Abstract
As fish move, they incur an energetic cost of transport (COT) from the use of aerobic muscles. Water currents are an integral component of the physical world of fishes, and if water currents are present, fish may pay higher costs fighting the currents, or may use the currents to facilitate movement and reduce COT. Some fish use “selective tidal stream transport” (STST) to move efficiently through tidal regions, swimming into the water column when the current is favorable, and returning to the bottom during opposing tides. This behavior has been reported in marine fish migrating through tidal habitats, but it is also likely of value in daily movements of fish residing in those habitats. It is extremely difficult to directly measure COT in wild fish; however, it is possible to combine analysis of field telemetry data of individual swimming efforts and measurements of metabolic costs of swimming from respirometry in order to estimate the COT in the wild, and to calculate the costs or savings of swimming with or against currents. We describe this novel analytical approach and demonstrate it using data from two green sturgeon tracked in San Francisco Bay, California. In this analysis, when moving at the surface and employing STST, the fish benefited from the current, swimming within 85.5% of optimal efficiency. When conducting non-STST movements near the bottom, swimming was less efficient, with a COT similar to swimming directly into the current. These results suggest that green sturgeon may opportunistically utilize stream transport in daily movements, swimming at the surface and orienting with currents to achieve substantial energetic savings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
All fishes, as they move within their respective habitats, interact with water currents (Webb 1994), particularly in the case of diadromous species that migrate between oceans, estuaries, and rivers (Arnold 1981). These currents may be either an impediment, requiring compensatory orientation and swimming, or an asset, reducing the energetic costs of transport. It is argued that there is strong selection for behavioral traits that maximize the efficiency of movements (Bernatchez and Dodson 1987) because energy not spent on activity is instead available for reproduction and growth (Harden Jones 1980).
Some fishes have been observed to take advantage of cyclical tidally-generated currents when migrating, as in selective tidal stream transport (STST: Greer-Walker et al. 1978; Arnold 1981). In this behavior, fish move up into the water column when the current is moving in a favorable direction, often aligning with and swimming in the direction of the current, using the flow to aid in transport and decrease the costs of travel. When the direction of the tidal current changes, the fish return to rest on the bottom rather than swim inefficiently against the current. Diverse species such as Atlantic cod (Gadus morhua L.) (Arnold et al. 1994), common sole (Solea solea L.) and small-spotted catshark (Scyliorhinus canicular L.)(Greer Walker et al. 1980), American eel (Anguilla rostrata Lesueur)(Parker and McCleave 1997), European eel (Anguilla anguilla L.)(Verhelst et al. 2018), and sockeye salmon (Oncorhynchus nerka Walbaum)(Levy and Cadenhead 1995) employ STST to move efficiently through tidally-dominated habitats.
Swimming into a current is metabolically demanding and adult fishes employing selective tidal stream transport may gain as much as a 40% energetic advantage compared to continuous swimming (Weihs 1978). This behavior is most efficient in areas where the current speed is high relative to the fish’s optimal swimming speed, but is less valuable at lower water speeds where there is less cost associated with fighting currents and less benefit gained by riding them. Further, small fishes are expected to gain the most benefit whereas larger individuals will benefit only if they are capable of closely orienting to and swimming with the current (Weihs 1978).
While both field telemetry studies of movements and laboratory studies of metabolism are common, it is rare that the energetic cost of movements have been calculated for free-swimming fish. To calculate the actual cost of observed movement, one must also account for the influence of currents on the total movement of the fish; however, most studies and energetic models have ignored this factor (Bernatchez and Dodson 1987). To date, only Metcalfe et al. (1990) have estimated the efficiency of this behavior in free-swimming animals. Those authors reported that when European plaice (Pleuronectes platessa (Linnaeus)) migrating in the English Channel swam in the direction of the current, they achieved an energetic saving of approximately 20%.
Previous reports of selective tidal stream transport in fish, including the energetic study of Metcalfe et al. (1990), have focused on adults engaged in spawning migrations; however, utilizing currents to save energy can also be an energy-saving strategy at other times during daily, non-migratory movements within a home range. While engaged in a multifaceted study of the biology of green sturgeon (Acipenser medirostris (Ayres)), we were presented with a unique opportunity to try a novel analytical approach, using a combination of field and laboratory data to examine the influence of currents on the energetic costs of movements in a tidally-complex estuary. This analysis is the first to determine the value of this behavior in the daily, non-migratory movements of a fish.
