Abstract
The distribution of Orestias (Cypriniforms) species in the southern western Altiplano (17ºS-22ºS) is allopatric; the seven species described inhabit different freshwater systems with extreme climatic characteristics and different ecological conditions, factors that would have enhanced interspecific differentiation. To analyze their head differences we compared jaw morphology of eight species of Orestias, seven southern ones and one from Puno Peru, using linear and geometric morphometrics. We found differences among the species with both methods. Nevertheless, none of the external measurements by themselves allows classification of any of the species or populations. The geometric analysis showed differences related to feeding structures such as a protractile jaw, separating the western species in two groups: a northern group with O. chungarensis, O. parinacotensis, O. piacotensis, O. laucaensis, O. puni and O. cf. agassii and a southern group, O. gloriae and O. ascotanensis. The results from this methodology reaffirmed the importance of the extreme environmental conditions of the Altiplano systems to explain the process of adaptation described for the specious genus Orestias.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Orestias (Cypriniforms), the endemic high Andean South American genus, is distributed from Lacsha Lake Perú, (9°S) to the Ascotán Salt pan in Chile (21º S’) (Parenti 1984a, b). Cyprinodontids fish are reported as tolerant to a wide range of temperature and salinity (Davenport and Sayer 1993); their small size is postulated as allowing them to tolerate extreme and unstable environments, permitting the survival of relict populations (Parker and Kornfield 1995; Cruz-Jofré et al. 2016). The evolution of lacustrine systems in the Altiplano to the present Titicaca and Chungará lakes has been strongly controlled by climate, geology and the size of paleo-lakes (Placzek et al. 2006). Their effluents and shallow associated freshwater systems would have contributed to the diversity of physical and chemical characteristics, promoting isolation, diversification and speciation through radiation of Orestias in Titicaca Lake and numerous wetlands (bofedales) and salt pans in the southwest Chilean Altiplano (SWA; 17ºS to 22ºS) (Parenti 1984b; Maldonado et al. 2009; Vila et al. 2010, 2013; Arratia et al. 2017). Diversification of Orestias in the SWA may be linked to historical vicariant events and contemporary and historical variation in water levels that could have affected the populations from the Plio-Pleistocene until the present (Arratia et al. 2017).
In the Holocene and late Pleistocene, repeated climatic changes and water levels, intense volcanic activity and major tectonic movements affected the Altiplano freshwater systems (Placzek et al. 2006). As a result of these geological events, large, closed basins were developed on the southwestern side of the Altiplano; with a more arid climate these basins have remained small and unconnected (i.e., Chungará, Cotacotani, Piacota, Lauca, Isluga, Carcote and Ascotán) where Orestias maintains small populations that have remained isolated. During all the Quaternary the Altiplano alternated between cold, wet glacial and dry interglacial periods. Due to the repetitive expansions and contractions of the freshwater systems, the populations of Orestias would have been subjected to drastic environmental variations, which last until today (Parker and Kornfield 1995; Márquez-García et al. 2009; Vila et al. 2010, 2013; Cruz-Jofré et al. 2016). These systems present high total irradiance above 300 W m− 2 (Rondanelli et al. 2015), high salinity, reaching conductivities of more than 100 µmhos/cm, pH range between 8.0 and 9.5 and significant diurnal temperature changes (Márquez-García et al. 2009). Consequently, the different populations experienced a wide variety of ecological conditions in their distribution range, probably with local adaptations and particular phenotypic expression as it has been described for Orestias from Titicaca Lake (Lauzanne 1982; Maldonado et al. 2009).
