Abstract
Herbivorous damselfish that cultivate algal farms frequently exhibit aggressive territorial behaviour to defend their investment from intruders, particularly against other herbivorous fish and invertebrates. On a coastal coral reef in New Caledonia, the response of the damselfish Hemiglyphidodon plagiometopon to intruders of varying type and level of threat to their algal farm was tested. Responses to live and dead coral fragments, carnivorous whelks, shells, and rocks varied from no response to biting and extrusion from farms. Damselfish elicited the strongest defensive response to the herbivorous sea urchin Echinometra mathaei, rapidly attacking the urchin by biting and lifting it from farms, before extruding it up to 3.5 m away. H. plagiometopon responded in a similar manner to dead urchins (no threat of herbivory) as to live urchins, but typically did not extrude them as far. Ultimately, damselfish responded to intruders in a manner largely consistent with the level of threat posed to their algal farm, with the similar response between live and dead urchins suggesting such strong defensive behaviours may be combination of ‘hard-wired’ and learned behaviours in reaction to the level of realised threat.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many animal species modify habitats through their behaviours and interactions, sometimes driving wholesale changes that have lasting consequences to habitat structure and functioning. Familiar examples include sea urchins that over-graze diverse kelp forests to create and maintain relatively unproductive coralline barrens (Steneck et al. 2002; Ling 2008), beavers that topple riverbank trees to dam rivers and create lakes (Naiman et al. 1988), and humans, who have extensively modified habitats in almost all biomes on Earth (Vitousek et al. 1997; Jackson 2001; Wohl et al. 2017). Sometimes cited as keystone species (Power et al. 1996) due to the far-reaching consequences of their actions for the broader community, such organisms often modify habitats directly (e.g., the creation of beaver dams), but may also indirectly facilitate habitat change by modifying behaviours in other species (e.g., reducing herbivory; Irving and Witman 2009).
Damselfish (Family Pomacentridae) are a common feature of coastal fish communities on tropical and temperate shores around the world (Frederich and Parmentier 2016). Many of the approximately 385 species are small, rocky/coral reef-dwelling fish that can locally modify habitats due to a combination of their own manipulation of the substratum, as well as their often aggressive defensive behaviour toward other species (Ceccarelli et al. 2001). Many herbivorous damselfish modify the primary rock or coral habitat by cultivating and maintaining discrete ‘farms’ of filamentous algae, akin to a marine turf or lawn (Lassuy 1980; Hata and Kato 2002; Hata and Ceccarelli 2016). Farms, and the small benthic invertebrates they contain, are typically the primary source of food for the damselfish (Wilson and Bellwood 1997; Hata and Umezawa 2011), may be used to attract mates and protect eggs (Navarrete-Fernandez et al. 2014), and have been observed to occupy up to 70% of the reef area (Ferreira et al. 1998; Ceccarelli 2007). As such, damselfish may present as a key determinant of reef habitat structure, capable of killing corals to increase the amount of suitable substrata for their farms (Wellington 1982), but also indirectly facilitating local coral establishment through aggression toward corallivores (Kamath et al. 2019).
Given the investment damselfish typically put into their farms, they are often vigorously defended from other herbivorous fish (including conspecifics) and invertebrates through aggressive territorial behaviours (Ceccarelli et al. 2001; Jan et al. 2003; Hattori and Shibuno 2013). Intruding fish are typically chased away from the local area by being charged at and/or bitten (Hourigan 1986), while invertebrates that encroach into farms are bitten, dragged out and/or picked up and swum away from the farm (Sammarco and Williams 1982; Glynn and Colgan 1988; Irving and Witman 2009). While such territorial behaviours of damselfish have been well documented (see review in Hata and Ceccarelli 2016), there is limited evidence showing how the type of response may be broadly intruder-specific (e.g. fish vs invertebrate), as well as species-specific (e.g. among species of sea urchin), eliciting responses ranging from no reaction to rapid and vigorous defence/removal (Jan et al. 2003; Irving and Witman 2009). One general explanation for such variation is that damselfish modify their response according to the level of perceived threat, with the most vigorous and aggressive responses most likely to be associated with intruders recognised as the most direct threat. While this hypothesis is largely untested, there is evidence that damselfish aggression is greater between conspecifics vs heterospecifics on coral reefs, with conspecifics likely to represent a greater threat to farms due to similarities in diet, farming behaviour, and territory requirements (Eurich et al. 2018; Robles et al. 2018).
