Abstract
Male guppies display an outstanding diversity of color patterns which is formed as a result of a complex interplay between sexual selection, predation, and other environmental factors. The heterogeneity of the environment affects the variability of ornamental traits in male guppies through genotype–environment interaction. Thyroid hormones (THs) are important regulators of the ontogeny of fish and serve as a link between environmental changes and phenotypic development. However, the role of THs in the formation of a variety of color patterns in male guppies remained poorly understood. In this work, an experimental assessment of the effect of THs on the variability of ornamental traits in Poecilia wingei males was carried out. The fish were reared from birth to the initial stages of the formation of melanistic elements in males and until the final formation of the color pattern; they were subjected to different hormonal regimes: euthyroidism (natural TH status), hyperthyroidism (high TH level, at a triiodo-L-thyronine concentration of 0.15 μg/mL), and hypothyroidism (TH-deficiency, at a thiourea concentration of 0.025%). Alterations in the TH status caused changes in the timing and rate of the development of coloration and affected the transformation of various elements of the color pattern in males. These changes led to an increase in phenotypic variability and the appearance of ornamental traits in the male color patterns that were characteristic of closely related species of Poecilia. Thus, the data obtained indicate a potentially important role of thyroid hormones in the diversification of guppy color patterns and open up new prospects for studying the role of endocrine regulatory mechanisms in the adaptive evolution of poeciliid fish.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The color of animals is an important morphological trait that plays a crucial role in the ecology and evolution of many taxonomical groups. Among vertebrates, the greatest variety of pigment patterns and chromatophore types is characteristic of teleost fishes, which makes them good model objects for studying the pathways and mechanisms of the formation of color diversity. Guppies are considered to be one of the most polymorphic in color groups of freshwater fish (Endler 1980, 1984). There are three species of guppies: the common guppy (Poecilia reticulata), the Endler’s (or Cumaná) guppy (P. wingei), and the Oropuche guppy (P. obscura) which have some genetic and morphological differences (Alexander and Breden 2004; Poeser et al. 2005; Schories et al. 2009). Only male guppies exhibit an outstanding degree of color pattern variability which is formed owing to a rapid evolutionary response to both sexual selection and predation, as well as a number of other environmental factors (Endler 1995; Houde 1997; Kemp et al. 2009; Magurran 2005; O'Steen et al. 2002).
The development of the color pattern in male guppies is affected by genetic and environmental effects. The formation of color patterns depends on the coordinated expression of genes associated with sex chromosomes in different types of pigment cells (Kottler et al. 2013; (Kottler and Schartl 2018; Tripathi et al. 2008, 2009). Male coloration is genetically correlated with female preference for the trait, and most of the elements of color patterns are inherited (Houde 1992; Brooks and Endler 2001). However, male ornamental patterns can also be affected by various environment-specific factors, such as food availability (Grether et al. 1999; Kodric-Brown 1989). Environmental factors can initiate alternative pathways of development, affect the range of expressed trait values, and alter the rate of their development over time (West-Eberhard 2005; Ruell et al. 2013). Novel traits emerging as a result of environmental influence are believed to have a far greater evolutionary potential than those induced by mutations (Levis and Pfennig 2016).
Thyroid hormones (THs) are important physiological mediators of developmental plasticity and effectively act at the interface between genes of an organism and the environment (Lema and Kitano 2013; Lema 2020). Environmentally induced shifts in the TH-signaling pathway can affect phenotypic expression both directly by altering gene expression and indirectly by modulating energy availability via changes in metabolism (Holzer and Laudet 2015; Zak and Manzon 2019; Lema 2014). Hormone-mediated plasticity can contribute to the emergence of novel phenotypes, and even minor alterations in hormonal signaling can underlie evolutionary changes (Lema 2020).
In male guppies, the formation of color patterns is mainly determined by three types of chromatophores (melanophores, xanthophores, and iridophores) (Kottler et al. 2014), and most of the identified genes involved in the development of ornamental traits are expressed before the appearance of the color pattern elements in the phenotype (Dick et al. 2018). THs are known to control very different activities of pigment cell lines during the formation of adult coloration (Saunders et al. 2019) and affect the development of pigment patterns in teleost fish (Clement et al. 2001; McMenamin et al. 2014; Prazdnikov and Shkil 2019a; Yoo et al. 2000). However, the role of THs in the formation of a variety of color patterns in male guppies remains poorly understood.
In the present work, using Poecilia wingei Poeser, Kempkes and Isbrucker, 2005 (Cyprinodontiformes, Poeciliidae, Poeciliinae) as a case study, I investigated the effect of alterations in TH status on the development of elements of the color pattern in males. The results obtained made it possible to experimentally assess the potential contribution of THs to the variability of color patterns in male guppies.
Materials and methods
Fish husbandry and hormonal treatment
A laboratory line of outbred P. wingei which are descendants of wild fish imported from Venezuela (Cumana region) was used as model fish. For experiments, offspring taken from females giving birth on the same day were randomly divided into three equal groups (24 specimens per group) which were reared under different hormonal regimes until the final formation of the adult pigment pattern: (i) the control or euthyroid group (C-1), natural TH status; (ii) the hyperthyroid group (TH-1), high TH level; and (iii) the hypothyroid group (Tio-1), TH deficiency. A month later, more offspring were obtained from the same group of females (37 specimens per each hormonal group); they were also reared under different hormonal regimes (control (C-2), hyperthyroidism (TH-2), and hypothyroidism (Tio-2), but only until the initial stages of the formation of the adult melanistic elements in males (Fig. 1d). Alterations in the TH status were induced by the traditional methods (Brown 1997; Shkil et al. 2012): hyperthyroid conditions were caused by the administration of the active form of TH – triiodo-L-thyronine (T3) (Sigma-Aldrich, China) at a final concentration of 0.15 μg/mL in aquarium water, and hypothyroid conditions were caused by the administration of thiourea (CS(NH2)2) (Solins, Russia), a goitrogen suppressing the thyroid hormone synthesis, at a final concentration of 0.025%. The concentrations of T3 and thiourea used in this study were chosen on the basis of preliminary optimization experiments (Prazdnikov 2020). A higher concentration of thiourea (≥ 0.045%) drastically delays the transition from juveniles to adults and often leads to high mortality of fish, while a lower dosage of thiourea (≤ 0.01%) does not cause distinguishable changes in timing of ontogenetic events and adult morphology. One-fourth of the water from each experimental aquarium was replaced daily. The necessary amounts of hormone and goitrogen were administrated into the water to maintain their predetermined concentrations for specific periods for each experimental group. The rest of the experimental conditions (temperature, feeding frequency, aeration, stocking density of fish, light regime, background of the bottom and side walls of the aquarium, presence of plants) were the same for all the groups. Found sick or injured fish were excluded from the experiment.
