Abstract
Fish and benthic invertebrates are important groups that inhabit shallow coastal areas. Many studies use these two groups separately to answer questions related to environmental relationships, but few assess the correlation between these two groups. This study aimed to assess correlation between richness and composition of fish and benthic invertebrate assemblages and to evaluate their responses to environmental variables in sandy beaches in a tropical coastal area in south-eastern Brazil. We tested for a correlation between fish and benthic invertebrates and evaluated which environmental variables influenced each group. Fish and invertebrate taxonomic richness were not correlated across sites. In addition, the two groups were not significantly correlated, even after controlling the effects of the environmental variables. The taxonomic richness of the two groups were influenced by different set of environmental drivers: fish were influenced mainly by the physicochemical variables, being positively correlated with salinity and dissolved oxygen, and negatively with temperature, whereas invertebrates richness were related mainly to granulometric variables, decreasing in fine and very fine sediment. Fish and invertebrate showed similar patterns with more influence of environmental variables than biotic variables that had comparatively more effects on the invertebrate than on the fish assemblage. Spatial segregation in species distribution along the beaches were found with the Gerreid fish predominating in semi-exposed beaches whereas the sparid Diplodus argenteus, the haemulid Orthopristis ruber and the clupeids Harengula clupeola and Sardinella brasiliensis predominated in the exposed beaches. The Polychaetae families Syllidae and Dorvilleidae, and isopod of the family Cirolanidae predominated in semi-protect beaches, whereas polychaetas of the families Glyceridae and Saccocirridae predominated in the exposed beaches. In this study, we showed that fish and benthic invertebrates are influenced by different environmental variables in tropical sandy beaches and no significant correlation was found between these two taxonomic groups. Our findings are a step to a better understanding of the fish-benthic invertebrate relationship and a contribution to management policies aiming the conservation of tropical coastal areas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sandy beach surf zones account for most of the world’s open shoreline (Defeo et al. 2009) and are inhabited by diverse assemblages that use this coastal zone as their only habitat, or as nursery areas or migratory path to other nurseries, or as a sink where they have drifted inadvertently (McLachlan and Brown 2006). Among these assemblages, the fish and the benthic invertebrate, mostly composed of polychaetes and shrimp-like macrocrustaceans (e.g., prawns and mysids) predominate in these areas (Crawley et al. 2006; Sato et al. 2008). However, there is still insufficient information on how and whether fish and invertebrates correlate, and how they respond to environmental variables. However, there is still insufficient information on whether and how, fish and benthic invertebrate correlates and how they respond to environmental changes.
Although several authors seek to understand patterns and changes in species diversity (e.g. Wolters et al. 2006), there is a difficulty in establishing which factors influence these patterns (Johnson and Hering 2010). The fish species that inhabit these systems use several strategies associated with tolerances to physicochemical variables. In addition, these variables seem to play an important role in the distribution of fish, which may be associated with an abundance of food resources (Whitfield and Elliott 2002) and provide protection from predators (Blaber and Blaber 1980). In general, fish communities are important components within a coastal ecosystem, being relevant in studies associated with benthic invertebrates relationships.
The benthic fauna of sandy beaches is composed of many phyla of invertebrates, with crustaceans, molluscs and polychaetes being the most dominant. In addition, they are fundamental in the bioturbation process, in which the nutrients stored in the sediment are taken to the water column (Josefson and Rasmussen 2000). Thus, nutrients are made available by mixing the decomposition of organic matter and sedimentary material (Nielson and Jernakoff 1996). This process mainly favours higher trophic levels, being a source for primary production in the water column with the availability of these nutrients (Human et al. 2015).
Concordance or cross-taxon congruence refers to similar assemblage structure among different taxa. Different mechanisms can drive cross-taxon concordance (Heino 2010) such as similar response to the same or correlated environmental gradient, co- loss of species along stress gradients and biotic interactions. Concordance of two assemblages are more likely to occur in freshwater ecosystems (Jackson and Harvey 1993; Paszkowski and Tonn 2000; Larsen et al. 2012), can reveal patterns with the environment and interactions among assemblages (Santoul et al. 2004). At local scale, environmental conditions are a main driver for the occurrence of species distribution. However, as different taxonomical groups use different habitats, they may be expected to respond in different ways to environmental influences with each community was associated with a different set of environmental factors. In addition, biotic interactions may involve direct processes such as fish predation on a particular invertebrate taxon or indirect factors, e.g., where fish limit the abundance of invertebrate predators, thereby limiting the impact of these invertebrate predators (Jackson and Harvey 1993). Identifying the patterns of different taxonomic groups simultaneously with similar environmental gradients can seem challenging, because it gives us a more robust answer on how agreement between different groups occurs.