Green sturgeon are an anadromous, iteroparous fish native to the west coast of North America (Moyle 2002). There are two distinct population segments (DPS), the northern DPS found in the Klamath and Rogue rivers (California and Oregon, respectively) and the southern DPS found only in the Sacramento River watershed (California). The southern DPS was listed as threatened under the United States Endangered Species Act in 2006. The species, which may grow in excess of three meters (Nakamoto et al. 1995), engages in long distance migrations, spawning in their natal rivers at intervals of two to five years (Erickson and Webb 2007). They are considered to be the most oceanic of the acipenserid fishes (Moyle 2002; Erickson and Hightower 2007); however, adults and sub-adults are routinely found in estuaries including San Francisco Bay (Lindley et al. 2011).
Detailed manual tracking of sturgeon movements in the San Francisco Bay Estuary yielded fine-scale spatial and temporal records of fish moving in three dimensions (Kelly et al. 2007). It was noted that when green sturgeon engaged in sustained directional travel, their distribution in the water column was bimodal, either near the surface (49.2% of records were in the upper 20% of the water column) or near the bottom. These results were combined with current vector analysis to determine the swimming behavior of the fish, both over ground and with respect to the movement of the water mass (Kelly and Klimley 2012). In these studies, it was reported that green sturgeon employed selective tidal stream transport during daily, non-migratory movements, moving at the surface in the deeper, high-current areas of the bay, where they oriented to and swam with the current. In contrast, movements near the bottom were usually conducted in shallow water, where currents were minimal, and were not specifically oriented in the direction of the current. The total speed over ground was higher in fish making movements at the surface, but the mean swimming speeds of the fish themselves within the water column were the same, indicating the increased rate of movement in surface swimming was the result of the current. Concurrent with the field study of movements, the metabolic performance characteristics of the species, including standard and active metabolic rates, were measured by flume respirometry as a component of a study of the effects of chronic stress on green sturgeon (Lankford et al. 2005).
Here, we describe an analytical approach to estimating the energetic costs of swimming influence of currents in free-swimming fish, and demonstrate it using data from previous field and laboratory studies. We were able to calculate the metabolic costs of the movement behaviors that were actually observed in the wild by combining the data sets of swimming metabolism and movement with respect to currents. We were also able to estimate the costs of making the same observed movements over ground in tidal currents opposite of those recorded or assuming the fish were aligned optimally to the flow, and presumably gaining the most net benefit. We expected that sturgeon moving near the surface would travel more efficiently than those swimming near the bottom, and that fish engaged in this behavior would be moving optimally, aligning with the current to minimize their energetic expenditure. This is the only attempt known to the authors to calculate the costs and efficiency of movement in a wide-ranging anadromous species that was not migrating during the time of the study.
Materials and methods
Study animals
The fish used in the tracking phase of this study were captured in the San Pablo Bay region of the San Francisco Bay Estuary, CA (Fig. 1a), using trammel nets. Depth-sensing, ultrasonic telemetry tags (Vemco, Ltd., V22) were implanted in the peritoneum of the sturgeon on the boat at the point of capture and the fish immediately released. Fish were followed upon release using a directional hydrophone (Vemco, Ltd., VH110), receiver (Vemco, Ltd., VR60) and GPS (Magellan, NAV5000 GLX) which were interfaced with a laptop computer. See Kelly et al. (2007) for additional details of the tagging and tracking process.
