Abstract
Many species of fishes use acoustic signals for a variety of purposes, and sciaenid are well-known producers of sound. The Southern King Weakfish (Macrodon atricuada- Sciaenidae) possesses sexually dimorphic bilateral sonic muscles used for sound production. The bilaterally paired muscles lie on the inner body wall of the male, surrounding the swimbladder. The Southern King Weakfish produces three different sounds: advertisement calls, disturbance calls and dual-knocks, these sounds were recorded from Rio de la Plata estuary, Uruguayan coastal waters and laboratory. The advertisement call, related to courtship, was recorded in the field and from spawning males in the laboratory. Disturbance calls were produced when captive M. atricauda were startled, chased with a net or grabbed by the tail. Disturbance calls consist of a burst of pulses produced at short intervals. In disturbance call interpulse interval increased and dominant frequency decreased linearly (P < 0.05), pulse duration did not change with fish size. M. atricauda start producing disturbance call at 14 cm LT (Total Length), but only individuals over than 25 cm LT were who produced the advertisement call. Dual-knocks, call compose of only two pulses together, was recorder only in captivity. Sounds are a valuable non-invasive tool for fisheries biologists can used to monitor on males in spawning populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fishes produce sounds to communicate with one another during feeding, mating, and aggression (Fine et al. 1977; Tavolga 1980; Amorim 2006; Parmentier et al. 2014). In a number of teleost families, such as Batrachoididae, Gadididae, Holocentridae, Sciaenidae, Pomacentridae, Gobiidae and Salmonidade, sound production is associated with agonistic and reproductive behaviour (Hawkins and Rasmussen 1978; Ladich 1997; Amorim et al. 2015; Fine and Parmentier 2015; Parmentier and Fine 2016; Bolgan et al. 2017). Sound plays an important role in species identification (Winn 1964; Fine et al. 1977; Ladich et al. 2006; Amorim 2008; Malavasi et al. 2008; Parmentier et al. 2009; Phillips and Johnston 2009; Bolgan et al. 2018). Passive acoustics has been used to record temporal and spatial patterns of fish reproduction by detecting sounds associated with spawning (Lobel and Mann 1995; Luczkovich et al. 2008). However, fish specific behavioral contexts of sound production during spawning are not known (Lobel 2002).
The family Sciaenidae is an important world fish resource (Nelson 2006), especially in the western Atlantic. With about 78 genera and 282 species this family is widely distributed in the tropical, subtropical and temperate seas, with some genera inhabiting freshwater habitats (Nelson 2006), and are fisheries species commonly found in shallow coastal waters (Shao 2005). Sciaenids (often called “croakers” or “drums”) produce sound by contracting specialized sonic swimbladder muscles (Schneider and Hasler 1960; Tavolga 1964; Ono and Poss 1981; Hill et al. 1987; Connaughton et al. 1997; Ladich and Fine 2006; Tellechea et al. 2010a, b; Locascio and Mann 2011), and most likely evolved the ability to produce acoustic signals in order to communicate in turbid estuaries where the water visibility is minimal (Holt et al. 1981; Holt 2008). Only a small fraction of the 282 sciaenid species have been recorded, and most of this research has been performed on species that inhabit the Eastern North American waters (Ramcharitar et al. 2006). Males form breeding aggregations and produce advertisement calls that exhibit species specific differences (Fish and Mowbray 1970; Myrberg 1981; Saucier and Baltz 1993; Luczkovich et al. 1999; Fine et al. 2004). Field-recorded sounds have been verified using voluntary sounds recorded in captivity from a small number of sciaenids including weakfish Cynoscion regalis, speckled trout Cynoscion nebulosus, Atlantic croaker Micropogonias undulatus, sand seatrout Cynoscion arenarius, whitemouth croaker Micropogonias furnieri, red drum Sciaenops ocellatus and Pogonias cromis (Fish and Mowbray 1970; Saucier and Baltz 1993; Luczkovich et al. 1999; Connaughton et al. 2000; Fine et al. 2004; Locascio and Mann 2008; Tellechea et al. 2010a, b; Parmentier et al. 2014; Montie et al. 2016), the orangemouth corvina Cynoscion xanthulus and white seabass Atractoscion nobilis from the Pacific coast (Fish and Cummings 1972; Aalbers and Drawbridge 2008), the brown meagre Sciaena umbra and the shi drum Umbrina cirrosa from Europe (Lagardère and Mariani 2006; Picculin et al. 2012, 2016), Japanese croaker Argyrosomus japonicus and blackspotted croaker Protonibea diacanthus from Taiwan (Ueng et al. 2007; Mok et al. 2009) are some examples. In most Sciaenids, males are the prominent sound producers; however, in the Atlantic croaker, black drum and whitemouth croaker, both male and female have sonic muscles and call (Hill et al. 1987; Tellechea et al. 2010a). In most cases, sound production is associated with defending territory and reproduction (Mok and Gilmore 1983).