Sandy beaches are physically dynamic habitats, inhabited by specialized biotic assemblages that are structured mainly by physical forces (Defeo 2003). Changes for beach ecosystems grain size becomes coarser, erosion-accretion dynamics more intense, and swash frequency and velocity increase as morpho dynamic conditions from dissipative to reflective extremes with reduction of species towards the reflective extreme that cause increasing environmental severity. In addition to this, water physicochemical variables and biotic interaction also contribute to large interspecific variability in the life history and ecological traits of sandy beach communities, since species with different characteristics could be controlled by different limiting factors (Alejandro 2001; Defeo et al. 2009; Qu et al. 2019). The identification of meaningful spatial and temporal scales of variability in population regulation mechanisms and processes, and also in the dynamic nature of the fishing process, are relevant for assessment and management (Defeo 2003; Meena et al. 2019)
The coast of Rio de Janeiro has sandy beaches with a range of environmental characteristics that provide a morpho-dynamic gradient of beaches, directly influencing the distribution of fauna. Thus, these systems are of great importance not only to investigate biotic relationships, but also to assess the distribution of biological groups in systems with different environmental characteristics. A small number of studies have sought to understand the concordance of fish and benthic invertebrates along environmental gradients; however, assuming that these two groups have different life forms and sizes, they may be expected to respond differently to environmental variables.
In this study, we seek to evaluate the important predictor variables for fish and benthic invertebrate distribution in sandy beaches in a nearshore tropical area in south-eastern Brazil, and whether these two groups have a significant correlation. We tested for a correlation between fish and benthic invertebrates and evaluated which environmental variables influenced each group. We hope to better understand which variables are important for each group, thus contributing to a better understanding of their relationships and supplying information for management programs of environmental conservation of tropical coastal systems.
Materials and methods
Study area
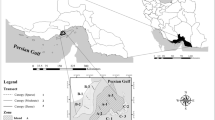
Six sandy beaches along the Rio de Janeiro coast were sampled for fishes, benthic invertebrates and environmental variables (Fig. 1), which is part of the transition region between the tropical and subtropical provinces in the South-eastern Brazilian coast. Two oceanic beaches (1 and 2) were located in an unprotected coastal area where the wave action is high. Each beach was approximately 2500 m long, with predominating medium to coarse sand (mean grain size of 0.43 mm), tide range of approximately 1 m, and intertidal slope varying from 1/5.29 to 1/17.82 (Veloso et al. 2006). Two beaches (3 and 4) were well protected within the Sepetiba Bay. These beaches are approximately 1800 m long and have low amplitude semidiurnal tides with 0.6 m of mean range with medium to fine sand grains size (mean diameter of 0.20 mm) and gentle slope profile (Vasconcellos et al. 2011).The other two beaches (5 and 6) were located in a semi-protected area in the Ilha Grande Bay, with comparatively less influence of anthropogenic activities and intermediary environmental conditions between the oceanic beaches and the protected beaches.
Sampling sites of collection of fish, benthic invertebrates and environmental data in sandy beaches along the Rio de Janeiro coast between 2014 and 2015: Oceanic exposed beaches: 1, Recreio and 2, Grumari; Protected beaches in the Sepetiba Bay: 3, Itacuruçá and 4, Muriqui; Semi-protected beaches in the Ilha Grande Bay: 5, São Gonçalo and 6, São Gonçalinho
Sampling design
Environmental and species assemblage data were collected quarterly from six beaches along the coast of Rio de Janeiro between March 2014 and December 2015 with four replicates at each site, totalling 192 samples (six sites × four seasons × two years × four replicates). In order to reduce the influence of diel and tidal amplitude, sampling was carried out at low tide, during quadrature tides, between 10:00 and 16:00 h.
Environmental data collection
The sediment samples for invertebrate composition, particle-size and nutrient analysis were obtained with the aid of a PVC corer (10 cm in diameter and 50 cm in length) with a collecting area of 0.00785 m2 at 1.5 m of water depth and 15 cm sediment depth with four replicates at each beach. According to Bally (1983), the first 15–20 cm of the substrate has the greatest abundance of benthic organisms.
The collected sediment was weighed (precision of 0.01 g) and dried at 80 °C in a stove. The samples were weighed on a precision scale (0.01 g) and 150 g were taken for analysis of nutrients and 300 g for granulometric analysis with the aid of the tampering device during 15 min for each sample. The silt and clay fractions were grouped together. The granulometric parameters were calculated according to Folk and Ward (1957) and classified according to Shepard (1954). The mean granules size was determined from each granulometric fraction weight retained in each sieve, using the software SysGran 3.0 (Camargo 2006). In total, seven classes of sediment size were determined: granules, very coarse sand, coarse sand, medium sand, fine sand, very fine sand, and silt + clay.
The concentrations of the following nutrients in the sediment were analysed: organic matter (g × cm−3), organic carbon (g × kg−1), total nitrogen (%) and total phosphorus (mg × dm−3). The concentration of organic carbon was determined using the method of Walkey and Black (1934). The concentration of total nitrogen in the sediment was determined using the Kjeldahl nitrogen method with a diffusion camera. The concentration of total phosphorus was determined using a spectrophotometer after digestion with HNO3–HCl (3:1, V/V) at 200 °C. The solubilization of the mineral and organic phosphate forms was conducted using 1:1 H2SO4 (Bowman 1988).
At each sampling occasion, water temperature (degree Celsius), salinity (ppt), dissolved oxygen (mg L−1) pH, turbidity (NTU) and conductivity (mS × cm−1) were measured. These measurements were performed using a Horiba U-23 multiprobe (Horiba Trading Co. Ltd., Shanghai) immersed approximately 0.5 m under the water surface. Transparency was measured with a Secchi disc.