The current species distribution in SWA includes O. chungarensis (Vila and Pinto 1986) in Chungará Lake (18º15’ S, 69º07’ W), Orestias piacotensis (Vila 2006) in Piacota (18º11’S, 69º15’W), O. laucaensis (Arratia 1982) in the Lauca River (18º11’ S, 69º16’ W) and Cotacotani Lake (18º12’ S, 69º14’ W) and O. parinacotensis (Arratia 1982), in the Parinacota Wetland, (18º10’ S, 69º20’ W); southern species cited as O. agassii (Mann 1954) currently are considered genetically as two different populations, one of these populations is located in Isluga River (19º15’S, 68º42’W) and the other one is in Huasco Salt pan (20º15’S, 68º52’W) (Scott 2010; Vila et al. 2013). The southernmost species are those that live associated with the springs that feed the southern salt pans, O. gloriae Vila et al. 2011 in Carcote (21°16’S, 68°19’W) and O. ascotanensis Parenti, 1984 in Ascotán Salt pan (21º29’S, 68º19’W). Interestingly, all southern species are allopatric (Vila et al. 2010), adapted to physical and chemical conditions, probably the volume and salinity of presently unconnected different systems such as deeper and shallow lakes rivers and wetlands locally called “bofedales”, systems that would have developed different aquatic biodiversity. Lakes sustain abundant zooplankton while rivers and wetlands with abundant macrophytes present diverse aquatic insects, snails and amphipods, characteristics that would have stimulated different adaptations for feeding (Riveros et al. 2012). In general, analyses of the Orestias diet have determined their preference for benthonic macroinvertebrates, which are associated with the different substrates and macrophytes of the different Altiplano systems (Guerrero et al. 2015). It still is no consensus on the degree of diet specialization of species (Vila et al. 2006; Guerrero et al. 2015).
Orestias morphology measurements and meristic specific characteristics overlap (Parenti 1984a; Vila et al. 2010). To improve morphological information, complementary methods have been developed such as landmark geometric morphometrics, allowing quantitative comparisons (Rohlf and Marcus 1993; Adams et al. 2004). Morphological studies of populations would show possible feeding strategies and morphological adaptations since these represent an important relationship between phenotype and environment (Wainwright 1991; Winemiller 1991; Douglas and Matthews 1992; Motta and Kortsckal 1992; Skúlason and Smith 1995; Winemiller et al. 1995; Svanbäck and Eklö 2002; Langerhans et al. 2003; Higham et al. 2006; Parsons and Robinson 2006, 2007; Maldonado et al. 2009). In fact, the mouth structure especially jaw protraction is an important factor in feeding habits and diversification of Teleostomi (Schaeffer and Rosen 1961; Westneat 2004, 2005; Westneat and Wainwright 1989; Waltzek and Wainwright 2003).
In this study, we compared linear and geometric morphometric of associated head structures, mainly mouth structures. We expected to find differences at the interspecific level within the genus Orestias, probably due to historical isolation and particular environmental conditions of each ecosystem in the southern western Altiplano. This information will enhance conservation and allow future comparisons among populations of the species of the genus Orestias.
Materials and methods
Specimens from the Limnology Laboratory University of Chile collection (LCUCHILE), included 108 adults Orestias from eight SWA localities, 13 specimens each of Lauca, Isluga Rivers, Piacota and Chungará Lakes, Parinacota wetland and Huasco, Carcote and Ascotan Salt pans and four specimens of O. puni from Titicaca Lake (Fig. 1; Table 1). The detailed collection of specimens were analyzed using linear and geometric morphometrics, firstly with the closed mouth and after that with the opened mouth. We opened the specimens’ mouth to the maximum jaw protrusion for the purposes of this study.
Location of the Southern Altiplano systems and geographic distribution of Orestias in the Southwestern Altiplano (Chile). O. piacotensis in Piacota lake, O. laucaensis in Lauca River and Cotacotani, O. parinacotensis in the Parinacota Wetland, O. chungarensis in Chungará Lake, O. cf. agassii in Isluga River and Huasco Salt pan, O. ascotanensis in Ascotán Salt pan, O. gloriae in Carcote Salt pan
Linear morphometric measurements
The following linear measurements were obtained with a digital Vernier (Fig. 2): Specimens with closed mouth: (1) Head length, (2) Snout length, (3) Tip of the snout to pectoral fin ventral insertion. Measurements with open mouth: (4) Snout length, (5) Tip of the snout to pectoral fin ventral insertion, (6) Mouth height, (7) Maxillary length. The morphometric data of the specimens was related to the standard length using the regression of Elliott et al. (1995).
To determine species differences a multivariate analysis of variance (one-way MANOVA) and a posteriori Tukey test for unequal samples were performed. With the measurements and matrix obtained, a canonical variation analysis was performed (CVA). A linear discriminant analysis (LDA) was also performed, using locality as the dependent variable. As independent variables each of the standardized variables of the measured specimens were used. This analysis allows to obtain the discriminant level of the set of characters used, as well as to identify the significant morphological variables of populations and species. To establish the morphological pattern among populations and the grouping of the specimens geomorph package was used (Adams et al. 2015) with the software “R” (Team 2014).