Map of study area showing directional movements of two green sturgeon in the San Francisco Bay Estuary. Circles (green sturgeon A) and triangles (green sturgeon B) indicate the position of the fish at 5-min intervals (black = near bottom, white = near surface). Inset A) location of study site relative to the San Francisco Bay Estuary, CA. Inset B) expanded view of movement during one interval (black box on main figure) between two points (P1, P2) showing total movement vector over ground (black line, t), the current vector (dashed arrow, c), and the calculated swimming vector of the fish (solid arrow, f)
Sturgeon were tracked intensively during the summer and autumn months of 2001 and 2002. During this period, sturgeon were recorded making directional, non-random movements and two individuals, GSA and GSB, were observed moving both at the surface and near the bottom (Fig. 1), permitting direct comparison of these two different behaviors in the same animals. GSA was 101 cm TL, 5.1 kg, and the mean water temperature at swimming depth during the track used in this study was 19.8 °C. GSB was 106 cm TL, 5.9 kg, and the mean water temperature at swimming depth was 16.4 °C.
Fish used in the respirometry phase of the project were age 0+ green sturgeon (61–72 cm TL, N = 12) obtained from artificially spawned, wild-caught brood stock from the Klamath river (Van Eenennaam et al. 2001). Fish were maintained in round, 2-m, outdoor, flow-through tanks supplied with 19 °C well-water under a natural photoperiod (38.6 N, 121.7 W) at the Center for Aquatic Biology and Aquaculture (CABA), University of California, Davis. Fish were fed Silver Cup extruded, non-floating trout chow diet at the rate of 1.0% body weight·d−1 via a 24-h belt feeder.
Movement analysis
Geographic positions of the fish at five-minute intervals were entered into a Geographical Information System (ESRI, ArcMap), and were plotted over 5-m grid bathymetry provided by the California Department of Fish and Wildlife, Information Technology Division. The recorded positions were used to calculate vectors (heading and speed) of the total movement over ground using the great circle method for calculating distances on a sphere (Kelly and Klimley 2012). The total vector includes both the movement attributed to the swimming activity of the fish and the movement derived from the currents in which the fish swam. Current vectors at the location of the fish were estimated using current and tide predictive modeling software (Local Knowledge, Inc., Force 2), and were subtracted from the recorded total movement in the manner described in Kelly and Klimley (2012) to reveal the component of the total movement over ground contributed solely by the swimming activity of the fish (Fig. 1b).
Metabolic measurements
Fish were transferred from holding tanks to a plastic and stainless steel Brett-type swim-tunnel respirometer (ca. 655 l) with a variable-speed motor and propeller. Fish were allowed to acclimate overnight (approximately 16 h) at a velocity of 10 cm·s−1, which promotes quiescence in green sturgeon yet does not require active swimming to maintain position.
The morning of the experiment following the overnight acclimatization, the oxygen partial pressure (PO2) in the respirometer was adjusted to 140–160 mmHg by diffusing oxygen into the water. PO2 was determined in duplicate water samples collected via micro-bore Tygon tubing, stopcock, and glass syringe and analyzed with a blood gas analyzer (Cameron Instruments, BGM 200). Water velocity was increased to 25 cm·s−1 for 20 min and then increased to 35 cm·s−1 over 10 min to induce steady swimming. The respirometer was then sealed and duplicate initial PO2 samples were collected. Final PO2 samples were collected after 45 min, and the respirometer was unsealed and flushed for 15 min, resulting in a 60-min velocity interval. The velocity was rapidly increased by 5 cm·s−1 increments and the protocol was repeated until the fish became exhausted, impinging three times on the posterior screen. The metabolic rate (MO2) for each swimming speed interval was calculated by converting PO2 into oxygen content (CO2) with a nomogram (Green and Carritt 1967) and using the equation in Cech (1990) for closed respirometers. Bacterial respiration was not accounted for, as 60-min “blank” runs resulted in no PO2 decreases and the entire respirometer was bleached weekly. See (Lankford et al. 2005) for additional details of the respirometry.
Cost of transport
The energetic expense of moving or cost of transport (COT), measured in oxygen consumed per unit weight per unit distance (mg O2·kg−1·km−1; Metcalfe et al. 1990) or units of energy per unit weight per unit distance (kJ·kg−1·km−1; Videler 1993), can be used as a measure of swimming efficiency.
