic fin, (12) intersection of the ventral-most margin of the operculum and ventrum, (13) anterodorsal point of lower lip, (14) ventroposterior point of descending process of premaxilla, (15) dorsal point of orbital, (16) posterior point of orbital, (17) ventral point of orbital, (18) anterior point of orbital, (19) dorsal-most point of operculum, (20) posteroventral point of operculum, (21) dorsal insertion of pectoral fin, and (22) ventral insertion of pectoral fin
Statistical analyses
Outliers were identified and removed using an outlier analysis in the geomorph package in R (Adams and Otrola-Castillo 2020; Collyer and Adams 2018, 2020; Adams et al. 2020; R Core Team 2020) prior to analysis. Generalized Procrustes analysis (GPA) was used to align specimens to an average shape, remove non-shape information, and convert each specimen to a point in shape space (Bookstein 1991), which was then visualized using principal component analysis (PCA) where each axis represents a specific set of morphological characteristics that summarizes the greatest variation in morphology among specimens (Rohlf and Marcus 1993). To test for shape differences between populations, a Procrustes analysis of variance (ANOVA) and post-hoc pairwise tests were completed separately for the dorsal and lateral images using the geomorph package in R (Adams and Otárola-Castillo 2020; R Core Team 2020). For both dorsal and lateral images, each Procrustes ANOVA was conducted comparing habitat type (lake, large river, river); habitat type was nested within waterbody of origin (e.g., Erie; included as a random effect) and time (i.e., time since invasion: early, mid, recent), and the logarithm of centroid size, a commonly used measure of size in morphometric analyses, was incorporated into the Procrustes ANOVA. If the Procrustes ANOVA results were statistically significant, a post-hoc pairwise test was completed using the pairwise function in geomorph, which conducted residual randomization permutation procedures, determined fitted values over 10,000 permutations, and calculated least squares means and pairwise statistics based on a grouping factor (i.e., habitat type and time since invasion).
Results
Dorsal shape analysis
Three individuals, identified as outliers, were excluded from dorsal image analyses. The plot of the first two principal components and corresponding deformation grids shows that the first principal component summarizes variation in shape between the dorsal insertion of the pectoral fins and the widest lateral points of the head while the second principal component summarizes variation in shape between the widest lateral points of the head and the dorsal points of the orbits (Fig. 4). Thus, specimens towards the bottom left of the plot have a more elongated head shape while specimens towards the top right have a more shortened head shape, reflecting the relative distance between the head and pectoral fins (Fig. 4). The results of the Procrustes ANOVA indicated that there is a significant association between shape and centroid size (p = 0.0001; Table 3). An ANOVA testing for allometric effects among habitats and waterbody of origin using residual randomizations supported a common allometry model. There is a large overlap of specimens from all habitats found in every quadrant, with more lake and river specimens in the upper quadrants and large river specimens in the lower quadrants generally. The Procrustes ANOVA showed no statistically significant differences between habitats (p = 0.9012; Table 3), but the variation in shape between habitats was significantly different based on waterbody of origin (p = 0.0001; Table 3) and time since invasion (p = 0.002; Table 3). No post-hoc pairwise tests were completed since the main effects of habitat type were not significant.