Geometric morphometric analysis
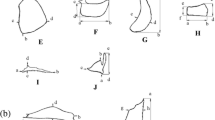
For the geometric morphology the left side of each specimen was photographed with the Canon SX 530 (16 Mega Pixel, with 1x optical zoom) camera in two different positions, one with the mouth closed and the other with it opened to maximum protrusion. For each group of specimens, we defined 12 anatomical landmarks corresponding to points or homologous structures that coincide between populations (Dryden and Mardia 2016). With closed and open mouth position with maximum protrusion the landmarks shown in Fig. 3, were: (1) The dorsal margin of head vertical to the center of eye, (2) Posteroventral corner of the maxilla, (3) Antero dorsal point of mouth in fleshy lip, (4) insertion of the operculum on the ventral profile, (5) Opercula width, (6) Supraoccipital, posteromedial tip, (7) Anterior middle eye border, (8) Posterior middle eye border, (9) Middle superior eye border, (10) Beginning of operculum, 11. Tip of the upper jaw, symphysis of the premaxillaries, 12. Tip of lower jaw, symphysis of the dentaries. These landmarks were digitized for all specimens with the software tpsDig2 and tpsRelW (Rohlf 2012).
The software Morpheus was used to analyze the anatomical configurations defined by the landmarks (Slice 1998). As a first step, landmark data from the 108 specimens were superposed with GPA (General Procrustes Analysis) (Rohlf 1990). This method scales all the specimens to one size, moving them to a common location and rotating them so the corresponding landmarks are best aligned, standardizing the landmark configurations for position, orientation, and size (Mitteroecker and Gunz 2009; Rohlf 1990). The analysis considered closed mouth and maximum protrusion independently for each group.
To determine differences among populations or species a PERMANOVA with adjustment of Euclidean distance (Anderson 2005) was developed, with 10000 permutation test using software Primer 6-Permanova 2 (Clarke and Gorley 2006).
To evaluate the influence of allometry on shape variation, a linear regression analysis was performed in MorphoJ (Klingenberg 2011). This regression included the Log of centroid and the scores of principal components. Centroid was obtained as the square root of the summed squared distances from each landmark to the centroid (Bookstein 1991). A canonical variation analysis (CVA) was performed to establish the morphological variation among species and populations, were grouped using the software Morpho J. (Klingenberg 2011).
We also integrated the matrices obtained from the superposition of the specimens and performed a cluster analysis with Euclidian distances. This summarized the similarities/differences among the analyzed groups. Additionally, we analyzed the partial distortions of the shape variables using Morpheus software, using the function TPS (thin-plate spline, Bookstein 1991). The specimens were compared using deformation grids as a graphic methodology.
Results
Table 2, shows the linear morphometric measurements and their standard deviations means. The multivariate analysis (MANOVA) indicated significant differences among the species (Wilk’s lambda = 0.038; F = 7.52; p < 0.05). The a posteriori analysis with the selected variables showed significant differences among groups (p < 0.05). The variables that had the greatest differences among species for both closed and open mouth specimens were snout length (2/4) and distance of the tip of the snout to the pectoral fin ventral insertion (3/5).
The CVA analysis showed that the first two axes explained 85.7% of the variance of the original data, 75.8% for the first axis and 9.9% for the second. For the first axis the variables with most weight among species for both closed and open mouth specimens were the tip of the snout to the pectoral fin ventral insertion (3/5). For the second axis, the variables with highest weight for both closed and open mouth were length of head and maxillary (1/7). The grouping of the specimens determined by the two first axes of the CVA analysis showed a separation of the O. gloriae (Carcote) specimens from all other specimens throughout the axis 1 and little gaps in the axis 2 (Fig. 4).