The southern king weakfish Macrodon atricauda (Günther, 1880) is a sciaenid fish that inhabits shallow coastal waters in soft bottom areas near estuaries from Espírito Santo state (Brazil) to northern Argentina. This species was recently discriminated from Macrodon ancylodon (Bloch and Schneider, 1801), which can be found from northeastern Brazil to Venezuela (Santos et al. 2006; Carvalho-Filho et al. 2010; Cardoso et al. 2012). Five population groups of M. atricauda have been genetically identified (Rodrigues et al. 2014). The largest one occurs between Rio de la Plata estuary (Lat. 36°) and Santa Marta Grande Cape in southern Brazil (Lat. 28°). It is commercially important and caught mainly in southern Brazil, Uruguay and Argentina by coastal trawl fisheries (Haimovici and Vieira 1986; Militelli and Macchi 2004; Jaureguizar and Milessi 2008).
In the Río de la Plata estuary and adjacent marine waters, the Sciaenidae family is the most important with respect to abundance and landings (Cousseau 1985) and M. atricauda is one of those species (Jaureguizar and Milessi 2008). M. atricauda is a coastal demersal species, euryhaline with a salinity range between 15.5 and 32.9% (Vizziano and Berois 1990). Adult specimens prefer the coastal zones of mixing between the Río de la Plata and the Atlantic Ocean, and spawns in the Rio de la Plata estuary from spring to late summer (October to March) (Vizziano and Berois 1990). Bimodal spawning is indicated by two mean GSI peaks, one during November followed by another in March (Militelli and Macchi 2004).
The aims of the present study were to describe for the first time the sounds produced by M. atricauda and the associated acoustic behavior, determine if there is a relationship between sizes and call characteristics and to examine the gross structure of the swimbladder muscle.
Material and methods
Field and captivity sound recordings
Recordings of M. atricauda were carried out during the spawning season (Militelli and Macchi 2004) between October 2017 and March 2018. Recordings were taken at the artisanal fishing ground in the Rio de la Plata estuary (34°79 S, 55°86 W) (Fig. 1) and at the adjacent coast on board a small boat in the town of Pinar in the second spawning peak (March 2018). First were recorded sounds in a known spawning area for M. atricauda (Advertisement call) adjacent to the coast. Water temperature was between 22 and 23 °C, and salinity was 20‰. The hydrophone was placed at depths between 2 and 3 m. during daylight.
(A) Regional distribution of Macrodon atricauda in the south-west Atlantic cean from Cardoso et al. (2012). (B) Station on the Uruguayan coast with recorded M. atricauda
Captivity recordings were carry out with captured fishes (N = 47; 26 males and 21 females) with a fishing rod and trawls. These fishes were kept alive on board in a plastic tank with estuarine water. For recording, individual fish were transferred to the laboratory polystyrene tanks (1.50 × 1 × 1 m) and the hydrophone was placed 50 cm from the fish. M. atricauda emit disturbance calls when held in the hand as do other sciaenids, i.e., weakfish C. regalis (Connaughton et al. 2000), Atlantic croaker Micropogonius undulatus (Fine et al. 2004) and whitemouth croaker Micropogonias furnieri (Tellechea et al. 2010a, b). The water temperature and salinity in the tanks were the same as in the estuary, 23 °C, and 20‰. Once the specimens died naturally were determine the sex, and the sonic muscles were also examined.
To witness sound production during courtship behavior were carried out additional captivity recordings of 10 marked fishes by small cuts in the tail fin (free-swimming individuals (five males and five females) polystyrene tanks (2 × 1.5 × 0.50 m) in the laboratory. These five males and five females were part of the fishes obtained previously (N = 47). The fishes in laboratory were monitored very close from 9 to 25 March 2018, started its voluntary calls (advertisement call) in 13 March and died one by one from day 25 naturally. For prolonged and continuous recording of sound, the same recording equipment was used and connected to an external battery.
Animal care protocols were approved by the Comisión Honoraria de Experimentación Animal (CHEA), Universidad de la República, Montevideo, Uruguay.