Generalized Procrustes analysis results of the dorsal shape analysis for round goby, visualized using a principal component analysis with 80% confidence ellipses, with each specimen is identified by habitat (lake, large river, river). Solid lines represent the convex hulls for each habitat. Each specimen (n = 182) is transformed into a point in shape space and compared to the mean shape across all specimens. The convex hulls indicate groupings by habitat. Deformation grids are displayed at the minimum and maximum of each principal component as compared to the mean shape for all specimens. Specimens at the maximum of each component have a shallow, broader head shape and specimens at the minimum of each component have a more elongated head shape
Lateral shape analysis
Eight individuals were excluded from lateral shape analyses because they were identified as outliers, primarily deformed by preservation. The plot of the first two principal components and corresponding deformation grids shows that the first principal component summarizes variation in body depth between the dorsal and ventral sides of the specimens with less deep-bodied specimens towards the minimum of the first component and more deep-bodied specimens towards the maximum of the first component (Figs. 5, 6). The second principal component summarizes longitudinal shape with less elongate specimens towards the minimum of the second component and more elongate specimens toward the maximum of the second component (Figs. 5, 6). The PCA plot of specimens has more elongate, less deep-bodied individuals to the upper left, and less elongate, deeper-bodied, individuals to the lower right (Figs. 5, 6). In the PCA grouping individuals by habitat, most lake specimens are in the right quadrants, the majority of large river specimens are in the top left and bottom right quadrants, and most river specimens are in the bottom quadrants (Figs. 5, 6). The results of the Procrustes ANOVA indicated that there is a significant association between shape and centroid size (p = 0.0001; Table 4). An ANOVA testing for allometric effects using residual randomizations supported a unique allometry model, with significant interactions between centroid size and habitats among waterbody of origin (p = 0.0011; Table 4). The Procrustes ANOVA based on habitat type is not different across time periods since invasion (p = 0.2748; Table 4), but habitat types (p = 0.0007; Table 4) and habitat type nested within waterbody of origin (p = 0.0001; Table 4) showed significant differences. No post hoc comparisons were completed to test for differences in shape variation within habitats across time since invasion as the Procrustes ANOVA showed no significant differences between time periods. A post hoc pairwise test was completed to compare the three categories of habitat (i.e., lake, large river, river) across waterbodies of origin (i.e., Erie, Huron, Michigan, Ontario, Simcoe). These comparisons showed that specimens in the lake habitat were significantly different in shape in Erie versus Michigan (p = 0.0157) and Huron versus Michigan (p = 0.0041). Specimens in the large river habitat were significantly different in shape in Erie versus Huron (p = 0.0084) and Huron versus Michigan (p = 0.0231). Specimens in the river habitat were significantly different in shape in Erie versus Huron (p = 0.0156) and Michigan versus Ontario (p = 0.0434). Specimens from Erie were significantly different in shape in lake versus large river (p = 0.0527). Specimens were also significantly different in shape across different habitats between waterbody of origin (Table 4).
Generalized Procrustes Analysis results of the lateral shape analysis for round goby, visualized using a principal component analysis with 80% confidence ellipses, with each specimen identified by habitat (lake, large river, river) and waterbody in which it was collected. Each specimen (n = 154) is transformed into a point in shape space and compared to the mean shape across all specimens type in which it was collected. The convex hulls indicate groupings by habitat. Deformation grids are displayed at the minimum and maximum of each principal component as compared to the mean shape for all specimens. Specimens toward the top left quadrant have an elongated, narrower body shape while specimens toward the bottom right have a shortened, deeper body shape
Generalized Procrustes analysis results of the lateral shape analysis for round goby, visualized using a principal component analysis with 80% confidence ellipses, with each specimen identified by time since invasion (i.e., early, mid, recent) and the habitat (i.e., lake, large river, river) in which it was collected. Each specimen (n = 152) is transformed into a point in shape space and compared to the mean shape across all specimens type in which it was collected. The convex hulls indicate groupings by time since invasion. Deformation grids are displayed at the minimum and maximum of each principal component as compared to the mean shape for all specimens. Specimens toward the top left quadrant have an elongated, narrower body shape while specimens toward the bottom right have a shortened, deeper body shape
Discussion
The round goby specimens exhibited substantial variation in dorsal and lateral body shapes across habitats and waterbody of origin. Our first hypothesis that round goby collected from large river and river habitats have a more streamlined body shape than individuals from lakes is supported by the distribution of specimens in multivariate space for lateral, but not dorsal head, shape. Our second hypothesis that round goby collected from lakes will be more similar in shape to round goby collected from large rivers than from rivers was not supported by the distribution of specimens in multivariate space for lateral or dorsal head shape analysis. Our third hypothesis that round goby collected from later stages of the invasion will be divergent in shape from earlier invasion stages was not supported by the lateral or dorsal shape analysis.
Habitat influences the body shape of the benthic round goby in the Great Lakes basin. Based on the lateral shape patterns, lake specimens appear to have an overall deeper body shape, whereas river specimens appear to have an overall shallow body shape and large river specimens appear to be in intermediate in shape (Figs. 4, 5, and 6). In general, round goby in both river and large river habitats would benefit from a more slender body shape to maneuver better and maintain position in the fast-moving waters, whereas round goby in lakes would not benefit from such a shape due to limited water flow, except potentially in high-energy zones (e.g., currents, surf). However, there may also be areas of large and small rivers with little to no flow (e.g., back eddies, wetlands). These results are consistent with those found for other more pelagic species but similar analyses on other benthic species are lacking. Brinsmead and Fox (2012) found that pumpkinseed and rock bass (Ambloplites rupestris) from stream habitats had more slender bodies than their lake counterparts. Similarly, Samways et al. (2015) found that brook trout (Salvelinus fontinalis) displayed different morphologies in streams and lakes to allow them to respond to different needs in swimming performance.