The discriminant analysis (LDA) showed differences among the studied species with the characters analyzed (Wilk’s λ: 0.03833, p < 0.05), correctly assigning a high percentage of the specimens in the classification matrix (Table 3). O. puni, O. chungarensis, O. laucaensis and O. cf. agassii (Huasco Salt pan) had more than 75% correct assignment and O. gloriae from Carcote Salt pan showed 100% correct assignment. Only O. parinacotensis showed less than 50% correct assignment; the specimens were assigned to five different groups although the majority was assigned to three species (Table 3).
According to the linear discriminant analysis, the significant variables among species for both open and closed mouth specimens were the snout length (4/2), mouth height (6) and maxillary length (7) with the head length (1).
Geometric morphometric analyses found significant difference among species morphology for the closed mouth group (PERMANOVA Pseudo-F = 5.5458, p = 0.001). The a posteriori analysis showed differences among all pairs (p < 0.05), with exception of the O. chungarensis – O. puni pair (p = 0.095).
The open mouth group showed significant differences among species (PERMANOVA pseudo-F = 6.872; p = 0.001). The a posteriori analysis showed differences among all populations (p < 0.05) except for four pairs: O. ascotanensis - O. gloriae (p = 0.077), O. chungarensis - O. laucaensis (p = 0.354), O. chungarensis - O. puni (p = 0.139) and O. cf. agassii (Huasco) - O. parinacotensis (p = 0.062).
The CVA analysis was performed with the coordinates obtained after the superimposition by GPA. As a result, the two first axes explained 52.5% of the original data variance for the group with closed mouth, 28.2% for the first axis and 24.3% for the second axis. The variables with most weight in the first axis were; insertion of the operculum on the ventral profile (4), Opercula width (5) and for the second axis were; anterior middle eye border (7) and posterior middle eye border (8). For the group open mouth, the first two axes explained 65.4% of the original data variance. The first axis explained 43.7% and the second 21.7%. The variables with most weight in the first axis were; tip of the upper jaw, symphysis of the premaxillaries (11) and tip of lower jaw, symphysis of the dentaries (12), instead of the second axis were; posterior middle eye border (9) and middle superior eye border (10).
The cluster analysis with the matrix of the integrated superposition of the distance data from both the closed mouth and opened mouth individuals (Fig. 5), showed a basal separation between O. puni from Lake Titicaca and the rest of the Orestias species studied. Among the SWA species, the separation was similar to that observed geographically (Fig. 1). The first division separated northern and southern groups: O. ascotanensis (Ascotán), O. gloriae (Carcote) and O. cf. agassii (Huasco); in the northern region, the most basal division separated O. piacotensis (Piacota), O. parinacotensis (Parinacota) and O. cf. agassii (Isluga) with O. laucaensis (Lauca) and O. chungarensis (Chungará) together.
The analysis of geometric morphometric differences may also be seen graphically with the thin plate spine function (TPS). This technique shows the change that the shape of a species must undergo to resemble the morphological shape of another species (Bookstein 1991; Toro et al. 2010). The greater differences were found among the more geographically distant groups. For the group closed mouth were O. gloriae - O. puni, O. gloriae - O. piacotensis and maximum protrusion were O. ascotanensis - O. chungarensis, O. chungarensis - O. piacotensis (Fig. 6). The evaluation of the Procrustes analysis of variance (ANOVA) showed that errors were negligible for the Orestias (p was always < 0.001).
Discussion
Orestias has been described as a specious genus (Parenti 1984a, b; Parker and Kornfield 1995), a characteristic that could have been enhanced by the climatic and volcanic variability occurring in central southern Altiplano (SWA) since the Pliocene. The fluctuations of the hydrological levels of basins due to a succession of dry and wet periods, in addition to the change in geology for volcanic activity have generated fractionated freshwater systems, which vary from shallow lakes to rivers and wetlands (Placzek et al. 2006) with different ionic contents varying from Ca-Mg-HCO3 to Na-Cl (Risacher et al. 2003a, b).
Studies have described the speciation of Orestias as the result of a process of fragmentation of one or more ancestral populations (Vila et al. 2013). The presence of Orestias in the isolated aquatic systems of the SWA region could be explained by the dispersion of the fish, which could have occurred with the formation of Paleolakes (Keller and Soto 1998). In this case, the morphological diversification of the groups could occur through allopatric speciation caused by habitat instability and very heterogeneous environmental conditions described for these systems, where the structure and composition of the aquatic biota would be determined by the physical and chemical variables of the water (Northcote 2000; Márquez-García et al. 2009).