On a coastal coral reef in Noumea, New Caledonia, the response of territorial damselfish to a variety of intruders was tested. Intruders represented varying levels of grazing threat to algal farms, ranging from no threat (e.g. rocks, coral fragments) to an explicit threat (e.g. live sea urchins). Damselfish response to each intruder was measured as i) the time taken for the damselfish to bite the intruder, ii) the number of bites given to the intruder, iii) whether the intruder was extruded from the farm (and how), and iv) the distance that the intruder was extruded. Intrusion by sea urchins was predicted to elicit the strongest aggressive response from damselfish given they are capable of rapidly over-grazing farms and are known to be forcibly removed by damselfish (Sammarco and Williams 1982), sometimes even cooperatively with neighbours (Irving and Witman 2009). While this response highlights that damselfish can perceive a significant threat from urchins, it was also tested whether damselfish were responding to a perceived or realised threat of sea urchins by comparing their response to dead urchin tests (no realised threat of herbivory) with that of live urchins.
Materials and methods
Study site and species description
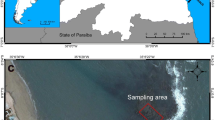
All research was done on a shallow (~ 4 m deep) coastal coral reef in Anse Vata, Noumea, New Caledonia (Fig. 1, 22° 18′ 26.70″ S, 166° 26′ 49.87″ E). The reef extends ~ 50 m out to sea, comprising a mix of predominantly branching staghorn coral (Acropora spp.), millepora spp., and large Porites spp. corals at the fore-reef, which give way to sand and sparse seagrass meadows. A wide diversity of herbivorous and carnivorous fish occupy the reef, including the territorial damselfish Hemiglyphidodon plagiometopon, which grows to ~ 20 cm and is known to actively cultivate and defend farms of filamentous algae from conspecifics and other intruders (Wilson and Bellwood 1997; Lassuy 1980; Hattori and Shibuno 2013).
The most abundant herbivorous invertebrate observed on the reef was the sea urchin Echinometra mathaei, which grows to a test diameter of ~ 5 cm and grazes on filamentous algae (Mills et al. 2000). While E. mathaei often creates a burrow for protection by using its spines and teeth to bioerode coral and rock (Mokady et al. 1996), urchins at the study site were instead mostly found sheltering among dead coral fragments collecting on the reef floor. E. mathaei typically forages at night to avoid predation by carnivorous fish (Mills et al. 2000; Young and Bellwood 2011), yet the presence of empty urchin tests, typically only broken on the oral surface where spines are small and the urchin is more readily accessible, suggests fish predators are a local threat for exposed urchins at the study site (see Dee et al. 2012). Herbivorous gastropods were not observed at the study site despite intensive searching, with carnivorous whelks observed only rarely.
Habitat sampling and species abundance
The abundance of H. plagiometopon farms was quantified on replicate 10 × 1 m belt transects (n = 8) oriented perpendicular to the shore and between 1 and 3 m depth. For each transect, a tape measure was laid over the reef and the number of individual farms encountered within 0.5 m either side of the tape was counted. Concurrently, the number of H. plagiometopon individuals was counted along each transect, up to 1 m in height above the reef substratum given their propensity for swimming close to the reef structure. To estimate the size of farms, 20 were haphazardly selected across the reef area and measured across their longest dimension. It was observed that the majority of farms were approximately circular, so the area of each farm was estimated as πr2.
The densities of E. mathaei sea urchins and gastropods were estimated by counting individuals within 1 m2 quadrats (n = 15) placed haphazardly on the reef. Coral rubble and corals with a branching architecture (e.g. Acropora, Millepora) were targeted since these habitats appeared to support the greatest densities, presumably due to more hiding spaces among branches and therefore greater protection from fish predators. Targeted sampling in this manner may over-estimate urchin abundance on a per-reef-area basis, but this may be countered somewhat by the cryptic day-time behaviour of sea urchins during the sampling period.