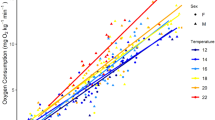
The regression equation derived from the relationship of the oxygen consumption rate (MO2: mg O2·kg−1·min−1) to swimming speed (body lengths [BL]·s−1), measured by swimming respirometry, was used to estimate the MO2 of the fish at the speed it was swimming during each 5-min track interval. The minimum MO2 was set at 3.17 mg O2·kg−1·min−1, the standard metabolic rate reported for green sturgeon by Lankford et al. (2005). The maximum MO2 was set at the rate measured at the maximum speed at which fish could be induced to swim in the respirometer.
COT was calculated by first multiplying the MO2 for each movement interval by the duration of that interval to calculate the oxygen consumed by the fish per unit weight (mg O2·kg−1) over that period, then assuming a value of 27 J·mg O2−1 (Metcalfe et al. 1990). The COT values for each track segment were summed and divided by the total distance moved over ground to calculate the cost of the movement for each fish (COTF). The hypothetical cost to move during an opposite tide (COTR) was estimated by reversing the actual current headings 180o and calculating the swimming vector of the fish that would be necessary to achieve the observed movement. The cost of optimally efficient movement (COTO) was estimated by calculating the fish vector when the current headings were normalized to the total movement heading, thus assuming the fish was perfectly aligned with the flow.
Results
When swimming near the bottom, GSA traveled over ground at an total mean rate of 0.64 m·s−1. The fish was oriented into the flow, maintaining a mean orientation of 217o with respect to the southwesterly current (a fish swimming directly into the current would be oriented 180o with respect to the current). During this time, the fish was swimming through the water at a mean speed of 0.63 m·s−1, which was flowing at 0.23 m·s−1. In contrast, when swimming near the surface, GSA followed the flow, deviating only 28o with respect to the direction of the current which was flowing to the east at a mean speed 0.53 m·s−1. During this time, the fish moved faster over ground (mean speed = 0.83 m·s−1) though it was swimming more slowly (mean speed = 0.51 m·s−1).
Similarly, GSB moved near the bottom at a total mean rate of movement over ground of 0.70 m·s−1 while swimming 0.84 m·s−1. The fish maintained a heading of 126o relative to the current that was flowing east at a mean speed 0.44 m·s−1. As with the first sturgeon, when at the surface, GSB moved more swiftly over ground (mean speed = 0.94 m·s−1) though it swam slower (mean speed = 0.45 m·s−1) and oriented in the direction of the easterly current (mean orientation to flow = 49o), which was flowing at a mean speed 0.73 m·s−1.
Based on results measured in the laboratory swimming respirometry trials, the relationship of the MO2 to swimming speed (BL·s−1) in green sturgeon is best described by the linear equation y = 15.244x − 2.232 (N = 12, R2 = 0.591) (Fig. 2). We used this equation to calculate the MO2 of the fish at the speed it was swimming during each 5-min track interval. The minimum MO2 was set at 3.17 mg O2·kg−1·min−1, the standard metabolic rate reported for green sturgeon by Lankford et al. (2005). The maximum sustained swimming speed observed in the respirometer was 0.90 BL·s−1, so maximum MO2 was set at 11.49 mg O2·kg−1·min−1.
In both fish, the mean metabolic rate during surface movements was lower than when moving at the bottom, though the disparity was considerably larger for GSB (Table 1). The mean COTF was also lower in both fish when swimming at the surface compared to swimming near the bottom (Fig. 3) with movements conducted at the surface costing 45.1% (GSA) and 35.7% (GSB) of movements at the bottom. The movements of sturgeon using selective tidal stream transport at the surface cost between 2.35–3.98 kJ·kg−1·km−1, substantially less than the costs of benthic movement (6.57–8.82 kJ·kg−1·km−1). If the observed movements had been conducted when the tides were reversed in direction, surface movements would have cost an additional 66.5% (GSA) or 156.1% (GSB), while benthic movements would have cost 17.4% (GSA) or − 9.1% (GSB). Had the fish aligned perfectly to the direction of the current, the savings in energy expended would have been 14.5% (GSA) and 21.0% (GSB) for surface movements and 8.4% (GSA) and 51.4% (GSB) for benthic movements.