In this context, linear morphometric analysis got a high assignment rate for the SWA specimens compared to the original description of these species showing that the measurements used have had good discriminant taxonomy for Orestias. The geometric morphometry analysis showed significant differences among the studied species. The interspecific and inter-population differences were supported by the linear classification, which designated a high percentage of the specimens to their corresponding species. The Tukey tests and the CVA analysis coincided in that the measurements evaluated that best described the interspecific differences were tip of the snout to pectoral fin ventral insertion closed mouth and open mouth height at maximum protrusion; Snout length and the height of the oral region. The importance of these characteristics would explain the different foraging strategies described for the genus (Maldonado et al. 2009; Riveros et al. 2012; Guerrero et al. 2015).
The biotic components of the SWA systems sustain abundant zooplankton, coastal macrophytes and macroinvertebrates that grow in shallower systems as aquatic insects, snails and crustaceans. Many authors have suggested that Orestias differentiated in habitat and feeding from small planktonic to bigger prey such as insects and mollusks, according to this their morphology would have evolved in accordance with their feeding habits (Lauzanne 1982; Parker and Kornfield 1995; Maldonado et al. 2009; Riveros et al. 2012; Guerrero et al. 2015). Along with their extreme physical and chemical characteristics, SWA systems have been fractionated through time and they have been described as each sustaining a small population of specific Orestias (Vila et al. 2010; Arratia et al. 2017). Seven species have been currently described as evolving in allopatry, however their validity has been argued, since morphological and meristic measurements overlap (Parenti 1984a; Vila et al. 2011). As it has been postulated, in allopatry morphological variation would be the result of adaptation to different habitat characteristics (Vila et al. 2010, 2013).
Using geometric morphometric was possible to detect interspecific differences among the studied groups. The group formed by specimens with closed mouth showed differentiation for the majority of the pairs of species, with the exception of O. chungarensis and O. puni. The landmarks that most contributed to differentiation in this group according to the CVA analysis were those related to the operculum (landmarks 10, 4 and 5). In the group with the maximum maxillary protrusion, there were more pairs of these species that could not be differentiated by the PERMANOVA. However, O. gloriae and O. chungarensis differed from the other species and populations being the capacity of protrusion important in the variation according to the CVA analysis (landmarks 11, 12 and 3).
In fact, the feeding of Orestias implies sucking a water flow generated by the operculum (Higham et al. 2006; Parsons and Robinson 2007). There is a compromise between maximizing the incoming water velocity and its volume, and this is reflected in the morphology of the oral structures. The optimum head shape for large and evasive prey should include a large oral region that maximizes the water volume, while for small and non-evasive prey the predator should have a small mouth that allows high frequency rapid movements (Maldonado et al. 2009). Thus, the relative head size, the protrusion capacity and in general the mouth structure should correlate with the diet of a species. This process has been amply described for cichlids; their adaptive radiation in Lake Malawi is one of the most studied and cited examples (Motta 1984; Albertson et al. 2003, 2005). There is still a lack of more detailed studies on SWA Orestias feeding, since there is not a consensus if they are specialists or generalists in feeding, even so, the major mouth protractibility of O. gloriae and O. chungarensis would show coincidence with the feeding of macroinvertebrate benthics such as larvae of aquatic insects, amphipods and mollusks (Vila et al. 2011). Both species live in lacustric habitat (Vila et al. 2006). Besides, springs and running water species such as O. ascotanensis has shown a larger head. This is of interest to mention that the detailed internal morphology description of one southern Orestias led to the recent description of a new genus and species Pseudorestias lirimensis (Arratia et al. 2017).
In this sense, the cluster analysis integrating the results of the closed mouth and maximum protrusion of specimens separated two groups of Orestias, those of the extreme north including O. chungarensis, O. parinacotensis, O. piacotensis and O. laucaensis from Lauca National Park and O. puni of Puno, Peru and O. cf. agassii from Isluga, and a group of more southern species, O. cf. agassii from Huasco, O. gloriae and O. ascotanensis from Carcote and Ascotán. This separation of northern and southern groups is similar to that described by Lüssen et al. (2003), who found a division between Orestias species using molecular tools (mitochondrial markers) and to the results of Scott (2010).