Benthic invertebrates
Immediately after collection, the organisms were fixed with 10% formalin for subsequent screening in the laboratory. The sediment samples were initially screened with the aid of a plastic tray (80 cm × 40 cm × 7 cm) using tap water for removal of the largest specimens, then sieved through a 0.5 mm mesh and examined under light stereo microscope for identification of the smallest specimens to family level. According to Kilgour and Barton (1999), benthic invertebrates have a better correlation with fish when identified at the family level, as there is no significant difference when identified at the species level. All identified specimens were preserved in 70% ethanol solution.
Fish collection
A beach seine net (12 m long × 2.5 m high; 8 mm stretched mesh at the wings and 4 mm at the cod end) was used for the fish sampling. The hauls were 30 m long and perpendicular to the shore, and they were taken out to a depth of approximately 1.5 m, covering a swept area of approximately 300 m2 (30 m long × 10 m wide opening). After capture, the fish were anesthetized in benzocaine hydrochloride (50 mg L−1) and subsequently fixed in 10% formalin solution. In the laboratory, fish were identified and transferred to a preservative liquid (70% alcohol). All fishes were identified to the species level, and voucher specimens were deposited in the Ichthyological Collection of the Fish Ecology Laboratory of the Universidade Federal Rural do Rio de Janeiro.
Data analyses
The fifteen environmental variables obtained were previously separated into three different categories: water physical-chemical variables, sediment nutrients, and sediment particle size (Table S1 in the Supplementary Information). These environmental variables were standardized (centred to the mean and reduced to units of standard deviation) to eliminate the effects of different units of measurements. A principal component analysis (PCA) was used to reduce each group of environmental variables into few independent and interpretable components (PCs). Three groups of environmental variables were considered: 1) water physicochemical variables (PCA pc); 2) sediment nutrients variables (PCA n); and 3) sediment granulometric variables (PCA g). The PCA was based on a correlation matrix of the centred and standardized data (Legendre and Legendre 2012). The first two axes that are the most relevant to explain the environmental variance were selected (Peres-Neto et al. 2003), and used as latent environmental variables. The variables that most contributed for the explained variance were considered as those with loadings larger than the hypothetical equal contribution of all variables.
Numerical abundances were used for fish and benthic invertebrate that were expressed at species and family levels, respectively. Taxa with less than 3% of occurrence were considered rare and omitted from these analyses to avoid overweighting their influence on the ordination results (Leps and Smilauer 2003), as incidental taxa diminish the response signal of the more abundant taxa to environmental gradients. Spearman rank correlation was used to assess relationship between fish and benthic invertebrate richness across sites.
Prior to analysis, the biological data were square root transformed to reduce the influence of abundant species but preserve information on their relative abundance. The relationship between taxonomic richness of each group with environmental variables and the taxonomic richness of the other group were assessed using hierarchical partitioning of R2 values. By taking a hierarchical approach in which, all orders of variables are used, the average independent contribution of a variable is obtained and an exact partitioning results (Chevan and Sutherland 1991). Randomization test was used to compare the observed independent contribution of variables to explain variance against a population of independent contribution drawn from 500 randomization of the data matrix. The statistical significance of the variables was determined using the upper 95% confidence limit (Mac Nally 2000). This approach is less affected by multi-collinearity between variables and was performed using the Hier-Part package (Walsh and Mac Nally 2007) within the R statistical package.
The partition variance in both fish or macroinvertebrates assemblages into unique and shared contribution of environmental and biological variables were determined using Canonical Correspondence Analysis (CCA) and partial CCA (pCCA). In this analysis, the unique contribution of one group in explaining variation in the assemblage of the other group is an estimate of the importance of biological interactions (Paszkowski and Tonn 2000). Partial CCA enables decomposition of variance and was used to partition the variance into: i) the unique or pure variation explained by environmental variables after removing the (co)variation associated with the other taxonomic group, ii) the pure variation explained by the other taxonomic groups after removing the (co)variation associated with environmental variables, iii) the common or shared variation between environmental and biological variables, and iv) unexplained variation.
CCA with no covariables (using both environmental variables and biological variables from the other group as explanatory variables) was used to calculate the total amount of variance explained. In these analyses the first two Detrended Correspondence Analysis (DCA) axes and taxonomic richness of one group were used as biological explanatory variables when analysing the other group. In a second step, the unique effect of environmental variables or biological variables was estimated by using one as a predictor and the other as a covariable. To evaluate this variation, we used the following procedure for the fish and for the benthic invertebrate variables: (1) CCA of the species matrix constrained by the environmental matrix; (2) CCA of the species matrix constrained by the biotic matrix of the other group; (3) CCA of the species matrix constrained by the environmental matrix but removing the effect of the other group biotic matrix; and (4) CCA of the species matrix constrained by the other group biotic matrix but removing the effect of the environmental matrix. With these four constraints of ordination and three data matrices, it was possible decompose the variance on fish and benthic invertebrate assemblages. We used a Monte Carlo permutation test (generating 999 permutations) to test the significance of each environmental and biotic variable. All ordinations and permutation tests were performed using CANOCO 4.0 for Windows software (Leps and Smilauer 2003).
The correlation between fish and benthic invertebrate was assessed using the Mantel test on the dissimilarity (Bray-Curtis) matrices of both taxa. In addition, a Partial Mantel test was also used to control for environmental variables (using Euclidean distance matrices), as concordance between taxa matrices could derive simply by their shared response to environmental variables. Random permutations (5000) were used to obtain the significance level for the correlation coefficients.