Cost of transport (kJ·kg−1·km−1) for green sturgeon during surface- and benthic-oriented swimming. White bars represent the cost of the total movement over ground assuming no currents are present, black bars represent actual recorded movements of fish, gray bars represent optimally efficient swimming if fish are oriented perfectly with the current, and crosshatched bars represent when current direction is reversed
Discussion
Water currents can have a substantial influence on the cost of movement for aquatic organisms, and fish that can selectively use currents to their advantage can realize significant energetic savings. In this study, the predicted cost of transport if fish had moved during opposite tides was high, within 17.4% of the costs of the actual benthic movements, but greater than double the cost of observed surface movements. This represents a possible savings of 20.2–50.3% for green sturgeon employing selective tidal stream transport at the surface. For comparison, plaice exhibiting selective tidal stream transport were estimated to save approximately 20% of the cost of transport (Metcalfe et al. 1990). The performance of green sturgeon reported here matches or exceeds the efficiency predicted by Weihs (1978), who calculated that adult fishes could save approximately 40% of the cost of moving continuously if they employed selective tidal stream transport. However, that author assumed fish were engaged in a lengthy migration over multiple tidal cycles – as was also the case with plaice (Metcalfe et al. 1990) – and were thus also accruing the cost of standard metabolism while waiting between favorable tides. The fish in this study were making shorter movements during single tides, and thus may have been able to achieve greater energetic savings.
Theoretical estimates of the savings benefits of selective tidal stream transport by Weihs (1978) assumed that fish were moving at an optimally efficient swimming speed, which he estimated at approximately 1 BL·s−1 (Weihs 1973). Green sturgeon were calculated in this study to be swimming at 0.45–0.84 BL·s−1 and we were unable to induce fish in the flume to swim continuously in excess of 0.90 BL·s−1. Fish will most likely move at optimum speeds during routine movement (Videler 1993), and it is assumed that the optimum speed should occur at approximately the point at which active metabolic rate is double the standard metabolic rate (Webb 1994). Based on our metabolic measurements, this point occurs in green sturgeon at 0.56 BL·s−1, which corresponds with the observed swimming speeds in the wild. This suggests that sturgeon may be less efficient swimmers than the salmonids postulated by Weihs (1978) and would have more to gain from utilizing currents in their daily movements.
Differences in responses to currents between salmonids and sturgeon have implications for the design of volitional fish passage structures. Fish passage criteria often focus primarily on salmonids and other more surface oriented swimmers (e.g. USFWS 2019); however, sturgeon are likely to use the water column and react to water currents in a different way. Based on our observations, one might expect that fish moving with the current, including out-migrating post-spawn adults, may move near the surface, selecting high flows to aid in transport. Conversely, fish moving against a current, including adults migrating up-river to spawn, are likely to move near the bottom in order to minimize energetic expenditures by taking advantage of the reduced water velocities in the benthic boundary layer. Further analysis of these behaviors could inform engineers when making design decisions such as determining attracting flows, fishway orifice shape and location, and substrate roughness.
The predicted costs of optimum movement were lower in all cases than what was actually observed, but were within 79% of the costs of the recorded surface movements, suggesting green sturgeon are benefiting from closely orienting in the direction of flow while at the surface. This degree of orientation was also reported in plaice, who achieved an average of 88% of optimum efficiency in their movements (Metcalfe et al. 1990). In contrast, the efficiency of benthic movements varied. The overall costs of benthic movement for GSA would have been high in all instances, even if the fish had been optimally swimming in the direction of the currents, suggesting that at the slower current speeds experienced by this fish, there was less benefit to orienting to current. This corresponds to Weihs’ (1978) argument that there is a threshold current velocity relative to the length of the fish, at which selective tidal stream transport does not represent a significant savings and it is more efficient to move without regard for current. The costs of movement for GSB were very high during benthic swimming, essentially the same as if the fish had moved on the opposite tide, and twice the cost of moving optimally with the current. During this time, the fish traveled roughly perpendicularly to the direction of the prevailing currents. Since this fish moved in this direction at this time, despite the energetic savings it could have realized at a different time or direction, it must be assumed that movement motivations are based on more factors than simple energetic efficiency.