Experimental tests
The response of H. plagiometopon to intruders was tested by placing rocks, bivalve shells, dead coral fragments, live coral fragments, predatory whelks, live E. mathaei, and dead E. mathaei tests (an almost-whole urchin test presumably left over from a fish predation event) into isolated algal farms maintained by a single adult H. plagiometopon. While rocks, shells, and coral fragments could not be considered functionally as intruders that deliberately choose to occupy algal farms, they were conceptually considered intruders for this study as a reference to any object not usually found within an algal farm. For each replicate trial (n = 10 per intruder type), a single intruder was placed into the centre of a haphazardly selected farm with the ensuing behaviour of the resident H. plagiometopon observed for a maximum of 60 s (preliminary observations suggested aggressive responses would occur within this time frame, if at all). While H. plagiometopon often retreated among the coral as the intruder was placed by the diver, they usually immediately returned to the farm once the diver retreated a metre or two. If the damselfish did not immediately return, the trial was abandoned and a new trial begun elsewhere. Individual farms/H. plagiometopon were only ever tested once to avoid biasing results (e.g. learned behaviours), and effort was made to use intruders of similar dimensions (approximately 4–5 cm across the longest axis) to minimise possible size-related influences on the damselfish response.
The time taken for H. plagiometopon to respond to the intruder was quantified as the duration between placing the intruder into a farm and the first bite of the intruder by the damselfish. The number of additional bites, if any, and whether the intruder was extruded by H. plagiometopon during the 60 s trial was subsequently recorded. Additionally, the method of extrusion (nudging and dragging vs picking up and swimming away) and the resulting distance of extrusion from the edge of the farm was recorded. Differences in the response of H. plagiometopon to this suite of intruders were analysed using one-way ANOVA, with post-hoc Student-Newman-Keuls tests used to identify the hierarchy of differences among intruder types where main effects were detected.
Results
Sampling of reef habitats revealed that there were 8.00 ± 1.21 (mean ± SE) H. plagiometopon algal farms per 10 m2 of reef (i.e. per transect). Each farm appeared to be maintained by a single H. plagiometopon, with the counted abundance of H. plagiometopon averaging 10.38 ± 1.48 per transect, highlighting that some individuals were not clearly associated with algal farms. Farms averaged 60.95 ± 3.06 cm across their longest axis, and being approximately circular, gives an average farm area of 3057.20 ± 292.64 cm2 per 10 m2 of reef (~ 3% of reef area).
The urchin E. mathaei was sparsely distributed, averaging 1.00 ± 0.40 individuals per m2. Given their cryptic day-time behaviour, however, they are likely more prevalent than what was observed and counted. Gastropods were very rare, averaging 0.13 ± 0.09 individuals per m2 and comprising only carnivorous whelks (surprisingly, no herbivorous gastropods were ever observed).
Experimentally adding intruders into H. plagiometopon farms resulted in clear behavioural responses dependent on the identity of the intruder. The intrusion of either live or dead urchins resulted in the shortest response times from H. plagiometopon (average time to first bite = 2.9–3.2 s), followed by dead and live coral fragments (15.4–17.8 s), and lastly rocks, shells, and whelks (42.2–54.7 s) (Fig. 2a, Table 1a). In contrast, the number of bites upon an intruder until extrusion or within the 60 s observation period (if extrusion did not occur) was statistically similar among all intruders, generally averaging 2–4 bites (Fig. 2b, Table 1b). Based on the rank magnitude of the number of bites, rocks were bitten on average more than other intruders, but this was highly variable among trials and may also reflect that rocks were never extruded from farms, thus allowing more opportunity for H. plagiometopon to bite during the 60 s trial.
Response of damselfish (Hemiglyphidodon plagiometopon) to different intruders into their algal farms, including the herbivorous sea urchin Echinometra mathaei. Data show the mean ± SE a) response time to each intruder (time to first bite), and b) the number of bites of the intruder over a 60 s observation period. Letters above bars indicate statistical similarities/differences in responses based on SNK tests
Among all types of intruders, rocks and whelks were never observed to be extruded from farms, and shells only extruded once, whereas live and dead urchins were always extruded (Fig. 3). The rapid extrusion of urchins was consistently characterized by H. plagiometopon biting onto an urchin spine and lifting the entire urchin off the farm before vigorously swimming it away, temporarily abandoning the farm in the process. Live and dead coral fragments were extruded in 90% of trials, most often by H. plagiometopon nudging and dragging the fragment out of the farm but occasionally by being lifted and swum away (Fig. 3).