Results
Environmental variables
The first two axes of the PCA showed more than 60% of the variation in each of the three categories of environmental physicochemical variables (Table 1). PCA 1 of the physicochemical variables represent mainly a gradient of high temperature in opposition to low salinity, whereas PCA 2 represent the effect of low dissolved oxygen and pH. These two axes were used in the subsequent analyses to synthetize environmental physicochemical variables. The PCA1 of the nutrient analysis was characterized by high influence of carbon and organic matter in opposition to nitrogen, whereas PCA 2 reflected mainly the effect of phosphorous. The PCA1 of the granulometry showed a gradient with positive contribution of fine sediment (fine and very fine sand, and silt + clay), whereas PCA 2 represented the positive effect of the coarse sediment in opposition to medium sediment (Table 1).
Hierarchical partitioning of taxonomic richness
A total of 59 species of fish and 24 families of benthic invertebrates were identified (Tables S2 and S3 in the Supplementary Information). The taxonomic richness between fish and invertebrates had no significant correlation (rs = 0.063 p = 0.58).
The water physicochemical variables had influence on the fish species richness. PCA2-pc and PCA1-pc were negatively correlated with richness, whereas the nutrient PCA2-n and the granulometric PCA1-g were positively correlated with richness (Table 2, Fig. 2). That is, fish species richness was higher in areas with high salinity and dissolved oxygen and low temperature. In addition, richness was also favoured by higher phosphorus content and presence of fine and very fine sand sediment.
Distribution of independent effects (I%) of predictor variables for fish and benthic invertebrate richness calculated with hierarchical partitioning. PCA-pc, PCA-n, PCA-g are the first two axes of the Principal Component Analysis (PCA) from water physicochemical (pc), sediment nutrients (n) and sediment granulometric (g) variables, respectively
For benthic invertebrates, the granolumetric variables PCA1-g were negatively correlate with invertebrate richness (Table 2, Fig. 2). In other words, benthic invertebrate richness was lower in fine and very fine sediment, and increased in the presence of medium and coarse sediment.
Variance partitioning in the community composition
Only 11.30% of the variation in the fish community was explained by the biotic benthic invertebrate (invertebrate richness) data and the environmental PCA1-pc, PCA2-g, PCA1-g and PCA2-pc. The partial CCA using the biotic matrix as covariate explained 10.01% of the explained variation in the analysis of CCA with PCA1-pc, PCA2-pc and PCA1-g as the strongest variable selected through the Monte Carlo test permutations (Table 3). Using the environmental matrix as a covariate, the partial CCA explained only 2.95% by the biotic variables of the fish variation (Table 4). The shared variation by environmental and biotic variables were only 1.29% of the fish.
For the benthic invertebrates, the initial CCA showed 17.9% of the variation explained by the environmental and biotic variables. The fish richness and all PCAs variables, except PCA1-pc were selected variables by the Monte Carlo test permutations explaining the benthic invertebrate variables (Table 3). In the of partial CCA analysis, removing the effect of the biotic variables, the selected variables explaining benthic invertebrate were PCA1-g, PCA2-n, PCA2-g and PCA1-n, explaining about 13.56 of the benthic invertebrate variables (Table 3). When the effect of environmental variables was removed, the explanation of the biotic variables was 4.43%, whereas the shared variation of the environmental and biotic variables was 4.31% of the benthic invertebrate variables (Table 4).
The Mantel and partial Mantel tests showed no significant correlation between fish and benthic invertebrates (r = 0.017; p = 0.17), even when controlling the effect of environmental variables (r = 0.013; p = 0.23).
For the fish community, the sum of all eigenvalues in the canonical correspondence analysis of the species matrix was 6.503 (Table 4). From here, we obtained the relative importance of each factor that controls the variation in the composition of species. The environmental variables explained about 11.30% of the variation of the species matrix (Step 1) (Table 4). About 1.29% out of this variation was related to biotic variables. Roughly, 14.25% of the explained variation was due to both spatial and biotic variables, while the amount of unexplained variation was 85.75% (Table 4, Fig. 3).
For the benthic invertebrates community, the sum of all eigenvalues in the canonical correspondence analyses of the data matrix was 3.832 (Table 4). The environmental variables explained 17.87% of the variation of the benthic invertebrate matrix (Step 1). From this variation, 4.31% was shared between environmental and biotic variables. A total of 22.3% of the benthic invertebrate variation was explained by both environmental and biotic variables, whereas the amount of unexplained variation was 77.7% (Table 4, Fig. 3).
The first two CCA axes of the relationship between fish relative abundance and environmental variables showed that PCA1-pc was positively associated with the semi-protected beaches (Fig. 4). Species of the family Gerreidae (e.g., Eucinostomus argenteus, Eucinostomus melanopterus) were abundant in these sites (5 and 6) and were influenced by high temperatures and low salinity, with sediment predominated by fine sand grains (PCA1-g). On the other hand, the protected beaches (sites 3 and 4) had high PCA2-n, characterized by high phosphorus concentration in the sediment. The opposite of these environmental conditions prevails in the unprotected oceanic beaches (sites 1 and 2) that had predominance of the sparid Diplodus argenteus, the haemulid Orthopristis ruber, and the clupeids Harengula clupeola and Sardinella brasiliensis.