The results of morphologic measurements and geometric explain the particular morphological divergence that has occurred in each of the studied localities. from 17º to 22º S in Chile, where seven species have been described and up to now with some populations still not specifically classified (Scott 2010; Vila et al. 2011, 2013; Arratia et al. 2017), this study has been of importance to corroborate the SWA Orestias species descriptions validity. This is also of present importance in considering that all these species are in danger of extinction according to the country regulations of the Environmental Ministry.
References
Adams DC, Rohlf FJ, Slice DE (2004) Geometric morphometrics: ten years of progress following the ‘revolution.’ Ital J Zool 71(1):5–16
Adams DC, Collyer M, Sherratt E (2015) Geometric morphometric analyses of 2D/3D landmark data
Albertson RC, Streelman JT, Kocher TD (2003) Directional selection has shaped the oral jaws of Lake Malawi cichlid fishes. Proc Natl Acad Sci 100(9):5252–5257
Albertson RC, Streelman JT, Kocher TD, Yelick PC (2005) Integration and evolution of the cichlid mandible: the molecular basis of alternate feeding strategies. Proc Natl Acad Sci USA 102(45):16287–16292
Anderson MJ (2005) Permutational multivariate analysis of variance. Department of Statistics, University of Auckland, Auckland
Arratia G (1982) Peces del Altiplano de Chile. In: Veloso A, Bustos E (eds) El hombre y los ecosistemas de montaña MAB-6. El ambiente natural y las poblaciones humanas de Los Andes del Norte Grande de Chile, Volumen I. La vegetación y los vertebrados inferiores de los pisos altitudinales entre Arica y El Lago Chungará. ROSTLAC, UNESCO, Montevideo, Uruguay, pp 93–133
Arratia G, Vila I, Lam N, Guerrero CJ, Quezada-Romegialli C (2017) Morphological and taxonomic descriptions of a new genus and species of killifishes (Teleostei: Cyprinodontiformes) from the high Andes of northern Chile. Plos One 12(8):e0181989
Bookstein FL (1991) Morphometric tools for landmark data: geometry and biology. Cambridge University Press, Cambridge
Clarke KR, Gorley RN (2006) PRIMER v6: User Manual/Tutorial. PRIMER-E, Plymouth
Cruz-Jofré F, Morales P, Vila I, Esquer-Garrigos Y, Hugueny B, Gaubert P, Poulin E, Méndez M (2016) Geographical isolation and genetic differentiation: the case of Orestias ascotanensis (Teleostei: Cyprinodontidae), an Andean killifish inhabiting a highland salt pan. Biol J Linn Soc 117(4):747–759. https://doi.org/10.1111/bij.12704
Davenport J, Sayer MDJ (1993) Physiological determinants of distribution in fish. J Fish Biol 43:121–145
Douglas ME, Matthews WJ (1992) Does morphology predict ecology? Hypothesis testing within a freshwater stream fish assemblage, Oikos: 213–224
Dryden IL, Mardia KV (2016) Statistical shape analysis: With applications in R, 2nd edn. Wiley, Hoboken
Elliott NG, Haskard K, Koslow JA (1995) Morphometric analysis of orange roughy (Hoplostethus atlanticus) off the continental slope of southern Australia. J Fish Biol 46:202–220
Guerrero CJ, Méndez MA, Vila I (2015) Caracterización trófica de Orestias (Teleostei: Cyprinodontidae) en el Parque Nacional Lauca. Gayana (Concepción) 79(1):18–25
Higham T, Day S, Wainwright P (2006) Multidimensional analysis of suction feeding performance in fishes: Fluid speed, acceleration, strike accuracy and the ingested volume of water. J Exp Biol 209:2713–2725. https://doi.org/10.1242/jeb.02315
Keller B, Soto D (1998) Hydrogeologic influences on the preservation of Orestias ascotanensis (Teleostei: Cyprinodontidae), in Salar de Ascotán, Northern Chile. Rev Chil Hist Nat 71:147–156
Klingenberg CP (2011) MorphoJ: an integrated software package for geometric morphometrics. Mol Ecol Resour 11(2):353–357