We did not find any significant differences in comparing habitats and time since invasion. This may be due to small or uneven sample sizes or coarse measure of time since invasion at particular locations. These results differ from Brandner et al. (2013), who found that late invasion-stage populations of round goby in the Danube River had diverged from the initial population in morphology and several other characteristics. However, Brandner et al. (2013) examined the morphology of round goby in the same habitat and geographic location over time, while our study did not have access to such a time series.
When accounting for general shape differences between waterbodies of origin and habitat type, round goby lateral shape was significantly different between waterbodies within a single habitat (i.e., lake, large river, river), and one instance where lateral shape was significantly different between two habitats within a single waterbody. There were also significant differences between various combinations of different habitats and waterbodies. Erie samples were included in several of our significant pairwise tests. Many of the Erie samples are from Early and Mid time since invasion periods, so it is possible that these were most diverged from other waterbodies due to time since invasion differences. However, due to the low sample sizes from waterbodies such as Michigan and Huron, and lack of significant differences between habitat and time since invasion, further sampling is needed to confirm this result. Round Goby in Michigan and Ontario waterbodies also had significant differences, which may have been the result of divergence into different morphological variations related to the great distance between the basins. Overall, these differences could also be due to the different environmental characteristics of each habitat and waterbody type, founder affects, or drift in populations among water bodies that could lead to nonadaptive morphological variation.
The results of this study show that there are morphological variations between habitats and within different waterbodies. Additional research (e.g., common-garden experiments) should be completed to determine if this is the result of phenotypic plasticity and divergence due to habitat and environmental differences. Morphological differences were found in round goby in habitat types across waterbodies of origin (i.e., basin), but not across time since initial invasion. Further studies should be completed using fresh samples from current round goby populations across the Great Lakes, which will allow for a more detailed examination on how waterbody of origin may impact round goby shape across habitats. Furthermore, using fresh samples would allow for larger sample sizes from multiple habitat types and would remove bias in shape variation caused by preservation effects, such as dehydration. Individuals should be sexed prior to analyses (Brinsmead and Fox, 2002) to control for sexual dimorphism in the species, which could potentially shift mean specimen shape due to skewed sex ratios. While males typically have a larger body size at age, darker or black colouration, and enlarged cheeks when compared to females (Kornis et al. 2012), and a genital pore difference, previous studies have shown sex-related differences in round goby to be absent or small (Polačik et al. 2012; Cerwenka et al. 2014), which we found particularly difficult to observe in preserved specimens. To further examine the influence on time since invasion, morphological variation should be measured using specimens sampled from the same location, particularly at invasion fronts, over many years. Genetic analysis could also be completed on these specimens to examine the rate of genetic divergence (Brown and Stepien 2008) and any potential genomic basis for adaptive phenotypic variation that could be used for control (Brown and Stepien 2008).
The results of this study help us better understand what mechanisms facilitate invasions and inform management decisions for invasive species entering the Great Lakes. Round goby has not yet been able to colonize most of Lake Superior because of its incompatible physiochemical properties (Grigorovich et al. 2003), nor most inland lakes in the Great Lakes basin because of limited movement by humans (Drake and Mandrak 2014). However, as global warming continues to alter landscapes, waterbodies, and human behavior, this may change or, alternatively, round goby could adapt to such environments as it has in many other habitats of the Great Lakes. We can use the morphological shape information found in this study to model and predict morphological variants of round goby that would be successful in areas of the Great Lakes basin in which it has not yet spread. This information can be used to increase mitigation efforts in areas of the Great Lakes that would be considered high risk for invasions of the morphological variants predicted to be successful.
Data Availability
The datasets and code generated for this study are available from the corresponding author on reasonable request.