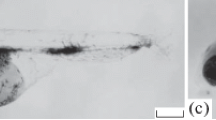
Development (a–f) of color pattern in P. wingei males. Scale: 2 mm. 1, black oblique bar; 2 and 3, anterior and posterior black stripes; 4, ventral black lining of the caudal peduncle; 5, anterior orange blotch; 6, posterior orange stripe; 7, blue iridescent blotch at the base of the dorsal fin; 8, orange-black lining of the caudal fin; 9, black pigment on the dorsal fin; 10, orange pigment on the dorsal fin
Since P. wingei belongs to ovoviviparous fish, days after birth (DAB) were used as a temporal characteristic to compare the timing of ontogenetic events in different hormonal groups.
Color pattern analysis
All photos of juvenile and adult fish were taken on live specimens without the use of anesthetics to prevent any distortion of the pigment pattern. Photos of juveniles and enlarged areas of adult pigmentation were taken using a Leica MS5 stereomicroscope with a Canon EOS 100D digital camera. Photos of adult males were taken in a narrow aquarium in the hormonal regime specific to their group using a Canon EOS 100D camera equipped with a macro lens. Each male was photographed from two sides. The type of chromatophores was identified by the color of the pigment: black, melanophores; yellow-orange, xanthophores; and from metallic to blue/green iridescent, iridophores. For analysis of pigment patterns, I used the classification of color traits proposed earlier for male guppies (Alexander and Breden 2004; Kottler et al. 2013): patches and stripes formed by various types of pigment cells (Fig. 1f). The patches were divided according to their shape: oblique bar, blotch (irregular shape), spot (regular round shape) and eyespot (eye-like shape with iridophore ring).
In the pigment pattern of males, changes in the shape, position, and bilateral asymmetry of color elements, as well as the occupied area on the body, were assessed. The area of color elements on the dorsal and caudal fins was not measured due to the impossibility of making a correct quantitative assessment. Analysis of all digital photographs was performed using the Fiji image processing package (Schindelin et al. 2012) based on the ImageJ 1.53 software (https://imagej.nih.gov/ij/download.html). The area occupied by color elements on the left side of the body was measured for each male from different hormonal groups. In the experimental groups of fish, the asymmetry of the color pattern elements (Ai) was assessed (ratio of the number of males with an asymmetric color element to the total number of all males in the group). Since some groups of fish did not follow a normal distribution, nonparametric Kruskal–Wallis tests were conducted. For statistical analysis of the results, the Excel 2016 software was used.
To compare the experimental male phenotypes with the phenotypes of closely related species of Poecilia, I used descriptions and photos from previously published studies (Alexander and Breden 2004; Endler 1984; Hughes et al. 2013; Kottler et al. 2013, 2014; Olendorf et al. 2006; Poeser et al. 2005; Schories et al. 2009; Tripathi et al. 2008).
Results
Development and variability of color pattern in euthyroid males
At birth, the pigment pattern of a euthyroid male guppy consists of clusters of melanophores on the head; on the trunk, melanophores form dorsal and ventral pigment lines, as well as a horizontal lateral line (Fig. 1a). Xanthophores are widely dispersed, while iridophores most often form small clusters in the area of the yolk sac remnants (Fig. 1a). By day 20 after birth (DAB 20), the juveniles developed a melanophore rhombic reticulate pattern on the body which persists throughout the entire ontogeny of fish and which is subsequently superimposed with various color elements. Then, clusters of iridophores appear in the region of the operculum and the region where a color pattern element (black oblique bar) will form in the future (Fig. 1b and Fig. 2). The first melanophore element of the adult male color pattern begins to develop by DAB 40 (Fig. 2). The black oblique bar is formed from a dense cluster of melanophores which is located over the reticulate pattern and a layer of iridophores (Fig. 1c). Iridophores precede the appearance of all melanophore and xanthophore color elements on the body of males (Fig. 2), gradually increasing their cell population during ontogeny towards the caudal peduncle (Fig. 1d). After the development of four melanophore elements of the color pattern on the fish body (black oblique bar, anterior and posterior stripes, and ventral lining of the caudal peduncle), xanthophore elements (anterior orange blotch and posterior orange stripe) begin to form by DAB 60 (Fig. 1e and Fig. 2). Later, the blue iridescent blotch appears on the body at the base of the dorsal fin (Fig. 2). Along with the development of the pigment pattern on the body, color elements are formed on the caudal and dorsal fin. The development of the orange-black lining of the tail fin begins with the appearance of melanophores, followed by the appearance of iridophores and xanthophores (Fig. 2). The order of development of chromatophores on the dorsal fin does not differ from the tail fin, ending with the appearance of an orange pigment (Fig. 2). The final formation of all color elements of the pigment pattern is completed by DAB 100 (Fig. 1f).