Where extrusion occurred, there was a clear pattern in the distance that intruders were removed from farms. Urchins were extruded the furthest distance of all intruders, with live urchins swum further away than dead urchins (mean distance = 2.15 ± 0.25 m vs 0.90 ± 0.22 m; Fig. 4, Table 1c). Live and dead coral fragments were only extruded by 0.13 ± 0.03 m, on average, by predominantly being nudged and dragged to the edge of the farm, while rocks, shells and whelks were rarely or never extruded.
Discussion
Damselfish that farm algal turfs are a common feature of tropical coral reefs and temperate rocky coasts, often defending their investment with vigour (Ceccarelli et al. 2001; Jan et al. 2003). On a New Caledonian coral reef, the damselfish Hemiglyphidodon plagiometopon defended its farm most actively against intruding sea urchins Echinometra mathaei, moderately against live and dead coral fragments, and least against rocks, shells and whelks. This strong reaction of damselfish to urchins is consistent with previous studies (Sammarco and Williams 1982; Irving and Witman 2009), and might be expected given the fervour with which urchins can consume algae (Chapman and Johnson 1990; Ling 2008) and thus threaten damselfish farms.
H. plagiometopon not only responded strongly to the presence of E. mathaei urchins through biting, but also rapidly extruded them by biting on to a spine, lifting the entire urchin off the farm, and then swimming it up to 3.5 m away before dropping it and rapidly returning to their farm. In contrast, live and dead coral fragments were dragged or nudged to the edge of the farm (approximately 0.10 m away) and were only occasionally swum further away. Robustly transporting urchins away from farms suggests they are perceived as a significant threat, especially as damselfish are prepared to temporarily abandon their closely-guarded farm as part of the extrusion process. Indeed, in approximately half of all live urchin trials, urchins were swum past the reef edge and deposited over bare sand, which likely represents an environment with greatly enhanced predation risk for an otherwise cryptic urchin that hides among coral branches and rubble (Young and Bellwood 2011). In doing so, it is possible that H. plagiometopon are not only removing the immediate threat to their farm, but also reducing the future risk of E. mathaei by increasing their exposure to predators. Previous studies have noted a different type of antagonistic response to urchins, whereby spines are successively broken over the urchin’s test until the urchin decides to leave the damselfish farm (Sammarco and Williams 1982). This type of territorial behaviour was not observed in this study, nor was cooperative removal of urchins by damselfish (Irving and Witman 2009).
Notably, H. plagiometopon responded to dead urchins in an almost identical way as to live urchins, suggesting that it is the perceived threat of the urchin to the algal farm that elicits such rapid and aggressive defensive behaviour in H. plagiometopon, rather than the realised threat of actual consumption of algae. Compared to live urchins, however, dead urchins were not swum as far away from the farm (less than half the distance). The reason for this difference is unknown, but one possible explanation is that H. plagiometopon are able to discern a dead urchin from a live one upon lifting it from the farm and recognising a difference in weight and associated ease of swimming with the urchin. The visual cue of an urchin shape, live or dead, is likely responsible for eliciting the primary defensive response from damselfish (Thresher 1976; Siebeck et al. 2009), but subsequent signals (e.g. weight of the urchin) may be important to determine continued defensive responses, such as swimming the intruder away, as opposed to renewed farm patrolling and maintenance.
H. plagiometopon rarely or never extruded rocks, shells, or whelks in this study, indicating little to no perceived threat from them. These objects were often investigated through multiple bites (Fig. 2b), but extrusion only occurred once (for a shell) in a total of 30 combined trials. When compared to a typical urchin shape, such intruders likely present very different visual cues to a damselfish, and may be tolerated inside farms because they may ultimately provide more surface area for farming algae (although whelks are likely transient). If so, the question must then be asked why H. plagiometopon wouldn’t deliberately add such structures to a farm to enhance their investment. The general absence of such structures in farms observed across the study site suggest that these ‘tolerated’ intruders are either dislodged by wave action, remove themselves (i.e. for whelks), or are eventually extruded (i.e. beyond the 60 s observation period used here). If the latter, it would suggest such intruders are not seen as an immediate threat like urchins are, but are still something undesirable inside farms (e.g., rocks rolling around because of wave action may still crush and abrade algae within farms). Live and dead coral fragments were often extruded, but it is presently difficult to ascertain what threat H. plagiometopon perceived from them. While potentially able to also abrade algae due to rolling from wave action, coral fragments may also present a physical barrier that reduces farming efficiency, especially if that barrier might also provide a sting (live coral).