Langerhans RB, Layman CA, Langerhans AK, Dewitt TJ (2003) Habitat-associated morphological divergence in two Neotropical fish species. Biol J Lin Soc 80(4):689–698
Lauzanne L (1982) Les Orestias (Pisces, Cyprinodontidae) du Petit lac Titicaca. Rev d’Hydrobiol Trop 15:39–70
Lüssen A, Falk TM, Villwock W (2003) Phylogenetic patterns in populations of Chilean species of the genus Orestias (Teleostei: Cyprinodontidae): results of mitochondrial DNA analysis. Mol Phylogenet Evol 29(1):151–160
Maldonado E, Hubert N, Sagnes P, De Mérona B (2009) Morphology–diet relationships in four killifishes (Teleostei, Cyprinodontidae, Orestias) from Lake Titicaca. J Fish Biol 74(3):502–520
Mann G (1954) Vida de los peces en aguas chilenas. Ministerio de Agricultura. Universidad de Chile. Imprenta y Litografía Stanley. Santiago. Chile, pp 342
Márquez-García M, Vila I, Hinojosa Lf, Méndez MA, Carvajal JL, Sabando MC (2009) Distribution and seasonal fluctuations in the aquatic biodiversity of the southern Altiplano. Limnol Ecol Manag Inland Waters 39(4):314–318
Mitteroecker P, Gunz P (2009) Advances in geometric morphometrics. Evol Biol 36(2):235–247
Motta PJ (1984) Mechanics and functions of jaw protrusion in teleost fishes: a review. Copeia: 1–18
Motta PJ, Kotrschal KM (1992) Correlative, experimental, and comparative evolutionary approaches in ecomorphology. Neth J Zool 42:400–415
Northcote TG (2000) Ecological interactions among an orestiid (Pisces Cyprinodontidae) species flock in the littoral zone of Lake Titicaca. Adv Ecol Res 31:399–420
Parenti LR (1984) A taxonomic revision of the Andean killifish genus Orestias (Cyprinodontiformes, Cyprinodontidae). Bull Am Mus Nat Hist 178:107–214
Parenti LR (1984) Biogeography of the Andean killifish genus Orestias with comments on the species flock concept. In: Echelle AA, Kornfield I (eds) Evol Fish Species Flocks. University of Maine at Orono Press, Orono
Parker A, Kornfield I (1995) Molecular perspective on evolution and zoogeography of Cyprinodontid killifishes (Teleostei; Atherinomorpha). Copeia: 8–21
Parsons KJ, Robinson BW (2006) Replicated evolution of integrated plastic responses during early adaptive divergence. Evolution 60(4):801–813
Parsons KJ, Robinson BW (2007) Foraging performance of diet-induced morphotypes in pumpkinseed sunfish (Lepomis gibbosus) favours resource polymorphism. J Evol Biol 20(2):673–684
Placzek C, Quade J, Patchett J (2006) Geochronology and stratigraphy of late Pleistocene lakes cycles on the southern Bolivian Altiplano: Implications for causes of tropical climate change. Geol Soc Am Bull 118(5/6):515–532
Risacher F, Alonso H, Salazar C (2003a) Hydrochemistry of two adyacent acid saline lakes in the Andes of northern Chile. Chem Geol 187:39–57
Risacher F, Alonso H, Salazar C (2003b) The origin of brines and salts in Chilean salars: a hydrochemical view. Earth Sci Rev 63:249–293
Riveros J, Vila I, Mendez MA (2012) Trophic niche of Orestias agassii (Cuvier and Valenciennes, 1846) in the streams system of salar de Huasco (20 degrees 05’S, 68 degrees 15’W). Gayana 76(2):79–91
Rohlf FJ (1990) Dennis slice, extensions of the procrustes method for the optimal superimposition of landmarks. Syst Biol 39:40–59. https://doi.org/10.2307/2992207
Rohlf FJ (2012) SB Morphometrics. Department of Ecology and Evolution, State University of New York , Stony Brook. http://life.bio.sunysb.edu/morph/
Rohlf FJ, Marcus LF (1993) A revolution morphometrics. Trends Ecol Evol 8(4):129–132
Rondanelli R, Molina A, Falvey M (2015) The Atacama surface solar maximum. Bull 625 Am Meteorl Soc 96:405–418. https://doi.org/10.1175/BAMS-D-13-00175.1