Order of appearance of chromatophore types in regions of color elements in P. wingei males reared under different hormonal regimes. Mel ret., rhombic reticulate pattern; Mel, melanophores (black); Ird, iridophores (blue); Xnt, xanthophores (orange). 1–10, regions of color pattern elements (see Fig. 1); DAB, day after birth
Melanophores and iridophores visibly contribute to all ornamental traits on the male body. In the composition of the reticulate pattern, melanophores are located under or above the main layers of chromatophores that form the color element. The second pigment cells, iridophores, are usually located in the adult pigment pattern at the periphery of the color element. Iridophores also make a significant contribution to the background coloration of the body, causing silvery-white to bluish-violet and blue-green hues, depending on the orientation of the reflecting platelets and combinations with melanophore and xanthophore regions (Fig. 3a–e).
Color pattern variability among P. wingei males reared under different hormonal regimes. a–e, euthyroid groups (C-1, C-2); f–j and k–o, hyperthyroid groups (TH-1 and TH-2); p–t and u–y, hypothyroid groups (Tio-1 and Tio-2). Scale: 2 mm; blue arrows, ornamental traits characteristic of P. reticulata; blue arrowheads, common traits for P. reticulata and P. obscura
Males from the two control groups (C-1 and C-2) showed changes in the size of color elements on the body, while the majority of specimens had the same shape of the elements. The largest color element in terms of occupied area on the body is the black oblique bar which was characterized by variations (Table 1 and Fig. 3a–e). Euthyroid males are characterized by variability in the number of orange patches and their area on the body (Fig. 4a and Fig. 5).
Development and variability of color pattern in males with elevated TH level
The development of pigment patterns in fish from two hyperthyroid groups (TH-1 and TH-2) within 7 days after birth did not differ from the control groups. The first differences between the hormonal groups were observed at the time of the onset of the development of the rhombic reticulate pattern which was formed in hyperthyroid fish earlier, by DAB 12. The timing of the subsequent appearance of all types of chromatophores in the color elements and the rate of development of the adult pigment pattern are accelerated in comparison with euthyroid males (Fig. 2).
In the TH-1 group, there were changes in the sequence of development of the iridophore blotch relative to the onset of the formation of melanophore elements, as well as anterior and posterior xanthophore elements on the body. In the TH-1 group, there are changes in the sequence of development of the iridophore blotch at base of the dorsal fin relative to the onset of the formation of melanophore elements, as well as anterior and posterior xanthophore elements on the body (Fig. 2). At the same time, the ventral black lining of the caudal peduncle did not develop in fish, while the posterior black stripe developed, but its coloration was poorly pronounced due to the low density of melanophores. The adult pigment pattern in males from the TH-1 group was finally formed by DAB 50.
In the TH-2 group, there are no changes in the sequence of the formation of color pattern elements (Fig. 2). Starting from the formation of anterior black stripe, there is a slowdown in the rate of development of the pigment pattern in males from the TH-2 group compared to the TH-1 group (Fig. 2). The formation of the color pattern in the TH-2 group was completed by DAB 85.
In hyperthyroid males, more pronounced changes in the shape of color elements and their area are observed than in euthyroid males (Table 1, Fig. 3f–o). The black oblique bar and anterior black stripe are transformed into blotches or/and spots (Fig. 3f–o), occupying different areas in the male color patterns (Fig. 5). All these changes lead to a significant increase in the number of melanophore blotches and spots (Fig. 4a) and a decrease in the total area of melanistic elements on the body of hyperthyroid males, especially from the TH-1 group, compared to males from other hormonal groups (Fig. 6) (Kruskal–Wallis test for number and area: P < 0.01). Males from the two hyperthyroid groups develop pigment patterns with novel color elements that were not found in the control groups of fish, in particular, with eyespots (Fig. 3f–o and Fig. 4b).
Development and variability of color pattern in males with TH deficiency
Fish from the two hypothyroid groups (Tio-1 and Tio-2) exhibit a delayed onset in the development of the color pattern elements in comparison with fish with other hormonal regimes (Fig. 2). In the Tio-1 group, the melanophore rhombic reticulate pattern is formed only by DAB 40; after that, there is a further slowdown in the development of the color pattern (Fig. 2). When the black and orange elements of the pattern were forming in the posterior part of the body, the order of the appearance of chromatophores changed; simultaneously with melanophores, xanthophores appeared in the black stripe region (Fig. 2). Orange pigment did not develop on the dorsal fin. The final color pattern in most specimens was formed by DAB 230.
Starting from the appearance of iridophores in the anterior black stripe region, there is an acceleration in the rate of development of the color pattern in fish from the Tio-2 group compared to fish from the Tio-1 group (Fig. 2). The order of the appearance of melanophores and xanthophores in color elements on the body did not change (Fig. 2). Orange pigment was present on the dorsal fin in 45% of the males from the Tio-2 group. The adult color pattern in most males finally developed by DAB 170.
In hypothyroid groups of fish, variability in the shape and area of black and orange elements of the color pattern is observed (Table 1, Fig. 3p–y and Fig. 6). Males from the Tio-1 group had significantly more orange blotches and spots in the pigment pattern (Fig. 4a) than males from the euthyroid and hyperthyroid groups (Kruskal–Wallis test: P = 0.02). In both hypothyroid groups, there is a transformation from black oblique bar to blotches and spots of different area (Fig. 5), as well as eyespots (Fig. 3p–y and Fig. 4b). Changes in the shape, number, and relative area of melanophore and xanthophore patches in hypothyroid males (Table 1, Fig. 4 and Fig. 5) lead to the appearance of color patterns distinct from fish with other hormonal regimes (Fig. 3p–y).