While efforts were made to keep the overall size of intruders similar among all trials, there were clearly broad differences in the overall shape among types of intruders. As such, an alternative explanation for the observed responses of H. plagiometopon, particularly the rapid response to urchins, is that urchins have numerous spines that may provide far more leverage points for extrusion than the other intruders tested. Previous research has shown that urchins with relatively blunt and thick spines (e.g., pencil urchins) are handled much faster by damselfish than urchins with sharp spines (Irving and Witman 2009). This would suggest that E. mathaei urchins, which possess many sharp and rigid spines, would be handled slower than intruders such as rocks and coral fragments (blunt ‘spines’) if extrusion was primarily a function of handling and leverage. In contrast, the rapid, consistent, and amplified response (e.g. swimming urchins up to 3.5 m away) of H. plagiometopon to urchins lends greater weight to the model that E. mathaei urchins are perceived as a critical threat to H. plagiometopon farms, at least above the other types of intruders tested.
In conclusion, the damselfish H. plagiometopon responded to intruders into its algal farm in a manner largely consistent with the threat posed. Herbivorous E. mathaei urchins were rapidly extruded, while inactive intruders posing no direct threat to algal farms (rocks and shells) were rarely extruded. Notably, the response to dead urchins was initially similar to live urchins in terms of response time, but the extrusion distance was approximately 42% of that measured for live urchins. These results suggest that H. plagiometopon initially responds to the perceived threat of urchins in general, perhaps as a ‘hard-wired’ behavioural response (also see Sammarco and Williams 1982), but that this behaviour can be subsequently modified upon discerning the realised threat of the intruder, perhaps as a learned response based on prior experience (Webster and Laland 2018). Prior research has shown that damselfish are able to discern an intruder’s identity under circumstances other than the guarding of an algal farm, such as when protecting egg clutches (Souza and Ilarri 2014). Collectively, this growing body of evidence suggests that intruder recognition is an essential trait for multiple elements in the life cycle of territorial damselfish.
References
Ceccarelli DM (2007) Modification of benthic communities by territorial damselfishes: a multi-species comparison. Coral Reefs 26:853–866
Ceccarelli DM, Jones GP, McCook LJ (2001) Territorial damselfishes as determinants of the structure of benthic communities on coral reefs. Oceanogr Mar Biol Annu Rev 39:355–389
Chapman ARO, Johnson CR (1990) Disturbance and organization of macroalgal assemblages in the Northwest Atlantic. Hydrobiologia 192:77–121
Dee LE, Witman JD, Brandt M (2012) Refugia and top-down control of the pencil urchin Eucidaris galapagensis in the Galapagos marine reserve. J Exp Mar Biol Ecol 416-417:135–143
Eurich JG, McCormick MI, Jones GP (2018) Habitat selection and aggressio as determinants of fine-scale partitioning of coral reef zones in a guild of territorial damselfishes. Mar Ecol Prog Ser 587:201–215
Ferreira CEL, Goncalves JEA, Coutinho R, Peret AC (1998) Herbivory by the dusky damselfish Stegastes fuscus (Cuvier, 1830) in a tropical rocky shore: effects on the benthic community. J Exp Mar Biol Ecol 229:241–264
Frederich B, Parmentier E (2016) Biology of damselfishes. CRC Press, Boca Raton
Glynn PW, Colgan MW (1988) Defense of corals and enhancement of coral diversity by territorial damselfishes. Proceedings of the 6th international coral reef symposium 2:157-163
Hata H, Ceccarelli DM (2016) Farming behaviour of territorial damselfishes. In: Frederich B, Parmentier E (eds) Biology of damselfishes. CRC Press, Boca Raton, pp 122–152
Hata H, Kato M (2002) Weeding by the herbivorous damselfish Stegastes nigricans in nearly monocultural algae farms. Mar Ecol Prog Ser 237:227–231
Hata H, Umezawa Y (2011) Food habits of the farmer damselfish Stegastes nigricans inferred by stomach content, stable isotope, and fatty acid composition analyses. Ecol Res 26:809–818