Schaeffer B, Rosen DE (1961) Major adaptive levels in the evolution of the actinopterygian feeding mechanism. Am Zool :187–204
Scott S (2010) Sistemática y filogenia de Orestias del complejo agassizii (Teleostei: Cyprinodontidae) de la Puna [Unpublished doctoral dissertation]. Santiago, Chile: Facultad de Ciencias, Universidad de Chile
Skulason S, Smith TB (1995) Resource polymorphisms in vertebrates. Trends Ecol Evol 10(9):366–370
Slice DE (1998) Morpheus et al.: software for morphometric research. Department of Ecology and Evolution. State University of New York, Stony Brook, New York
Svanbäck R, Eklöv P (2002) Effects of habitat and food resources on morphology and ontogenetic growth trajectories in perch. Oecologia 131(1):61–70
Team RC (2014) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. 2013. http://www.R-project.org
Toro IMV, Manriquez SG, Suazo GI (2010) Morfometría geométrica y el estudio de las formas biológicas: de la morfología descriptiva a la morfología cuantitativa. Int J Morphol 28(4):977–990
Vila I (2006) A new species of killifish in the genus Orestias (Teleostei: Cyprinodontidae) from the southern high Andes, Chile. Copeia 2006(3):472–477
Vila I, Pinto M (1986) A new species of Killifish (Pisces, Cyprinodontidae) from the Chilean Altiplano. Rev d’Hydrobiol Trop 19:233–239
Vila I, Pardo R, Habit E, Dyer B (2006) Peces límnicos: diversidad, origen y estado de conservación. In: Vila I, Veloso A, Schlatter R, Ramirez C (eds) Macrófitas y vertebrados de los sistemas límnicos de Chile. Editorial Universitaria, Santiago
Vila I, Scott S, Lam N, Iturra P, Mendez MA (2010) Karyological and morphological analysis of divergence among species of the killifish genus Orestias (Teleostei: Cyprinodontidae) from the southern Altiplano. Origin and phylogenetic interrelationships of Teleosts, pp 471–480
Vila I, Scott S, Mendez MA, Valenzuela F, Iturra P, Poulin E (2011) Orestias gloriae, a new species of cyprinodontid fish from saltpan spring of the southern high Andes (Teleostei: Cyprinodontidae). Ichthyol Explor Freshwaters 22:345–353
Vila I, Morales P, Scott S, Poulin E, Véliz D, Harrod C, Méndez MA (2013) Phylogenetic and phylogeographic analysis of the genus Orestias (Teleostei: Cyprinodontidae) in the southern Chilean Altiplano: the relevance of ancient and recent divergence processes in speciation. J Fish Biol 82(3):927–943
Wainwright PC (1991) Ecomorphology: experimental functional anatomy for ecological problems. Am Zool 31(4):680–693
Waltzek TB, Wainwright PC (2003) Functional morphology of extreme jaw protrusion in neotropical cichlids. J Morphol 257(1):96–106
Westneat MW (2004) Evolution of levers and linkages in the feeding mechanisms of Fishes1. Integr Comp Biol 44(5):378–389
Westneat MW (2005) Skull biomechanics and suction feeding in fishes. Fish Physiol 23:29–75
Westneat MW, Wainwright PC (1989) Feeding mechanism of Epibulus insidiator (Labridae; Teleostei): evolution of a novel functional system. J Morphol 202(2):129–150
Winemiller KO (1991) Ecomorphological diversification in lowland freshwater fish assemblages from five biotic regions. Ecol Monogr 61(4):343–365
Winemiller KO, Kelso-Winemiller LC, Brenkert AL (1995) Ecomorphological diversification and convergence in fluvial cichlid fishes. In: Ecomorphology of fishes (pp 235–261). Springer Netherlands, Dordrecht
Acknowledgements
I.V.Fondecyt 1140543, S.S. Fondecyt 3130575.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rojas, P., Scott, S., Tobar, I. et al. Head morphometry of Orestias (Cyprinodontiformes). Response to extreme Southern Altiplano systems?. Environ Biol Fish 103, 953–964 (2020). https://doi.org/10.1007/s10641-020-00997-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-020-00997-2