Discussion
THs and variability of male ornamental traits in Poecilia wingei
The results obtained indicate that changes in the hormonal status in P. wingei result in changes in the timing and rate of development of elements of the color pattern in males. The increased level of THs in experimental fish caused a premature appearance and changes in the order of development of color elements and in general accelerated the ontogeny of the pigment pattern. At the same time, a deficiency of hormones led to a deceleration in the rate of development and a change in the order of appearance of chromatophore lines in the color pattern. These ontogenetic changes in the experimental groups of fish led to changes in the shape, area, and number of melanophore and xanthophore elements in pigment patterns.
The greatest variability among hormonal groups was in melanistic color elements. The pigment pattern of hyper- and hypothyroid fish acquired blotches or/and spots of different area and position on the body, including eyespots. Alterations in the TH status caused an increase in the frequency of asymmetry of these melanophore patches. Depending on the hormonal regime, the relative area occupied by melanistic elements on the body was changing; e.g., it significantly decreased in males developing under conditions of excess hormones until the final formation of adult color.
Variability in xanthophore elements among hormonal regimes was most pronounced in the shape and number of orange patches on the body, as well as in the presence of orange pigment on the dorsal fin. Males developing under TH deficiency until the final development of adult pigment pattern had more orange blotches and spots in the posterior part of the body than those from other hormonal groups. The increase in the number of orange patches was associated with the specifics of the development of the adult color pattern, with the simultaneous appearance of xanthophore and melanophore populations at the sites of formation of color elements in the posterior part of the body. Differences between hormonal groups in the relative area occupied by xanthophore elements on the body are less pronounced compared to melanophore elements (Fig.6).
Large variability in the melanistic elements of the color pattern in males may be associated with the specific features of the regulation of melanophores in ontogeny. The guppy is known to have at least three temporally and genetically distinct populations of melanophores: the first population differentiates during embryogenesis; the second mainly differentiates after birth, participating in the development of the reticulate pattern; and the third population differentiates during puberty, participating in the formation of the adult melanophore pattern in males (Kottler et al. 2013). The interrelation between the phenotypic variability of black color elements in males and the differential expression of genes involved in the development of melanophores and the regulation of melanin synthesis were also confirmed (Dick et al. 2018). The results obtained indicate that the melanophore population participating in the formation of the adult color pattern in males is the most sensitive to changes in the TH level.
The identified dependence of the population of melanophores on THs in adult P. wingei was similar to the previously described results obtained in experiments with zebrafish (McMenamin et al. 2014; Saunders et al. 2019) and cichlids (Prazdnikov and Shkil 2019a, 2019b). However, the reaction of another type of pigment cells, xanthophores, to the hormone level was different from those described earlier. Interestingly, populations of xanthophores on the body and dorsal fin showed different responses to changes in the hormonal status. In general, the deficiency and excess of THs caused a similar reaction of xanthophores on the body, while on the dorsal fin of males reared under hypothyroidism, a reduction in pigment cells of this type was observed, in contrast to fish reared under other hormonal regimes. This may indicate that THs coordinate cellular behavior on the body and the fin in different ways, which can also be associated with different chromatophore precursors. Moreover, it is known that populations of chromatophore precursors on fish fins can differ from those found on the body which are capable of generating both xanthophores and melanophores (Parichy and Spiewak 2015). The orange ornament on the body of male guppies depends on the diet (the amount of carotenoid intake) and the ability to synthesize pteridines in xanthophores (Grether et al. 2001, 2005; Karino and Shinjo 2004). In this regard, as shown in my other experiments (unpublished data), males eating only food rich in carotenoids (live Artemia nauplii and spirulina), despite their development under conditions of artificial suppression of the synthesis of endogenous THs, had large areas on their bodies occupied by xanthophore elements (Fig. 7a).
Variability in color pattern in P. wingei males with altered TH status: (a) reared under TH deficiency on a diet rich in carotenoids (mean area of xanthophore elements on the body is 12.24 ± 0.70 mm2); (b) reared under excess of THs; scale: 2 mm. (c) spot formation as a result of transformation of melanistic element; scale: 0.5 mm; i, iridophores; m, melanophores; x, xanthophores
In the last decade, there has been growing evidence that both short- and long-range interactions between different types of chromatophores are required for the formation of complex pigment patterns in fish (Eom et al. 2015; Hamada et al. 2014; Kottler et al. 2013; Mahalwar et al. 2016; Patterson and Parichy 2019). In P. wingei, iridophores contribute to all ornamental traits in males. It is assumed that iridophores accumulate in juveniles at the sites of future black and orange patches, whereas subtle differences in the migration of iridophores and their differential interaction with other types of pigment cells (melanophores and xanthophores) can lead to variability in the color pattern in males (Kottler et al. 2014). THs regulate the expression of pigment genes and coordinate the state of differentiation or morphogenetic behavior of various chromatophore lineages, affecting the total number of cells and the cascade of interactions (Eskova et al. 2020; Guillot et al. 2016; McMenamin et al. 2014; Saunders et al. 2019) and thereby playing an important role in color variability. The results obtained in the current work support the assumption that iridophores precede the appearance of melanophore and xanthophore elements on the body (Fig. 1b–e and Fig. 2), and alterations in TH signaling cause changes in the shape of color elements (in some specimens, with asymmetry). As a result, instead of bars or stripes, blotches or/and spots are most often developing in the pigment pattern of experimental P. wingei males (Fig. 3 and Fig. 7b, c).
The important role of iridophores in intercellular interactions and the formation of adult pigment pattern have been demonstrated in another model species of fish (Frohnhöfer et al. 2013; Patterson and Parichy 2013). S-iridophores in zebrafish possessing a strong signaling capacity have been shown to predetermine stripes and interstripe space, as well as affect changes in the shape of pattern elements in these sites (Fadeev et al. 2015; Krauss et al. 2014; Patterson and Parichy 2019; Singh 2015; Watanabe and Kondo 2015). Further exploration of this model has shown the mechanism of how stripes become broken into spots during the formation of adult pattern in mutant zebrafish lines (Owen et al. 2020). The similarity of transformations of color elements in two phylogenetically distant fish species indicates the commonality of the mechanisms of intercellular interactions and hormonal regulation of chromatophores during the development of adult pigment patterns.