References
Adams DC, Otrola-Castillo E (2020) Geomorph: an R package for the collection and analysis of geometric morphometric shape data. Methods Ecol Evol 4:393–399. https://doi.org/10.1111/2041-210X.12035
Adams D, Collyer M, Kaliontzopoulou A (2020) Geomorph: software for geometric morphometric analyses. R Package Version 3(2):1
Azzurro E, Tuset VM, Lombarte A, Maynou F, Simberloff D, Rodríguez-Pérez A, Solé RV (2014) External morphology explains the success of biological invasions. Ecol Lett 17:1455–1463. https://doi.org/10.1111/ele.12351
Baker HG (1965) Characteristics and modes of origin of weeds. In: Baker HG, Stebbins GL (eds) The genetics of colonizing species. Academic Press, New York, pp 147–169
Balshine S, Verma A, Chant V, Theysmeyer T (2005) Competitive interactions between round gobies and logperch. J Great Lakes Res 31:68–77. https://doi.org/10.1016/S0380-1330(05)70238-0
Binning SA, Roche DG (2015) Water flow and fin shape polymorphism in coral reef fishes. Ecology 96:828–839. https://doi.org/10.1890/14-0426.1
Bookstein FL (1991) Morphometric tools for landmark data: geometry and biology. Cambridge University Press, Cambridge
Brandner J, Cerwenka AF, Schliewen UK, Geist J (2013) Bigger is better: characteristics of round gobies forming an invasion front in the Danube River. PLoS ONE 8:9. https://doi.org/10.1371/journal.pone.0073036
Brandner J, Cerwenka AF, Schliewen UK, Geist J (2018) Invasion strategies in round goby (Neogobius melanostomus): is bigger really better? PLoS ONE 13:1. https://doi.org/10.1371/journal.pone.0190777
Brinsmead J, Fox MG (2002) Morphological variation between lake- and stream-dwelling rock bass and pumpkinseed populations. J Fish Biol 61:1619–1638. https://doi.org/10.1111/j.1095-8649.2002.tb02502.x
Brown JE, Stepien CA (2008) Invasion genetics of the Eurasian round goby in North America: tracing sources and spread patterns. Mol Ecol 18:64–79. https://doi.org/10.1111/j.1365-294X.2008.04014.x
Carman SM, Janssen J, Jude DJ, Berg MB (2006) Diel interactions between prey behaviour and feeding in an invasive fish, the round goby, in a North American river. Freshw Biol 51:742–755
Cerwenka AF, Alibert P, Brandner J, Geist J, Schliewen UK (2014) Phenotypic differentiation of Ponto-Caspian gobies during a contemporary invasion of the upper Danube River. Hydrobiologia 721:269–284. https://doi.org/10.1007/s10750-013-1668-5
Cerwenka AF, Pagnotta A, Böker C, Brandner J, Geist J, Schliewen UK (2017) Little association of biological trait values with environmental variables in invasive alien round goby (Neogobius melanostomus). Ecol Evol 7:4076–4085. https://doi.org/10.1002/ece3.2942
Collyer ML, Adams DC (2018) RRPP: an R package for fitting linear models to high-dimensional data using residual randomization. Methods Ecol Evol 9:1772–1779. https://doi.org/10.1111/2041-210X.13029
Collyer ML, Adams, DC (2020) RRPP: linear model evaluation with randomized residuals in a permutation procedure, R package version 0.5.2
Cross EE, Rawding RS (2009) Acute thermal tolerance in the round goby, Apollonia melanostoma (Neogobius melanostomus). J Therm Biol 34:85–92
Dashinov D, Czerniejewski P, Balshine S, Synyshyn C, Tasheva-Terzieva E, Stefanov T, Ivanova P, Mandrak N, Uzunova E (2020) Variation in external morphology between the native and invasive populations of the round goby, Neogobius melanostomus (Actinopterygii: Gobiidae). Zoomorphology 139:361–371. https://doi.org/10.1007/s00435-020-00480-7
Drake DAR, Mandrak NE (2014) Bycatch, bait, anglers, and roads: quantifying vector activity and propagule introduction risk across lake ecosystems. Ecol Appl 24:877–894. https://doi.org/10.1890/13-0541.1
Gaston KA, Lauer TE (2014) Morphometric variation in bluegill Lepomis macrochirus and green sunfish Lepomis cyanellus in lentic and lotic systems. J Fish Biol 86:317–332. https://doi.org/10.1111/jfb.12581