Acoustics analyses
A calibrated omnidirectional Aquarian hydrophone H1a (Useful range: <1 Hz to >100 KHz, 100 KHz = −220 dB re: 1 V/μPa), connected to an amplifier with antialiasing filter and a TASCAM DR-HP-d2 digital recorder (sampling frequency of 44.1 kHz) was used for recordings. The recordings were analyzed using Audacity 1.2.3 free software, (Mazzoni 2006) power spectra were calculated using a 1024 point Fast Fourier Transform (FFT) with a Hanning window. Pulse duration, interval between pulses (interpulse interval), and dominant frequency were measured for each sound recorded.
Statistical analyses
The relationship between mean pulse duration, pulse interval and dominant frequency with LT (Total Length) were analyzed using segmented linear regression (Sokal and Rohlf 1995), nonparametric tests including the Kruskal-Wallis test and Mann-Whitney test were used. The critical α level for these analyses was 0.05. Past (version 1.95), using a free statistical software package (Hammer et al. 2001).
Results
Sound production
Advertisement choruses were recorded at sites that match the known distribution for the artisanal fishermen of M. atricauda in the Rio de la Plata, Uruguay. Calls were recorded at depths of 2–4 m from October to March over the coast. Individual sound pulses, consisting of multiple cycles, often overlapped and were difficult to distinguish in field recordings (Fig. 4D). Trawls indicated that M atricauda was the dominant species at recording sites although occasional M. furnieri was also caught along with other demersal species. Dissection of the 20 specimen (10 males recorded and 10 females) indicated that only males possessed sonic muscles and produced sound.
Disturbance calls were recorded from 26 captive males obtained from the field and advertisements call from the five males recorded during the spawning time in the lab belonging to the 26 males.
Disturbance call
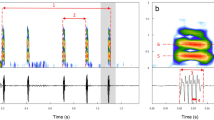
Disturbance calls, were evoked when the male fishes were handled or under disturbance condition as try to grab them with a hand net. They were recorded in 26 individuals that were not yet ripe (size range: 14 to 28 cm LT) and from five (size range: 26 to 28.9 cm LT) males in spawning condition as determined by sound production or sperm release upon gentle abdominal pressure. Were analyzed a total of 111 calls, with a total of 973 pulses. The disturbance call is a train of pulses, and the number of pulses per burst varied from 6 to 32, with each specimen emitting 1–4 bursts per call (Fig. 2). Pulses consisted of about five cycles, and most energy occurred in the second and third cycle, with the fourth cycle often greatly attenuated (Fig. 2B). The average pulse duration was 19 ± 2.95 ms, the interval was 18 ± 1.05 ms, and the dominant frequency was 466 ± 36.6 Hz for fish recorded in captivity (Table 1). Interpulse interval increased and dominant frequency decreased linearly (P < 0·05), but pulse duration did not change with LT (Fig. 3).
Disturbance call of a 26 cm total length (LT). Macrodon atricauda recorded in the laboratory tank. (A) Oscillogram (uncalibrated relative amplitude on the y-axis) of a disturbance burst, below sonogram of pulses (Hann window function and fast Fourier transformation size of 1024 points), (B) single pulse further expanded to illustrate the multicycle waveform and (C) typical power spectrum of a single pulse indicating dominant frequency (Hann window function and fast Fourier transformation size of 1024 points)
Relationship of (A) pulse duration, (B) interpulse interval, and (C) dominant frequency to total length (LT) in Macrodon atricauda disturbance call. Curves of significant regressions are shown: (B) y = 0.0408x + 18.951 (r2 = 0.0312, P = 4.45E-05) and (C) y = − −4.1873x + 557.18 (r2 = 0.2727, P = 0.00036). □ ripe U. canosai; ■ U. canosai not in spawning condition
Advertisement call
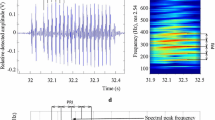
During March (spawning season) a voluntary train of pulses was recorded only for mature spawning individuals (n = 5) in laboratory tanks. Was verified that individuals of ≥21 cm LT were mature spawning (running-ripe) (Militelli and Macchi 2004; Militelli et al. 2013) by the release of sperm when stripped. These fishes (five males and five females; the females was added for a possible stimulation) were acclimatized on the tanks for four days, after which was began recording sounds. The fishes were in the tanks three weeks. The water temperature and salinity in the tanks were the same as those at the site of field recordings. After day five the captive fishes, the two sexes were in spawning condition (running-ripe, i.e., males release sperm and females release hydrated oocytes when stripped). At the moment, the males started to emit the advertising calls that consisted of a series of individual pulses that occurred at an average of 230 ± 92.4 ms (Fig. 4A). The average pulse duration was 25.8 ± 6.18 ms, the dominant frequency was 403 ± 1.66 Hz, and the number of pulses was 13 ± 2.38 in a period of recording of 3 h (Fig. 4). Advertising calls were heard when fish swam freely, alone or in groups, the males continued producing advertisement calls when females were near.