First two axes from CCA analysis of fish (a) and benthic invertebrate (b) relative abundances at sand beaches in South-eastern Brazil. Environmental variables are represented as vectors. Species of fishes and families of benthic invertebrate abbreviations are in Table S2 and S3 in the Supplementary Information. Sites codes: oceanic exposed beaches: 1, Praia do Recreio and 2, Grumari; Protected beaches in Sepetiba bay: 3, Itacuruçá and 4, Muriqui; Semi-protected beaches in Ilha Grande Bay: 5, São Gonçalo and 6, São Gonçalinho. PCA-pc, PCA-n, PCA-g are the first two axes of the Principal Component Analysis (PCA) from water physicochemical (pc), sediment nutrients (n) and sediment granulometric (g) variables, respectively
In relation to the benthic invertebrates, the semi-protected sandy beaches (sites 5 and 6) had high PCA2-pc and PCA1-pc, characterized by high temperature, low salinity with predominance of polychaetes of the families Syllidae and Dorvilleidae, and isopods of the family Cirolanidae (Fig. 4). The opposition condition characterized the protected beaches (sites 3 and 4) with high PCA2-n, PCA1-n and PCA1-g, representing sediment rich in organic matter, organic carbon and phosphorus with fine and very fine sediment, where predominate Tanaidacea and polychaetes of the families Spionidae, Sigalionidae and Ophellidae. The unprotected beaches (sites 1 and 2) had environmental conditions between the protected and unprotected beaches, with predominance of polychaetes of the families Glyceridae and Saccocirridae.
Discussion
In this study, we found no correlation between the fish and benthic invertebrate richness in this area that represent the transition region between the tropical and subtropical provinces along the South-eastern Brazilian coast. The lack of correlations is generally attributed to taxon specific responses to environmental gradients, and this appears to be the case also in this study. However, it is necessary to consider that the lack of correlation between fish and invertebrate richness may be due to the local scale of this study, with only six sites being analyzed in a relatively close distance. The fish richness was most influenced by the salinity and temperature, and to a lesser extent by the dissolved oxygen, as reflected in PCA1-pc and PCA2-pc, whereas the benthic invertebrate richness was mostly influenced by the sediment granulometry decreasing in fine and very fine sediment, as reflected by the physicochemical PCA1-g. This seems to be a common finding in other biodiversity studies. Wolters et al. (2006), in a meta-analysis covering 43 taxa, concluded that no taxon appeared to be a good predictor of the richness of other taxa. In a similar study for Mediterranean streams evaluating richness and composition of fish and macroinvertebrates, only a weak correlation between these two groups was found (Larsen et al. 2012). Therefore, we confirmed in this study that the fish richness is more related to physicochemical variables whereas the benthic invertebrate nicheness was more associated to sediment granulometry, rather than physicochemical variables. Other studies also have pointed to the importance of the sediment on the distribution of benthic invertebrate in sandy beaches (e.g., Lercari and Defeo 2006; Mclachlan and Brown 2006; Defeo and Mclachlan 2011; Cardoso et al. 2012).
Regarding the agreement between the two assemblages, no significant correlation was found between the fish and benthic invertebrates according to the Mantel and partial Mantel tests after controlling the influences of environmental variables. That is, we found no indication of influences from one taxon on the other, or other type of interactions such as common assemblage responses to the effects of environmental variables. In addition, the results of CCA and partial CCA for the relationship between each assemblage with both environmental variables and biological variables from the other group as explanatory variables, also showed weak correlation. In fact, each assemblage was influenced by different environmental variables with the effects varying at species-specific levels. Physicochemical variables, namely temperature and salinity, have been identified as important drivers of fish assemblages, whereas sediment granulometry influenced benthic invertebrate assemblages. Salinity and temperature are the most important predictors in the distribution of fish in estuarine areas of temperate coastal ecosystems (Thiel et al. 1995; Martino and Able 2003). In the tropics, salinity is one of the most important drivers for assemblage composition (Barletta et al. 2005), and turbidity is an essential factor providing protection against predators (Blaber and Blaber 1980). Araújo et al. (2002) found that depth, followed by transparency and salinity, were the primary factors influencing assemblage structure in different areas of the Sepetiba Bay, south-eastern Brazil.
It is well established that local ecological interactions are important in shaping the local assemblages (e.g., Cornell and Harrison 2013). The amount of ‘strictly biotic’ variation can be of particular importance in ecological investigations when there is a strong ecological relationship, such as prey-predator, interference or exploitation competition or other significant biotic interaction. Our approach of estimating biological interactions by using the unique contribution of one group (DCA1, DCA2 and species richness) in explaining variation in the assemblage of the other group, showed a weak relationship. The influence of the benthic invertebrates on the fish community was very low and the reverse situation was similar. However, the environmental influences explained a relevant part of the variance and were much more relevant than the biotic interactions, although a large proportion of the variance was unexplained for both taxa, suggesting that part of it was due to nondeterministic fluctuations. The large unexplained variance can be attributed to stochasticity that prevails in this type of environment and also to some spurious effect of an extraneous variable, not included in the model, that influence these communities. In most studies, it is difficult to discriminate between the part that is potentially explained and the part that is real stochasticity (Borcard et al. 1992). However, different patterns can be found at large scale. Highly significant similarities in the spatial pattern of distribution of benthos and fishes, despite their differences in motility and other ecological traits was found in the Barent Sea (Johannesen et al. 2017), with fish and benthos communities having a similar relationship to the environmental gradients at the scale of hundreds to thousands of kilometres.