Alterations in TH signaling lead to phenotypic diversity with the emergence of novel male ornamental traits
Alterations in the TH level in experimental fish led to a change in the timing and rate of formation of the adult pigment pattern, which can be considered as heterochrony (sensu Müller (2007). Heterochronic shifts in the development of color patterns (Fig. 2) cause an increase in phenotypic variability. The range of variability in the ornamental patterns in males with altered hormonal status exceeded the natural range of pattern variability described for the natural population of P. wingei (Alexander and Breden 2004). Some specimens of experimental fish were lacking the male-specific traits of P. wingei (Alexander and Breden 2004; Kottler et al. 2013; Tripathi et al. 2008), such as black oblique bar, posterior horizontal stripe, and ventral lining of the caudal peduncle, as well as orange pigment on the dorsal fin. In all hyper- and hypothyroid groups, there were pronounced changes in the color elements of the pigment pattern, leading to the formation of patches that differed in shape, position, occupied area, and color. In fish with altered hormonal status, novel combinations of color elements appeared in the pigment pattern. As a result of hormonally induced heterochrony, the male phenotype acquired a number of ornamental traits which are present in the color patterns of phylogenetically close species, Poecilia obscura and P. reticulata (Fig. 3).
Taking into account the individual variability in the TH responsiveness in experimental fish, it is obvious that manipulating the TH status and increasing the sample size will make it possible to obtain a greater variety of color patterns, especially melanistic patterns characteristic of three species of male guppies.
In natural populations, male guppies exhibit complex polymorphism in color patterns which forms as a result of interactions between sexual selection and predation regime (Brooks and Endler 2001; Endler 1980, 1984; Weese et al. 2010), as well as other environmental factors (Millar et al. 2006; Rahman et al. 2013; Ruell et al. 2013; Schwartz and Hendry 2010). For example, depending on the intensity of predation, the number, size, and area of spots in the male color pattern change (Endler 1995; Kemp et al. 2009). Color variability in guppies can be further supported by genotype-specific effects of variable levels of resources, the interaction of the genotype with changing environmental conditions, in particular, resource availability, and light intensity (Hughes et al. 2005; Kemp et al. 2018; Kolluru 2014).
THs are important regulators of fish development and act as molecular links between the genotype of the organism and the environment (Lema and Kitano 2013; Lema 2014). TH regulatory pathways act as mechanistic links between environmental change and phenotypic development (Lema 2020). For example, changes in feeding conditions, temperature, and light intensity cause alterations in the TH levels in fish (Eales et al. 1982; Karagic et al. 2018; Leiner & Mackenzie 2003), which can affect phenotypic expression (Lema 2020). It has been shown that plasticity in endocrine signaling may play an important role in evolutionary changes in different taxa of teleost fish (Kitano et al. 2010; Lema 2020; Prazdnikov and Shkil 2019a; Shkil and Smirnov 2015). The habitats of guppy populations undergo changes of varying degree in the availability of resources, lighting, predation, and parasitism (Kolluru 2014), which are likely to affect the TH dynamics. In addition, other environmental changes associated with anthropogenic pollution of freshwater ecosystems cause changes in the functioning of the endocrine system (Shenoy and Crowley 2011) and lead to variability of ornamental traits in males (Shenoy 2012).
The experimental results obtained demonstrate how alterations in the hormonal status during development in P. wingei lead to the formation of phenotypic diversity with the appearance of new ornamental traits in males. Individual alterations in TH signaling induce an increase in pigment pattern variability in offspring from the same parents. Hormone-mediated plasticity can probably contribute to the rapid adaptation of guppies to changing environmental conditions, as well as to generation of novel developmental variants. The appearance of new pigment patterns, including those with asymmetric color elements, can provide advantages for males and become fixed in the population. The preference of female guppies for rare and unfamiliar male color patterns is well known (Hughes et al. 2013; Valvo et al. 2019); moreover, the lateral asymmetry of signaling traits gives males an advantage during courtship (Řežucha and Reichard 2015). Thus, THs can make a significant contribution to the maintenance of extensive color polymorphism in guppy populations.
Conclusions
Experimental data indicate that the development of color patterns in P. wingei males is regulated by THs. Alterations in the TH status cause heterochronic shifts in the development of pigment pattern, which lead to an increase in phenotypic variability. The discovered similarity of the ornamental traits of experimental fish with closely related species of Poecilia suggests the participation of the TH-signaling pathway in the diversification of guppy color patterns. The emergence of new hormonal phenotypes indicates the potentially important role of alterations in endocrine regulation induced by the environment for the evolutionary origin of novel traits in fish. Furthermore, the data on the effect of THs on the variability of ornamental traits in males open up new prospects for future studies of the role of endocrine regulatory mechanisms in the adaptive evolution of guppies.