Advertisement call of a 26 cm total length (LT) male Macrodon atricauda courting females in a laboratory tank. (A) Oscillogram of pulse call with an interpulse interval of 210 ms, (B) expanded oscillogram of a pulse indicating multicycle waveform, (C) typical power spectrum of a single pulse indicating dominant frequency (Hann window function and fast Fourier transformation size of 1024 points and (D) sonogram of courtship chorus recorded in the Pinar town, Rio de la Plata coast
Field and captivity studies indicated a close link between male voluntary drumming behavior (Advertisement call) and reproductive activity. In the Rio de la Plata coastal estuary (Pinar), at 23 °C and 20‰ salinity) M. atricauda were recorded during part of their spawning season (from Oct. to Mar.) in shallow inshore waters (at depths of <5 m; Fig. 4D). Outside of the spawning season, this species was not fished, probably only approaches the coast in the reproductive season in the two spawning periods in November and March (Militelli and Macchi 2004), therefore was not detect this call in the field. In captivity after spawning the fishes stopped emit advertisement call. The choruses of advertisement calls were heard and recorded in the night (18:00–21:00) in captivity and in the field. After each recording session was verified the presence of M. atricauda by netting. No significant differences in pulse duration (pulses n = 29 p = 0.23) and dominant frequency (p = 0.12) occurred between advertisement calls in captivity and in the field (Table 1). The pulse interval and number of pulses could not be distinguished in field recordings due to overlapping sounds (Fig. 4).
The number of pulses produced by disturbance calls (n = 26 fishes) were significantly greater (Mann-Whitney test, p < 0.0001) than those produced by advertisement calls (n = 5 fishes) (Table 2).
Dual-knocks
Dual-knocks was recorder only in captivity, was composed of only two pulses, with an interpulse dual-knock to dual-knocks of 109 ± −39.5 s. The average duration of pulse was 24 ± 1.8 ms, the interval between two pulses was 81 ± 14.21 s, and the dominant frequency was 601 ± 23.51 Hz for the fishes recorded. The dual-knocks were emitted in periods of 2 to 3 h in the course of the day. One day after spawning the five specimens began to issue dual-knocks (Fig. 5).
Dual knock of a 26 cm total length (LT) male Macrodon atricauda in a laboratory tank. (A) Oscillogram of a double pulse with a duration of 67 ms, (B) double pulsed expanded oscillogram indicating multicycle waveform, (C) typical power spectrum of a single pulse indicating dominant frequency (Hann window function and fast Fourier transformation size of 1024 points
Sonic muscles
The M. atricauda possesses sexually dimorphic extrinsic sonic muscles. Bilaterally paired muscles were observed on the inner body wall of males, surrounding the swimbladder. They extended nearly the entire length of the body cavity and attached to the lateral body wall musculature by connective tissue (Fig. 6). The muscle changed seasonally, increasing in vascularization and deepening to a dark red during the spawning season as others sciaenids.
Upper: illustration of the location and sexual dimorphism of sonic muscles in the M. atricauda. Lower: photograph of the body cavity of a sperm-producing male, showing the darkred sonic muscle during the spawning season; also photograph of the right body wall of a pre-spawning female without sonic muscle. sw, swimbladder; sm, sonic muscle; g, gonad
Discussion
The male of southern king weakfish (M. atricauda) possess sonic muscles that are absent in females as in other sciaenid (Smith 1905; Chao 1978; Hill et al. 1987; Connaughton et al. 2000; Tellechea et al. 2010a, b; Tellechea and Norbis 2012; Borie et al. 2014). The sounds emitted by M. atricauda are related to spawning and disturbance behaviors.
Disturbance calls is start producing at 14 cm LT, similar to C. regalis (Connaughton et al. 2000), C. guatucupa (Tellechea and Norbis 2012) and U. canosai (Tellechea et al. 2017). However the advertisement call is emitted by only in spawning condition, this suggest a correlation of sonic-muscle development (color change and increment mass) with puberty, the length at first maturity for M. atricauda is 21 cm LT for both sexes (Militelli and Macchi 2004; Militelli et al. 2013).