We found, at local scale, evidence that fish and benthic invertebrate assemblages were weakly correlated to each other and that each group responded more strongly to different types of environmental variables that biotic interactions. These results coincide with other studies (e.g., Herlihy et al. 2020), in which most of the environmental variables were related to either fish and/or macroinvertebrates in some fashion and that the factors involved, and the strength of the relationship, varied between the assemblages. Although the sampled locations are relatively distant from sources of pollution that are common around the city of Rio de Janeiro, it is important to keep in mind the great potential sources of environmental degradation nearby. Sandy beach urbanization is an increasing phenomenon that contributes to decreasing composition and density of benthic organisms by affecting sediment characteristics and consequently the macroinfaunal community (Veloso et al. 2006; Defeo et al. 2009). Main threats are linked with the social and economic use of beaches such as, sand nourishment to counter erosion, enrichment of coastal waters with nutrients, the mechanical cleaning of beaches and disturbance by tourist pressure. In cases of degradation, the complex associations among individuals of macroinvertebrates and benthic algae revealed relatively greater stability and resistance to human impacts, with faster community recovery than fish (Qu et al. 2019). Differences in the local habitat structure associated to environmental variables are widely known to influence spatial distribution in biotic assemblages (Auster et al. 2001; Cardoso et al. 2012; Cornell and Harrison 2013; Meena et al. 2019). The type of sediment, local geomorphology, organic input and wave exposure are important drivers determining habitat characteristics at local scale (within a few kilometres).
Wave exposure may have acted together with the other factors such as the type of sediment, which tended to be muddy in sites located in the bays, and sandy in the unprotected oceanic beaches, a common pattern reported elsewhere (e.g., Defeo et al. 2009; Di Domenico et al. 2014). In this study, the protected (sites 3 and 4) and semi protected (sites 5 and 6) beaches inside the bays with comparatively lesser wave exposure than the unprotected sandy beaches (sites 1 and 2) could have influenced the presence of different types of fish and benthic invertebrates across the beaches. The fish species Diplodus argenteus, Harengula clupeola, Sardinella brasiliensis and Trachinotus carolinus were more frequent and abundant in the unprotected oceanic beaches, whereas Atherinella brasiliensis, Anchoa januaria and Anchoa tricolor were common in the protected beaches of Sepetiba Bay, and Eucinostomus argenteus in the semi-protected beaches of the Ilha Grande Bay. In relation to benthic invertebrates, the family Saccocirridae occurred in high abundance in all beaches, but predominated in the unprotected oceanic beaches. Phyllodocidae, Syllidae and Cirolanidae occurred mainly in the semi-protected beaches of the Ilha Grande Bay, whereas Capitellidae and Tanaidacea in the protected beaches of the Sepetiba Bay. According to Mariani (2001), the structure of the fish assemblages of bays and coastal areas usually reflects the physical, geochemical and hydrological characteristics of the area, and the distribution of the species is consistent with the degree of marine influence in the system. Sand movement and, potentially, the influence of adjacent habitats (Jarrin and Miller 2016) can change local environmental conditions of each beach having particular influence on habitat structure that determine and discriminate the community structure (Bally 1983; Araújo et al. 2002; Cardoso et al. 2012; Cornell and Harrison 2013). Di Domenico et al. (2009, 2014) and Cardoso et al. (2012) observed the dominance of a family of polychaetes in coarse-grained coastal environments.
Fish distribution data (species or communities) have been used as a proxy for habitat distribution to develop precautionary conservation strategies for habitat protection (e.g., marine protected areas, restrictions on fishing gear) (Auster et al. 2001; Araújo et al. 2017). However, other taxonomic groups are neglected despite the importance for the overall functioning of the ecosystem. For example, Araújo et al. (2017) and Gomes-Gonçalves et al. (2020) evaluated the temporal changes of the ichthyofauna in Sepetiba Bay and observed contrasting differences in composition and abundance over time. However, the temporal variations of other taxonomic groups (e.g., benthic invertebrates) that could be associated with the ichthyofauna were not investigated. It is not an easy task to assess different taxonomic groups and their biotic and environmental constraints that are relevant in an ecological study. The amount of unexplained variation when considering biotic and environmental predictors are proportionally high. However, such predictors can still be considered as important factors in structuring the assemblages of fish and benthic invertebrates.
Our study showed that fish and benthic invertebrates are influenced by different environmental variables in tropical sandy beaches and no significant correlation was found between these two taxonomic groups. These results contribute to a better understanding of the functioning of sandy beaches, encompassing two important components of the biotic community and providing ecological information that are complementary and that can assist in conservation measures. Thus, the present study provides baseline information on assemblage patterns of fish and benthic invertebrates in tropical sandy beaches that would help in policies for sustainable management of these aquatic resources.