Grigorovich IA, Korniushin AV, Gray DK, Duggan IC, Colautti RI, MacIsaac HJ (2003) Lake Superior: an invasion coldspot? Hydrobiologia 499:191–210. https://doi.org/10.1023/A:1026335300403
Hôrková K, Kováč V (2013) Different life-histories of native and invasive Neogobius melanostomus and the possible role of phenotypic plasticity in the species’ invasion success. Knowl Manag Aquat Ecosyst 412:01. https://doi.org/10.1051/kmae/2013081
MacInnis AJ, Corkum LD (2000a) Fecundity and reproductive season of the round goby Neogobius melanostomus in the Upper Detroit River. Trans Am Fish Soc 129(1):136–144. https://doi.org/10.1577/1548-8659(2000)129%3c0136:FARSOT%3e2.0.CO;2
Hôrková K, Kováč V (2015) Ontogenetic phenomena, temporal aspect, and ecological factors in the successful invasion of round goby Neogobius melanostomus in the River Danube. Aquat Invasions 10:227–235. https://doi.org/10.3391/ai.2015.10.2.11
Imre I, Mclaughlin RL, Noakes DL (2002) Phenotypic plasticity in brook charr: changes in caudal fin induced by water flow. J Fish Biol 61:1171–1181. https://doi.org/10.1111/j.1095-8649.2002.tb02463.x
Istead A, Yavno S, Fox M (2015) Morphological change and phenotypic plasticity in response to water velocity in three species of Centrarchidae. Can J Zool 93:879–888. https://doi.org/10.1139/cjz-2015-0096
Jude DJ, Reider RH, Smith GR (1992) Establishment of Gobiidae in the Great Lakes basin. Can J Fish Aquat Sci 49:416–421. https://doi.org/10.1139/f92-047
Kornis MS, Mercado-Silva N, Zanden MJ (2012) Twenty years of invasion: a review of round goby Neogobius melanostomus biology, spread and ecological implications. J Fish Biol 80:235–285. https://doi.org/10.1111/j.1095-8649.2011.03157.x
Kováč V (2010) Developmental plasticity and successful fish invasions. In: Proceedings of 17th International Conference on Aquatic Invasive Species, 29 August – 2 September 2010, San Diego, 159
L’avrinčíková M, Kováč V (2007) Invasive round goby Neogobius melanstomus from the Danube mature at small size. J Appl Ichthyol 23:276–278. https://doi.org/10.1111/j.1439-0426.2007.00851.x
MacInnis AJ, Corkum LD (2000b) Age and growth of round goby Neogobius melanostomus in the upper Detroit River. Trans Am Fish Soc 129:852–858. https://doi.org/10.1577/1548-8659(2000)129%3c0852:AAGORG%3e2.3.CO;2
Novomeská A, Katina S, Copp GH, Pedicillo G, Lorenzoni M, Pompei L, Cucherousset J, Kováč V (2013) Morphological variability of black bullhead Ameiurus melas in its non-native European populations. J Fish Biol 82:1103–1118. https://doi.org/10.1111/jfb.12035
Pettitt-Wade H, Wellband KW, Heath DD, Fisk AT (2015) Niche plasticity in invasive fishes in the Great Lakes. Biol Invasions 17:2565–2580. https://doi.org/10.1007/s10530-015-0894-3
Pohlman CL, Nicotra AB, Murray BR (2005) Geographic range size, seedling ecophysiology and phenotypic plasticity in Australian Acacia species. J Biogeogr 32:341–351. https://doi.org/10.1111/j.1365-2699.2004.01181.x
Polacik M, Janac M, Vassilev M, Trichkova T (2012) Morphometric comparison of native and nonnative populations of round goby Neogobius melanostomus from the River Danube. Folia Zoology 61:1–8. https://doi.org/10.25225/fozo.v61.i1.a2.2012
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Version 3.6.3
Ren M, Zhang Q (2009) The relative generality of plant invasion mechanisms and predicting future invasive plants. Weed Res 49:449–460. https://doi.org/10.1111/j.1365-3180.2009.00723.x
Robinson BW, Wilson DS, Margosian AS, Lotito PT (1993) Ecological and morphological differentiation of pumpkinseed sunfish in lakes without bluegill sunfish. Evol Ecol 7:451–464. https://doi.org/10.1007/BF01237641
Robinson BW, Wilson DS (1994) Character release and displacement in fishes: a neglected literature. Am Nat 144:596–627. https://doi.org/10.1086/285696