In M. atricauda, as in other weakfish (e.g., C. regalis and C. guatucupa), drumming behavior (advertisement calls) was correlated with spawning in the field (Connaughton and Taylor 1994; Tellechea and Norbis 2012; Tellechea et al. 2017) and in captivity (Connaughton and Taylor 1996; Tellechea and Norbis 2012; Montie et al. 2017). Herein, was confirmed that the M. atricauda advertisement call recorded in the field was similar to voluntary calls recorded by fish in tanks during the spawning season. The pulses produced during advertisement calls have longer inter-pulse intervals, suggesting that disturbances cause a more-rapid pacing of pattern generators in the central nervous system than courtship vocalizations (Tellechea et al. 2010a, 2017; Tellechea and Norbis 2012). Which implicates control of contraction timing to a pattern generator in the central nervous system (Bass et al. 2015) as in toadfish Opsanus tau and piranha Pygocentrus nattereri (Kner, 1858) (Fine et al. 2001; Millot et al. 2011).
The sounds of sciaenids exhibit ontogenetic changes that correlate with development of the sonic muscles, the swimbladder, and gonads (Hill et al. 1987). Larger C. regalis produce sounds with lower dominant frequencies (Connaughton et al. 2000) and it has been argued that decreasing frequency reflects a forced rather than a resonant response because larger sonic muscles require more time to complete a twitch (Hill 1950; Wainwright and Barton 1995; Fine et al. 2001) such that slower movement of the bladder decreases the dominant pulse frequency among larger fish, as found in M. furnieri (Tellechea et al. 2010a). In the same way, the disturbance call characteristics of M. atricauda varied with size, interpulse interval increased and dominant frequency decreased linearly, but pulse duration did not change with LT, similar sound characteristic of others sciaenids reported (Connaughton and Taylor 1994; Connaughton et al. 1997; Tellechea et al. 2010a, b, 2017; Tellechea and Norbis 2012). The development of sonic muscles, together with a fish’s size and water temperature, determines characteristics of the sound parameters (Connaughton et al. 1997).
Sonic muscles of the M. atricauda undergo a yearly hypertrophyatrophy cycle whereby muscles increase in thickness and mass during the spawning season (Connaughton and Taylor 1994; Connaughton et al. 1997). Sound production by male M. atricauda is also seasonal and correlated with spawning activities in natural conditions and with courtship behavior observed prior to spawning in captivity as in other sciaenids (Connaughton and Taylor 1995, 1996; Tellechea and Norbis 2012; Tellechea et al. 2017). The coloration of the sonic muscles of the male M. atricauda was darker in the spawning season. This darkening occurs simultaneously with dramatic seasonal variations in the sonic muscle size, when the sonic muscle triples in mass during the spring spawning season and decreases to its pre-spawning mass by the end of the summer in response to changing androgen titers (Merriner 1976; Connaughton and Taylor 1994, 1995, 1996; Tellechea and Norbis 2012; Tellechea et al. 2017). The observations of the reproductive status, including male and female gonad conditions (females with hydrated oocytes and males producing milt when handled and stripped), suggest that spawning activities for this species coincide with peak drumming activity.
Number of pulses was lower in advertisement than in disturbance calls in M. atricuada as in C. regalis, M. furnieri and U. canosai (Connaughton et al. 2002; Tellechea et al. 2010a, 2017). Overlap of advertisement choruses with population locations from different places along the Rio de la Plata estuary suggests that M. atricuada is reproductively active in multiple sites on the shallow continental shelf throughout the species range in Uruguay. It has been suggested that time between pulses could be used for species-specific recognition in sciaenids (Locascio and Mann 2008) and in other species (Winn 1972; Spanier 1979; Mann and Lobel 1997). Their suggestion agrees with the separation of disturbance and advertisement calls in M. atricauda.