Hattori A, Shibuno T (2013) Habitat use and coexistence of three territorial herbivorous damselfish on different-size patch reefs. J Mar Biol Ass UK 93:2265–2272
Hourigan TF (1986) An experimental removal of a territorial pomacentrid: effects on the occurrence and behavior of competitors. Environ Biol Fish 15:161–169
Irving AD, Witman JD (2009) Positive effects of damselfish override negative effects of urchins to prevent an algal habitat switch. J Ecol 97:337–347
Jackson JBC (2001) What was natural in the coastal oceans? Proc Natl Acad Sci U S A 98:5411–5418
Jan RQ, Ho CT, Shiah FK (2003) Determinants of territory size of the dusky Gregory. J Fish Biol 63:1589–1597
Kamath A et al (2019) Potential feedback between coral presence and farmerfish collective behavior promotes coral recovery. Oikos 128:482–492
Lassuy DR (1980) Effects of "farming" behavior by Eupomacentrus lividus and Hemiglyphidodon plagiometopon on algal community structure. Bull Mar Sci 30:304–312
Ling SD (2008) Range expansion of a habitat-modifying species leads to loss of taxonomic diversity: a new and impoverished reef state. Oecologia 156:883–894
Mills SC, Peyrot-Clausade M, Fontaine MF (2000) Ingestion and transformation of algal turf by Echinometra mathaei on Tiahura fringing reef (French Polynesia). J Exp Mar Biol Ecol 254:71–84
Mokady O, Lazar B, Loya Y (1996) Echinoid bioerosion as a major structuring force of Res Sea coral reefs. Biol Bull 190:367–372
Naiman RJ, Johnston CA, Kelley JC (1988) Alteration of north American streams by beaver. Bioscience 38:753–762
Navarrete-Fernandez T, Landaeta MF, Bustos CA, Perez-Matus A (2014) Nest building and description of parental care in a temperate reef fish, Chromis crusma (Pisces: Pomacentridae). Rev Chil Hist Nat 87:30
Power ME et al (1996) Challenges in the quest for keystones. Bioscience 46:609–620
Robles LE, Cabaitan PC, Aurellado MEB (2018) Effects of competition on the territorial behaviour of a farmer damselfish, Plectroglyphidodon lacrymatus (Perciformes: Pomacentridae). J Fish Biol 93:1197–1206
Sammarco PW, Williams AH (1982) Damselfish territoriality: influence on Diadema distribution and implications for coral community structure. Mar Ecol Prog Ser 8:53–59
Siebeck UE, Litherland L, Wallis GM (2009) Shape learning and discrimination in reef fish. J Exp Biol 212:2113–2119
Souza AT, Ilarri MI (2014) Behavioural changes of a Brazillian endemic damselfish Stegastes rocasensis when guarding egg clutches. Environ Biol Fish 97:1295–1303
Steneck RS et al (2002) Kelp forest ecosystems: biodiversity, stability, resilience and future. Environ Conserv 29:436–459
Thresher RE (1976) Field experiments on species recognition by the threespot damselfish, Eupomacentrus planifrons, (Pisces: Pomacentridae). Anim Behav 24:562–569
Vitousek PM, Mooney HA, Lubchenco J, Melillo JM (1997) Human domination of Earth's ecosystems. Science 277:494–499
Webster MM, Laland KN (2018) Experience shapes social information use in foraging fish. Anim Behav 146:63–70
Wellington GM (1982) Depth zonation of corals in the Gulf of Panama: control and facilitation by resident reef fishes. Ecol Monogr 52:223–241
Wilson S, Bellwood DR (1997) Cryptic dietary components of territorial damselfishes (Pomacentridae, Labroidei). Mar Ecol Prog Ser 153:299–310
Wohl E, Lininger KB, Baron J (2017) Land before water: the relative temporal sequence of human alteration of freshwater ecosystems in the conterminous United States. Anthropocene 18:27–46
Young MAL, Bellwood DR (2011) Diel patterns in sea urchin activity and predation on sea urchins on the great barrier reef. Coral Reefs 30:729–736
Acknowledgements
R. Astaman provided helpful field support. Travel to New Caledonia was funded by the School of Health, Medical and Applied Sciences, Central Queensland University. This research complied with all international and national laws for animal research, and the guidelines of the animal ethics committee of Central Queensland University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Irving, A.D. Intruder identity alters the response of territorial damselfish protecting algal farms. Environ Biol Fish 102, 1281–1289 (2019). https://doi.org/10.1007/s10641-019-00906-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-019-00906-2