Robinson BW, Wilson DS, Margosian AS (2000) A pluralistic analysis of character release in pumpkinseed sunfish (Lepomisgibbosus). Ecol 81:2799–2812. https://doi.org/10.2307/177342
Robinson BW, Parsons KJ (2002) Changing times, spaces, and faces: tests and implications of adaptive morphological plasticity in the fishes of northern postglacial lakes. Can J Fish Aquat Sci 59:1819–1833. https://doi.org/10.1139/f02-144
Rohlf JF (2016) TPSUtil64 Ecology and Evolution. SUNY at Stony Brook, New York, USA
Rohlf JF (2017) tpsDig2 Ecology and Evolution and Anthropology. Stony Brook University, New York, USA
Rohlf JF, Marcus LF (1993) A revolution in morphometrics. Trends Ecol Evol 8:129–132. https://doi.org/10.1016/0169-5347(93)90024-J
Samways KM, Leavitt PR, Magnan P, Rodríguez MA, Peres-Neto PR (2015) Convergent polymorphism between stream and lake habitats: the case of brook char. Can J Fish Aquat Sci 72:1406–1414. https://doi.org/10.1139/cjfas-2015-0116
Sisneros JA, Alderks PW, Leon K, Sniffen B (2009) Morphometric changes associated with the reproductive cycle and behaviour of the intertidal-nesting, male plainfin midshipman. Porichthys notatus 74:18–36. https://doi.org/10.1111/j.1095-8649.2008.02104.x
Skόra K, Olenin S, Gollasch S (1999) Neogobius melanostomus (Pallas, 1811). In: Gollasch S, Minchin D, Rosenthal H (eds) Case histories on introduced species: their general biology, distribution, range expansion and impact. Logos-Verlag, Berlin, pp 69–73
Simonovic P, Paunović M, Popović S (2001) Morphology, feeding, and reproduction of the round goby, Neogobius melanostomus (Pallas), in the Danube River basin, Yugoslavia. J Great Lakes Res 27:281–289. https://doi.org/10.1016/S0380-1330(01)70643-0
Tomeček J, Kováč V, Katina S (2005) Ontogenetic variability in external morphology of native (Canadian) and non-native (Slovak) populations of pumpkinseed (Lepomis gibbosus, Linnaeus 1758). J App Ichythyol 21:335–344. https://doi.org/10.1111/j.1439-0426.2005.00678.x
Tomeček J, Kováč V, Katina S (2007) Biological flexibility of pumpkinseed, a successful coloniser throughout Europe. In: Gherardi F (ed) Freshwater bioinvaders: profiles, distribution, and threats. Springer, Verlag, pp 307–336
Yavno S, Fox MG (2014) Morphological plasticity of native and non-native pumpkinseed sunfish in response to habitat type. Evol Ecol Res 16:373–395. https://doi.org/10.1111/jeb.12230
Záhorská E, Kováč V, Falka I, Beyer K, Katina S, Copp GH, Gozlan RE (2009) Morphological variability of the Asiatic cyprinid, topmouth gudgeon Pseudorasbora parva, in its introduced European range. J Fish Biol 74:167–185. https://doi.org/10.1111/j.1095-8649.2008.02121.x
Záhorská E, Švolíková K, Kováč V (2013) Do invasive populations of topmouth gudgeon (Pseudorasbora parva, Temminck and Schlegel) from disturbed and undisturbed habitats follow different life-histories? Int Rev Hydrobiol 98:61–70. https://doi.org/10.1002/iroh.201201446
Acknowledgements
We thank the Royal Ontario Museum for use of their equipment and specimens.
Author information
Authors and Affiliations
Contributions
N.E.M. and C.C.R. co-conceived the idea for the paper. N.E.M. reviewed, edited the work, and mentored throughout the completion of the project. C.C.R. completed the literature review of the subject, took the photographs, landmarked the images in TPS software, helped complete the analysis, and wrote the manuscript. S.E.C. helped complete statistical analysis and edited the manuscript.
Corresponding author
Ethics declarations
Ethics
This study did not include any research on living animals requiring ethical clearance.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rawlings, C.C., Campbell, S.E. & Mandrak, N.E. Body shape variation in round goby Neogobius melanostomus in the Laurentian Great Lakes basin. Environ Biol Fish 104, 1089–1102 (2021). https://doi.org/10.1007/s10641-021-01138-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-021-01138-z