Green sturgeon often do swim at the bottom, unaligned to the current (Kelly and Klimley 2012). This tactic may not incur much additional energetic cost in areas of slower currents, or may be the unavoidable consequence of other motivations (e.g. foraging, directional travel). When possible though, particularly in areas of high flow, fish stand to gain sizeable energy savings by employing selective tidal stream transport, and our results suggest that green sturgeon do indeed use this strategy to move in an energy-efficient manner in non-migratory movements. We are aware of only one other report of a fish using this behavior in daily, non-migratory movements. American eels were observed to use tides to make round-trip movements in an estuary (Parker and McCleave 1997), though the eels were thought to be carried passively by the current rather than actively orient with it, and energetic estimates were not reported.
While selective tidal stream transport has been explored in some detail in migratory behavior, little attention to date has been focused on its role in daily movements. Yet behavioral strategies that maximize energy efficiency are likely to confer strong fitness benefits since that energy can instead be directed towards other critical functions (Bernatchez and Dodson 1987), and there is no reason to assume this is only true during migratory periods. It seems reasonable that fishes, particularly wide-ranging yet less-efficient swimmers inhabiting tidally-complex habitats such as green sturgeon, may utilize selective tidal stream transport for energetic gain. Unfortunately, determining the metabolic expenditures of free-swimming fishes and ascertaining the positive and negative influence of currents on those costs presents a substantial challenge. We acknowledge that the data presented here are a small snapshot of the behavior of two individuals from one such species, made possible by the serendipitous co-occurrence of studies involving both high resolution telemetry in the field and swimming performance in the laboratory. Based on our observations, we believe this subject merits additional exploration, and we hope that the approach presented here can be adapted to examine this behavior in detail in other species. Further, as telemetry, remote sensing, and swim respirometry technologies continue to improve and become more accessible, focused studies of this kind become more feasible. High resolution movement data from manual tracking or three-dimensional telemetry arrays, combined with in situ current measurements via acoustic doppler current profilers or high resolution tidal models, coupled with lab respirometry present exciting opportunities to examine these behaviors in detail. Of particular interest may be comparative studies that explore the differences in movement strategies between functional groups such as pelagic and demersal fishes, or migratory and non-migratory species. Additionally, since metabolic rate is influenced heavily by temperature, understanding factors that alter the cost of movement may have significant utility as we attempt to predict the impact of increasing water temperatures due to changing weather patterns.
We encourage further studies of this kind in order to understand the scope and importance of selective tidal stream transport in the energy budget of fishes. Examining how, when, and why species move in complex current environments will both expand our understanding of the basic ecology and physiology of fishes, and help inform managers as they grapple with decisions about conservation and restoration, regional water management, and responses to climate change.
References
Arnold GP (1981) Movements of fish in relation to water currents. In: Aidler DJ (ed) Animal migration. Cambridge University Press, Cambridge, pp 55–79
Arnold GP, Greer-Walker M, Emerson LS, Holford BH (1994) Movements of cod (Gadus morhua L.) in relation to the tidal streams in the southern North Sea. ICES J Mar Sci 51:207–232
Bernatchez L, Dodson JJ (1987) Relationship between bioenergetics and behavior in anadromous fish migrations. Can J Fish Aquat Sci 44:399–407
Cech JJ (1990) Respirometry. In: Schreck CB, Moyle PB (eds) Methods for fish biology. American Fisheries Society, Bethesda, pp 335–362
Erickson DL, Hightower JE (2007) Oceanic distribution and behavior of green sturgeon. Am Fish S S 56:197–211