References
Alexander HJ, Breden F (2004) Sexual isolation and extreme morphological divergence in the Cumana guppy: a possible case of incipient speciation. J Evol Biol 17:1238–1254
Brooks R, Endler JA (2001) Direct and indirect sexual selection and quantitative genetics of male traits in guppies (Poecilia reticulata). Evol 55:1002–1015
Brown DD (1997) The role of thyroid hormone in zebrafish and axolotl development. Proc Natl Acad Sci 94(24):13011-13016. https://doi.org/10.1073/pnas.94.24.13011
Clement SE, Lichtenbert JH, Kohler CC (2001) Stripping clowns: induced meristic changes in common clownfish (Amphiprion ocellaris). Bull Inst Océanogr Monaco 20:283–288
Dick C, Arendt J, Reznick DN, Hayashi CY (2018) The developmental and genetic trajectory of coloration in the guppy (Poecilia reticulata). Evol Dev 20:207–218. https://doi.org/10.1111/ede.12268
Eales JG, Chang JP, Van Der Kraak G, Omeljaniuk RJ, Uin L (1982) Effects of temperature on plasma thyroxine and lodide kinetics in rainbow trout, Salmo gairdneri. Gen Сomp Endocrin 47:295–307
Endler JA (1980) Natural selection on color patterns in Poecilia reticulata. Evol 34:76–91
Endler JA (1984) Natural and sexual selection on color patterns in poeciliid fishes. In: Zarct TM (ed) Evolutionary ecology of neotropical freshwater fishes. Springer, Dordrecht, pp 95–111
Endler JA (1995) Multiple-trait coevolution and environmental gradients in guppies. Trends Ecol Evol 10:22–29
Eom DS, Bain EJ, Patterson LB, Grout ME, Parichy DM (2015) Long-distance communication by specialized cellular projections during pigment pattern development and evolution. Elife. 4. https://doi.org/10.7554/eLife.12401
Eskova A, Frohnhöfer HG, Nüsslein-Volhard C, Irion U (2020) Galanin signaling in the brain regulates color pattern formation in zebrafish. Curr Biol 30:298–303. https://doi.org/10.1016/j.cub.2019.11.033
Fadeev A, Krauss J, Frohnhöfer HG, Irion U, Nüsslein-Volhard C (2015) Tight junction protein 1a regulates pigment cell organisation during zebrafish colour patterning. Elife. 4. https://doi.org/10.7554/eLife.06545
Frohnhöfer HG, Krauss J, Maischein HM, Nüsslein-Volhard C (2013) Iridophores and their interactions with other chromatophores are required for stripe formation in zebrafish. Devel 140:2997–3007. https://doi.org/10.1242/dev.096719
Grether GF, Hudon J, Millie DF (1999) Carotenoid limitation of sexual coloration along an environmental gradient in guppies. Proc R Soc Lond B Biol Sci 266:1317–1322. https://doi.org/10.1098/rspb.1999.0781
Grether GF, Hudon J, Endler JA (2001) Carotenoid scarcity, synthetic pteridine pigments and the evolution of sexual coloration in guppies (Poecilia reticulata). Proc R Soc Lond B Biol Sci 268:1245–1253. https://doi.org/10.1098/rspb.2001.1624
Grether GF, Cummings ME, Hudon J (2005) Countergradient variation in the sexual coloration of guppies (Poecilia reticulata): drosopterin synthesis balances carotenoid availability. Evol 59:175–188. https://doi.org/10.1111/j.0014-3820.2005.tb00904.x
Guillot R, Muriach B, Rocha A, Rotllant J, Kelsh RN, Cerdá-Reverter JM (2016) Thyroid hormones regulate zebrafish melanogenesis in a gender-specific manner. PLoS One 11:e0166152. https://doi.org/10.1371/journal.pone.0166152
Hamada H, Watanabe M, Lau HE, Nishida T, Hasegawa T, Parichy DM, Kondo S (2014) Involvement of Delta/notch signaling in zebrafish adult pigment stripe patterning. Devel 141:318–324. https://doi.org/10.1242/dev.099804
Holzer G, Laudet V (2015) Thyroid hormones: a triple-edged sword for life history transitions. Curr Biol 25:344–347. https://doi.org/10.1016/j.cub.2015.02.026
Houde AE (1992) Sex-linked heritability of a sexually selected character in a natural population of Poecilia reticulata (Pisces: Poeciliidae) (guppies). Heredity 69:229–235
Houde AE (1997) Sex, color, and mate choice in guppies. Princeton Univ, Press, Princeton, NJ
Hughes KA, Rodd FH, Reznick DN (2005) Genetic and environmental effects on secondary sex traits in guppies (Poecilia reticulata). J Evol Biol 18:35–45. https://doi.org/10.1111/j.1420-9101.2004.00806.x
Hughes KA, Houde AE, Price AC, Rodd FH (2013) Mating advantage for rare males in wild guppy populations. Nat 503:108–110. https://doi.org/10.1038/nature12717
Karagic N, Härer A, Meyer A, Torres-Dowdall J (2018) Heterochronic opsin expression due to early light deprivation results in drastically shifted visual sensitivity in a cichlid fish: possible role of thyroid hormone signaling. J Exper Zool Part B: Mol and Devel Evol 330:202–214. https://doi.org/10.1002/jez.b.22806
Karino K, Shinjo S (2004) Female mate preference based on male orange spot patterns in the feral guppy Poecilia reticulata in Japan. Ichthyol Res 51:316–320. https://doi.org/10.1007/s10228-004-0234-6
Kemp DJ, Reznick DN, Grether GF, Endler JA (2009) Predicting the direction of ornament evolution in Trinidadian guppies (Poecilia reticulata). Proc R Soc B Biol Sci 276:4335–4343. https://doi.org/10.1098/rspb.2009.1226
Kemp DJ, Batistic FK, Reznick DN (2018) Predictable adaptive trajectories of sexual coloration in the wild: evidence from replicate experimental guppy populations. Evol 72:2462–2477. https://doi.org/10.1111/evo.13564
Kitano J, Lema SC, Luckenbach JA, Mori S, Kawagishi Y, Kusakabe M, Swanson P, Peichel CL (2010) Adaptive divergence in the thyroid hormone signaling pathway in the stickleback radiation. Curr Biol 20:2124–2130. https://doi.org/10.1016/j.cub.2010.10.050