References
Alejandro B (2001) Relationship between species richness and morphodynamics in sandy beaches: what are the underlying factors? Mar Ecol Prog Ser 224:35–44
Araújo FG, Azevedo MCC, Silva MA, Pessanha ALM, Gomes ID, Cruz-Filho AG (2002) Environmental influences on the demersal fish assemblages in the Sepetiba Bay, Brazil. Estuaries 25:441–450
Araújo GF, Pinto MS, Neves LM, Azevedo MCC (2017) Inter-annual changes in fish communities of a tropical bay in southeastern Brazil: what can be inferred from anthropogenic activities? Mar Poll Bull 114:102–113
Auster PJ, Joy K, Valentine PC (2001) Fish species and community distributions as proxies for seafloor habitat distributions: the Stellwagen Bank National Marine Sanctuary Example (Northwest Atlantic, gulf of Maine). Environ Biol Fish 60:331–346
Bally R (1983) Intertidal zonation on sandy beaches of the west coast of South Africa. Cah Biol Mar 24:85–103
Barletta M, Barletta-Bergan A, Saint-Paul U, Hubold G (2005) The role of salinity in structuring the fish assemblages in a tropical estuary. J Fish Biol 66:45–72
Blaber SJM, Blaber TG (1980) Factors affecting the distribution of juvenile estuarine and inshore fish. J Fish Biol 17:143–162
Borcard D, Legendre P, Drapeau P (1992) Partialling out the spatial components of ecological variation. Ecology 73:1045–1955
Bowman RA (1988) A rapid method to determine total phosphorus insoils. Soil Sci Soc Am J 52:1301–1304
Camargo MG (2006) SYSGRAN: Um sistema de código aberto para análises granulométricas. Rev Brasil Geoc 36:371–378
Cardoso RS, Mattos G, Caetano CHS, Cabrini TMB, Galhardo LB, Meireis F (2012) Effects of environmental gradients on sandy beach macrofauna of a semi-enclosed bay. Mar Ecol 33:106–116
Chevan A, Sutherland M (1991) Hierarchical partitioning. Am Stat 45(2):90–96
Cornell HV, Harrison SP (2013) Regional effects as important determinants of local diversity in both marine and terrestrial systems. Oikos 122:288–297
Crawley KR, Hyndes GA, Ayvazian SG (2006) Influence of different volumes and types of detached macrophytes on fish community structure in surf zones of sandy beaches. Mar Ecol Progr Ser 307:233–246
Defeo O (2003) Marine invertebrate fisheries in sandy beaches: an overview. J Coast Res 35:56–65
Defeo O, Mclachlan A (2011) Coupling between macrofauna community structure and beach type: a deconstructive meta-analysis. Mar Ecol Prog Ser 433:29–41
Defeo O, McLachlan A, Schoeman DS, Schlacher TA, Dugan J, Jones A, Lastra M, Scapini F (2009) Threats to sandy beach ecosystems: a review. Estuar Coast Shelf Sci 81:1–12
Di Domenico M, Lana PC, Garraffoni ARS (2009) Distribution patterns of interstitial polychaetas in sandy beaches of southern Brazil. Mar Ecol 30:47–62
Di Domenico M, Martinez A, Almeida TCM, Martins MO, Worsaae K, Lana PC (2014) Response of the meiofaunal annelid Saccocirrus pussicus (Saccocirridae) to sandy beach morphodynamics. Hydrobiologia 734:1–16
Folk RL, Ward WC (1957) Brazos river bar: a study of significant of grain size parameters. J Sediment Petrol 27:3–26
Gomes-Gonçalves RS, Aguiar FS, Azevedo MCC, Araújo FG (2020) Functional stability despite anthropogenic influences on the ichthyofauna of a tropical bay. Mar Environ Res 159:105016
Heino J (2010) Are indicator groups and cross-taxon congruence useful for predicting biodiversity in aquatic ecosystems? Ecol Indic 10:112–117
Herlihy AT, Sifneos JC, Hughes RM, Peck DV, Mitchell RM (2020) The relation of lotic fish and benthic macroinvertebrate condition indices to environmental factors across the conterminous USA. Ecol Indic 112:105958
Human LRD, Snow GC, Adams JB, Bate GC, Yang S (2015) The role of submerged macrophytes and macroalgae in nutrient cycling: a budget approach. Estuar Coast Shelf Sci 154:169–178
Jackson DA, Harvey HH (1993) Fish and benthic invertebrates: community concordance and community–environment relationships. Can J Fish Aquat Sci 50:2641–2651
Jarrin JRM, Miller JA (2016) Spatial variability of the surf zone fish and macroinvertebrate community within dissipative sandy beaches in Oregon, USA. Mar Ecol 37(5):1027–1035
Johannesen E, Jørgensen LL, Fossheim M, Primicerio R, Greenacre M, Ljubin PA, Dolgov AV, Ingvaldsen RB, Anisimova NA, Manushin IE (2017) Large-scale patterns in community structure of benthos and fish in the Barents Sea. Polar Biol 40:237–246
Johnson RK, Hering D (2010) Spatial congruency of benthic diatom, invertebrate, macrophyte, and fish assemblages in European streams. Ecol Appl 20:978–992
Josefson AB, Rasmussen B (2000) Nutrient retention by benthic macrofaunal biomass of Danish estuaries: importance of nutrient load and residence time. Estuar Coast Shelf Sci 50:205–216
Kilgour BW, Barton DR (1999) Associations between stream fish and benthos across environmental gradients in southern Ontario, Canada. Freshw Biol 41:553–566
Larsen S, Mancini L, Pace G, Scalici M, Tancioni L (2012) Weak concordance between fish and macroinvertebrates in Mediterranean streams. PLoS One 7:e51115