Erickson DL, Webb MAH (2007) Spawning periodicity, spawning migration, and size at maturity of green sturgeon, Acipenser medirostris, in the Rogue River, Oregon. Environ Biol Fish 79:255–268
Green EJ, Carritt DE (1967) New tables for oxygen saturation of seawater. J Mar Res 25:140–147
Greer Walker M, Riley JD, Emerson L (1980) On the movements of sole (Solea solea) and dogfish (Scyliorhinus canicula) tracked off the east Anglian coast. Neth J Sea Res 14:66–77
Greer-Walker M, Jones FRH, Arnold GP (1978) The movements of plaice (Pleuronectes platessa L.) tracked in the open sea. ICES J Mar Sci 38:58–86
Harden Jones FR (1980) The nekton: production and migration patterns. In: Barnes RK, Mann KH (eds) Fundamentals of aquatic ecosystems. Blackwell Scientific Publications, Oxford, pp 119–142
Kelly JT, Klimley AP (2012) Relating the swimming movements of green sturgeon to the movement of water currents. Environ Biol Fish 93:151–167
Kelly JT, Klimley AP, Crocker CE (2007) Movements of green sturgeon, Acipenser medirostris, in the San Francisco Bay estuary, California. Environ Biol Fish 79:281–295
Lankford SE, Adams TE, Miller RA, Cech JJ Jr (2005) The cost of chronic stress: impacts of a nonhabituating stress response on metabolic variables and swimming performance in sturgeon. Physiol Biochem Zool 78:599–609
Levy DA, Cadenhead AD (1995) Selective tidal stream transport of adult sockeye salmon (Oncorhynchus nerka) in the Fraser River estuary. Can J Fish Aquat Sci 52:1–12
Lindley ST, Erickson DL, Moser ML, Williams G, Langness OP, McCovey BW Jr, Belchik M, Vogel D, Pinnix W, Kelly JT, Heublein JC, Klimley AP (2011) Electronic tagging of green sturgeon reveals population structure and movement among estuaries. T Am Fish Soc 140:108–122
Metcalfe J, Arnold G, Webb P (1990) The energetics of migration by selective tidal stream transport: an analysis for plaice tracked in the southern North Sea. J Mar Biol Assoc UK 70:149–162
Moyle PB (2002) Inland fishes of California. University of California Press, Berkeley
Nakamoto RJ, Kisanuki TT, Goldsmith GH (1995) Age and growth of Klamath River green sturgeon (Acipenser medirostris). U.S. Fish and Wildlife Service Report 93-FP-13, Yreka, CA, USA
Parker SJ, McCleave JD (1997) Selective tidal stream transport by American eels during homing movements and estuarine migration. J Mar Biol Assoc UK 77:871–889
USFWS (U.S. Fish and Wildlife Service) (2019) Fish passage engineering design criteria. USFWS, Northeast Region R5, Hadley, Massachusetts
Van Eenennaam JP, Webb MA, Deng X, Doroshov SI, Mayfield RB, Cech JJ Jr, Hillemeier DC, Willson TE (2001) Artificial spawning and larval rearing of Klamath River green sturgeon. T Am Fish Soc 130:159–165
Verhelst P, Bruneel S, Reubens J, Coeck J, Goethals P, Oldoni D, Moens T, Mouton A (2018) Selective tidal stream transport in silver European eel (Anguilla anguilla L.) – migration behaviour in a dynamic estuary. Estuar Coast Shelf Sci 213:260–268
Videler JJ (1993) Fish swimming fish and fisheries series 10. Chapman & Hall, London
Webb P (1994) Exercise performance of fish. Adv Vet Sci Comp Med 38:1
Weihs D (1973) Optimal fish cruising speed. Nature 245:48–50
Weihs D (1978) Tidal stream transport as an efficient method for migration. J Conseil 38:92–99
Acknowledgements
GIS bathymetry data were provided by W. Patterson, California Department of Fish and Wildlife (CDFW). The authors are indebted to C. Crocker for support and assistance during the field phase of this study, and to R. Kihslinger, N. Kogut, R. Schaffter, D. Kohlhorst, M. Silva for their help. Fish used in this study were generously provided by the Yurok Tribe (brood stock) and CDFW Bay Delta Region (telemetry).
Funding
This research was funded by grants from the U.S. Fish and Wildlife Service Anadromous Fish Restoration Program (to JJC Jr., Grant # 113321G005) and CALFED Ecosystem Restoration Program (to APK, Grant # ERP-02D-P57).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All fish were handled in full compliance with University of California, Davis, Institutional Animal Care and Use Committee protocols.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kelly, J.T., Lankford, S.E., Cech, J.J. et al. Estimating the energetic savings for green sturgeon moving by selective tidal stream transport. Environ Biol Fish 103, 455–463 (2020). https://doi.org/10.1007/s10641-020-00969-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-020-00969-6