Kodric-Brown A (1989) Dietary carotenoids and male mating success in the guppy: an environmental component to female choice. Behav Ecol Sociobiol 25:393–401
Kolluru GR (2014) Genotype by environment interactions and sexual selection in guppies. In: Hunt J, Hosken D (eds) Genotype by environment interactions and sexual selection. Wiley, NJ, pp 282–311
Kottler VA, Schartl M (2018) The colorful sex chromosomes of teleost fish. Genes 9:233. https://doi.org/10.3390/genes9050233
Kottler VA, Fadeev A, Weigel D, Dreyer C (2013) Pigment pattern formation in the guppy, Poecilia reticulata, involves the Kita and Csf1ra receptor tyrosine kinases. Genet 194:631–646. https://doi.org/10.1534/genetics.113.151738
Kottler VA, Koch I, Flötenmeyer M, Hashimoto H, Weigel D, Dreyer C (2014) Multiple pigment cell types contribute to the black, blue, and orange ornaments of male guppies (Poecilia reticulata). PLoS One 9:e85647. https://doi.org/10.1371/journal.pone.0085647
Krauss J, Frohnhöfer HG, Walderich B, Maischein HM, Weiler C, Irion U, Nüsslein-Volhard C (2014) Endothelin signalling in iridophore development and stripe pattern formation of zebrafish. Biol Open 3:503–509. https://doi.org/10.1242/bio.20148441
Leiner KA, Mackenzie DS (2003) Central regulation of thyroidal status in a teleost fish: Nutrient stimulation of T4 secretion and negative feedback of T3. J Exper Zool Part A: Compar Exper Biol 298(1):32-43. https://doi.org/10.1002/jez.a.10255
Lema SC (2014) Hormones and phenotypic plasticity in an ecological context: linking physiological mechanisms to evolutionary processes. Integr Comp Biol 54:850–863. https://doi.org/10.1093/icb/icu019
Lema SC (2020) Hormones, developmental plasticity, and adaptive evolution: endocrine flexibility as a catalyst for ‘plasticity-first’phenotypic divergence. Mol Cell Endocrinol 502:110678. https://doi.org/10.1016/j.mce.2019.110678
Lema SC, Kitano J (2013) Hormones and phenotypic plasticity: implications for the evolution of integrated adaptive phenotypes. Curr Zool 59:506–525. https://doi.org/10.1093/czoolo/59.4.506
Levis NA, Pfennig DW (2016) Evaluating ‘plasticity-first’evolution in nature: key criteria and empirical approaches. Trends Ecol Evol 31:563–574. https://doi.org/10.1016/j.tree.2016.03.012
Magurran AE (2005) Evolutionary ecology: the Trinidadian guppy. Oxford University Press on Demand
Mahalwar P, Singh AP, Fadeev A, Nüsslein-Volhard C, Irion U (2016) Heterotypic interactions regulate cell shape and density during color pattern formation in zebrafish. Biol Open 5:1680–1690. https://doi.org/10.1242/bio.022251
McMenamin SK, Bain EJ, McCann AE et al (2014) Thyroid hormone–dependent adult pigment cell lineage and pattern in zebrafish. Science 345:1358–1361. https://doi.org/10.1126/science.1256251
Millar NP, Reznick DN, Kinnison MT, Hendry AP (2006) Disentangling the selective factors that act on male colour in wild guppies. Oikas 113:1–12. https://doi.org/10.1111/j.0030-1299.2006.14038.x
Müller GB (2007) Evo-devo: extending the evolutionary synthesis. Nat Rev Genet 8:943–949. https://doi.org/10.1038/nrg2219
Olendorf R, Rodd FH, Punzalan D, Houde AE, Hurt C, Reznick DN, Hughes KA (2006) Frequency-dependent survival in natural guppy populations. Nat 441:633–636. https://doi.org/10.1038/nature04646
O'Steen S, Cullum AJ, Bennett AF (2002) Rapid evolution of escape ability in Trinidadian guppies (Poecilia reticulata). Evol 56:776–784. https://doi.org/10.1111/j.0014-3820.2002.tb01388.x
Owen JP, Kelsh RN, Yates CA (2020) A quantitative modelling approach to zebrafish pigment pattern formation. Elife 9:e52998. https://doi.org/10.7554/eLife.52998
Parichy DM, Spiewak JE (2015) Origins of adult pigmentation: diversity in pigment stem cell lineages and implications for pattern evolution. Pigment Cell Melanoma Res 28:31–50. https://doi.org/10.1111/pcmr.12332
Patterson LB, Parichy DM (2013) Interactions with iridophores and the tissue environment required for patterning melanophores and xanthophores during zebrafish adult pigment stripe formation. PLoS Genet 9:e1003561. https://doi.org/10.1371/journal.pgen.1003561
Patterson LB, Parichy DM (2019) Zebrafish pigment pattern formation: insights into the development and evolution of adult form. Annu Rev Genet 53:505–530. https://doi.org/10.1146/annurev-genet-112618-043741
Poeser FN, Kempkes M, Isbrücker IJ (2005) Description of Poecilia (Acanthophacelus) wingei n. sp. from the Paría peninsula, Venezuela, including notes on Acanthophacelus Eigenmann, 1907 and other subgenera of Poecilia Bloch and Schneider, 1801 (Teleostei, Cyprinodontiformes, Poeciliidae). Contrib Zool 74:97–115. https://doi.org/10.1163/18759866-0740102007
Prazdnikov DV (2020) Effect of thyroid hormones on the development of asymmetric pigment patterns in teleost fish: experimental data on the example of Amatitlania nigrofasciata (Cichlidae) and Poecilia wingei (Poeciliidae). Biol Bull 47:198–204. https://doi.org/10.1134/S1062359020020065
Prazdnikov DV, Shkil FN (2019a) Experimental evidence of the role of heterochrony in evolution of the Mesoamerican cichlids pigment patterns. Evol Dev 21:3–15. https://doi.org/10.1111/ede.12272
Prazdnikov DV, Shkil FN (2019b) The experimental heterochronies in a green terror cichlid Andinoacara rivulatus (Teleostei: Cichlidae: Cichlasomatinae) indicate a role of developmental changes in the cichlids coloration evolution. Biol Bull 46:56–64. https://doi.org/10.1134/S1062359019010102