Legendre P, Legendre L (2012) Numerical ecology, 3rd, English edn. Elsevier Science BV, Amsterdam
Leps J, Smilauer P (2003) Multivariate analysis of ecological data using CANOCO Cambridge University Press, Cambridge
Lercari D, Defeo O (2006) Large-scale diversity and abundance trends in sandy beach macrofauna along full gradients of salinity and morphodynamics. Estua Coast Shelf Sci 68:27–35
Mac Nally R (2000) Regression and model-building in conservation biology, biogeography and ecology: the distinction between and reconciliation of ‘predictive’ and ‘explanatory’ models. Biodivers Conserv 9:655–671
Mariani S (2001) Can spatial distribution of ichthyofauna describe marine influence on coastal lagoons? A Central Mediterranean case study. Estua Coast Shelf Sci 52:261–267
Martino E, Able KW (2003) Fish assemblages across the marine to low salinity transition zone of a temperate estuary. Estua Coast Shelf Sci 56:969–987
Mclachlan A, Brown AC (2006) The ecology of sandy shores. Academic Press, Burlington, USA
Meena DJ, Lianthuamluaia P, Mishal HS, Swain BK, Naskar S, Saha KM, Sandhya S, Kumari T, Tayung UK, Sarkar BKD (2019) Assemblage patterns and community structure of macro-zoobenthos and temporal dynamics of eco-physiological indices of two wetlands, in lower Gangetic plains under varying ecological regimes: a tool for wetland management. Ecol Eng 130:1–10
Nielson J, Jernakoff P (1996) A review of the interaction of sediment and water quality with benthic communities. Technical report 25, CSIRO environmental projects Yarralumla, ACT, Australia Australia, 30p
Paszkowski CA, Tonn WM (2000) Community concordance between the fish and aquatic birds of lakes in northern Alberta, Canada: the relative importance of environmental and biotic factors. Fresh Biol 43:421–437
Peres-Neto PR, Jackson DA, Somers KM (2003) Giving meaningful interpretation to ordination axes: assessing the significance of eigenvector coefficients in principal component analysis. Ecology 84:2347–2363
Qu X, Peng W, Liu Y, Zhang M, Ren Z, Wu N, Liu X (2019) Networks and ordination analyses reveal the stream community structures of fish, macroinvertebrate and benthic algae, and their responses to nutrient enrichment. Ecol Indic 101:501–511
Santoul F, Soulard A, Figuerola J, Céréghino R, Mastrorillo S (2004) Environmental factors influencing local fish species richness and differences between hydroregions in South-Western France. Int Rev Hydrobiol 89(1):79–87
Sato N, Asahida T, Terashima H, Hurbungs MD, Ida H (2008) Species composition and dynamics of larval and juvenile fishes in the surf zone of Mauritius. Environ Biol Fish 81:229–238
Shepard FP (1954) Nomenclature based on sand-silt-clay ratios. J Sediment Petrol 24:151–158
Thiel R, Sepúlveda A, Kafemann R, Nellen W (1995) Environmental factors as forces structuring the fish community of the Elbe estuary. J Fish Biol 46:47–69
Vasconcellos RM, Araújo FG, Santos JNS, Silva MA (2011) Diel seasonality in fish biodiversity in a sandy beach in South-Eastern Brazil. J Mar Biol Assoc UK 91:1337–1344
Veloso VG, Silva ES, Caetano CHS, Cardoso RS (2006) Comparisons between the macroinfauna of urbanized and protected beaches in Rio de Janeiro state, Brazil. Biol Conserv 27:510–515
Walkey A, Black JA (1934) An examination of the Degtjareff method for determining soil organic matter, and proposed modification of the chronic acid titration method. Soil Sci 37:29–38
Walsh C, Mac Nally R (2007) Hierarchical partitioning. Version 1-2.0. R Foundation for statistical computing Vienna, Austria. Available: http://cran.rproject.org/
Whitfield AK, Elliott M (2002) Fishes as indicators of environment and ecological changes within estuaries: a review of progress and some suggestions for the future. J Fish Biol 61:229–250
Wolters V, Bengtsson J, Zaitsev AS (2006) Relationships among the species richness of different taxa. Ecology 87:1886–1895
Acknowledgements
The authors thank technicians from the Laboratory of Fish Ecology for helping in fieldwork. This work was financially supported by CNPq—Brazilian National Agency for Scientific and Technological Development (Proc. 302878/05-0 and 302555/08-0) and by FAPERJ (Rio de Janeiro State Agency for Research Development (Proc. E-26/170.258/01). This research was conducted under SISBIO Collection of Species Permit number10707 issued by ICMBio, Brazilian Environmental Agency.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted (Universidade Federal Rural do Rio de Janeiro, Brazil, Animal Care and Use Committee, Protocol # 11874).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 63 kb)
Rights and permissions
About this article
Cite this article
de Aguiar, F.S., de Sousa Gomes-Gonçalves, R. & Araújo, F.G. Fish and benthic invertebrate relationship and their association to environmental variables in tropical sandy beaches. Environ Biol Fish 103, 1309–1321 (2020). https://doi.org/10.1007/s10641-020-01024-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-020-01024-0