Rahman MM, Kelley JL, Evans JP (2013) Condition dependent expression of pre- and postcopulatory sexual traits in guppies. Ecol Evol 3:2197–2213. https://doi.org/10.1002/ece3.632
Řežucha R, Reichard M (2015) Strategic exploitation of fluctuating asymmetry in male Endler's guppy courtship displays is modulated by social environment. J Evol Biol 28:356–367. https://doi.org/10.5061/dryad.9hk3g
Ruell EW, Handelsman CA, Hawkins CL, Sofaer HR, Ghalambor CK, Angeloni L (2013) Fear, food and sexual ornamentation: plasticity of colour development in Trinidadian guppies. Proc R Soc B Biol Sci 280:20122019. https://doi.org/10.1098/rspb.2012.2019
Saunders LM, Mishra AK, Aman AJ, Lewis VM, Toomey MB, Packer JS, Qiu X, McFaline-Figueroa JL, Corbo JC, Trapnell C, Parichy DM (2019) Thyroid hormone regulates distinct paths to maturation in pigment cell lineages. Elife 8:e45181. https://doi.org/10.7554/eLife.45181
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. https://doi.org/10.1038/nmeth.2019
Schories S, Meyer MK, Schartl M (2009) Description of Poecilia (Acanthophacelus) obscura n. sp.,(Teleostei: Poeciliidae), a new guppy species from western Trinidad, with remarks on P. wingei and the status of the Endler s guppy. Zootaxa 2266:35–50
Schwartz AK, Hendry AP (2010) Testing the influence of local forest canopy clearing on phenotypic variation in Trinidadian guppies. Funct Ecol 24:354–364. https://doi.org/10.1111/j.1365-2435.2009.01652.x
Shenoy K (2012) Environmentally realistic exposure to the herbicide atrazine alters some sexually selected traits in male guppies. PLoS One 7:e30611. https://doi.org/10.1371/journal.pone.0030611
Shenoy K, Crowley PH (2011) Endocrine disruption of male mating signals: ecological and evolutionary implications. Funct Ecol 25:433–448. https://doi.org/10.1111/j.1365-2435.2010.01787.x
Shkil FN, Kapitanova DV, Borisov VB, Abdissa B, Smirnov SV (2012) Thyroid hormone in skeletal development of cyprinids: effects and morphological consequences. Journal of Applied Ichthyology 28:398–405. https://doi.org/10.1111/j.1439-0426.2012.01992.x
Shkil FN, Smirnov SV (2015) Experimental approach to the hypotheses of heterochronic evolution in lower vertebrates. Paleontol J 49:1624–1634. https://doi.org/10.1134/S0031030115140178
Singh AP (2015) Nüsslein-Volhard C. Zebrafish stripes as a model for vertebrate colour pattern formation Curr Biol 25:R81–R92. https://doi.org/10.1016/j.cub.2014.11.013
Tripathi N, Hoffmann M, Dreyer C (2008) Natural variation of male ornamental traits of the guppy, Poecilia reticulata. Zebrafish 5:265–278. https://doi.org/10.1089/zeb.2008.0548
Tripathi N, Hoffmann M, Willing EM, Lanz C, Weigel D, Dreyer C (2009) Genetic linkage map of the guppy, Poecilia reticulata, and quantitative trait loci analysis of male size and colour variation. Proc R Soc B Biol Sci 276:2195–2208. https://doi.org/10.1098/rspb.2008.1930
Valvo JJ, Rodd FH, Hughes KA (2019) Consistent female preference for rare and unfamiliar male color patterns in wild guppy populations. Behav Ecol 30:1672–1681. https://doi.org/10.1093/beheco/arz134
Watanabe M, Kondo S (2015) Is pigment patterning in fish skin determined by the Turing mechanism? Trends Genet 31:88–96. https://doi.org/10.1016/j.tig.2014.11.005
Weese DJ, Gordon SP, Hendry AP, Kinnison MT (2010) Spatiotemporal variation in linear natural selection on body color in wild guppies (Poecilia reticulata). Evol: Intern J Organ Evol 64:1802–1815. https://doi.org/10.1111/j.1558-5646.2010.00945.x
West-Eberhard MJ (2005) Phenotypic accommodation: adaptive innovation due to developmental plasticity. J of Experl Zool Part B: Mol Devel Evol 304:610–618. https://doi.org/10.1002/jez.b.21071
Yoo JH, Takeuchi T, Tagawa M, Seikai T (2000) Effect of thyroid hormones on the stage-specific pigmentation of the Japanese flounder Paralichthys olivaceus. Zool Sci 17:1101–1106. https://doi.org/10.2108/zsj.17.1101
Zak MA, Manzon RG (2019) Expression and activity of lipid and oxidative metabolism enzymes following elevated temperature exposure and thyroid hormone manipulation in juvenile lake whitefish (Coregonus clupeaformis). Gener Comp Endocrin 275:51–64. https://doi.org/10.1016/j.ygcen.2019.02.001
Acknowledgments
I am grateful to anonymous reviewers for their valuable comments and suggestions. This study was partially supported by the Russian Foundation for Basic Research, project no. 18-34-00685.
Availability of data and material
The datasets generated during the current study are available on reasonable request.
Code availability
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no competing interest.
Ethics approval
All experimental procedures with fish were carried out according to the guidelines and following the laws and ethics of the Russian Federation and approved by the ethics committee of the Severtsov Institute of Ecology and Evolution, Russian Academy of Sciences (Moscow).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Prazdnikov, D.V. Role of thyroid hormones in color diversity of male guppies: experimental data on Endler’s guppy (Poecilia wingei). Environ Biol Fish 104, 675–688 (2021). https://doi.org/10.1007/s10641-021-01102-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-021-01102-x