There is not much information about the dual knock call. As was described by Mok and Gilmore (1983) in spotted seatrout (Cynoscion nebulosus- Sciaenidae) and Lin et al. (2007) in the big-snout croaker (Johnius macrorhynus- Sciaenidae) the dual-knocks seem not to be a fright-response sound. In C. nebolosus this call was produced in spawning aggregation (Mok and Gilmore 1983). The big-snout croaker dual-knocks often being placed in the initial phase of a series of call and the purrs (disturbance call) often being chained together suggest the communication signal of this species is actually not very diverse. However in the case of M. atricauda, this call was issued in a state of calm and free swimming in the laboratory tanks without any stimulus of threat. This sound was recorded solo without being part of disturbance call as is described by Lin et al. (2007). It would seem that double knocks is a common call in some sciaenid species sound producers, in personal observation in later acoustic studies of whitemouth croaker M. furnieri (Tellechea et al. 2010a) were recorded dual knocks. Perhaps this call could be more related to spawning or courtship, since the specimens in captivity in this study were spawning conditions, in addition to this Mok and Gilmore (1983) recorded this sound in spawning groups in C. nebolosus. In this way it would be necessary to make a deeper study about sciaenid sound emissions in captivity or in the wild to know if this call is common and what would be its roll.
Sounds produced by sciaenid have been associated with the mating season since the early twentieth century (Smith 1905), and are now routinely used to monitor spawning populations in the field (Mok and Gilmore 1983; Saucier and Baltz 1993; Connaughton and Taylor 1995; Rountree et al. 2006).
References
Aalbers SA, Drawbridge MA (2008) Spawning behavior and associated sound production of the white seabass Atractoscion nobilis. Trans Am Fish Soc 137:542–550. https://doi.org/10.1577/T04-058.1
Amorim MCP (2006) Diversity of sound production in fish. In: Ladich F, Collin SP, Moller P, Kapoor BG (eds) Communiation in fishes. Science Publishers, Enfield, pp 71–105
Amorim MCP (2008) Species differences in courtship acoustic signals among five Lake Malawi cichlid species (Pseudotropheus spp.). J Fish Biol 72:1355–1368
Amorim MCP, Vasconcelos RO, Fonseca PJ (2015) Fish sounds and mate choice. In: Ladich F (ed) Sound communication in fishes. Springer Verlag, Wien, pp 1–33
Bass AH, Chagnaud BP, Feng NI (2015) Comparative neurobiology of sound production in fishes. In: Ladich F (ed) Sound communication in fishes. Springer Verlag, Wien, pp 35–75
Bolgan M, O'Brien J, Picciulin M, Manning L, Gammell M (2017) Behaviour of Arctic charr Salvelinus alpinus during an induced mating season in captivity: how male relative size influences male behavioural investment and female preference over time. J Fish Biol 90:1479–1505. https://doi.org/10.1111/jfb.13244
Bolgan M, Amorim MCP, Fonseca PJ, Di Iorio L, Parmentier E (2018) Acoustic complexity of vocal fish communities: a field and controlled validation. Sci Rep 8(1):10559. https://doi.org/10.1038/s41598-018-28771-6
Borie A, Mok H, Chao N, Fine M (2014) Spatiotemporal variability and sound characterization in silver croaker Plagioscion squamosissimus (Sciaenidae) in the Central Amazon. PLoS One 9:e99326. https://doi.org/10.1371/journal.pone.0099326
Cardoso LG, Santos S, Haimovici M (2012) Differences in the otoliths support the distinction of the genus Macrodon into two species in the South-Western Atlantic Ocean. Mar Biodivers Rec 5:e93
Carvalho-Filho A, Santos S, Sampaio I (2010) Macrodon atricauda (Günther, 1880) (Perciformes: Sciaenidae), a valid species from the southwestern Atlantic, with comments on its conservation. Zootaxa 2519:48–58
Chao LN (1978) A basis for classifying Western Atlantic Sciaenidae (Teleostei: Perciformes). NOAA technical report circular 415. Washington DC: US Department of Commerce, National Oceanic and Atmospheric Administration, national marine fisheries service. 65pp
Connaughton MA, Taylor MH (1994) Seasonal cycles in the sonic muscles of the weakfish, Cynoscion regalis US Fish Bull 92: 697–703
Connaughton MA, Taylor MH (1995) Seasonal and daily cycles in sound production associated with spawning in the weakfish, Cynoscion regalis. Environ Biol Fish 42:233–240
Connaughton MA, Taylor MH (1996) Drumming, courtship and spawning behavior in captive weakfish, Cynoscion regalis. Copeia 1996:195–199
Connaughton MA, Fine ML, Taylor MH (1997) The effects of seasonal hypertrophy and atrophy on fiber morphology, metabolic substrate concentration and sound characteristics of the weakfish sonic muscle. J Exp Biol 200:2449–2457
Connaughton MA, Taylor MH, Fine ML (2000) Effects of fish size and temperature on weakfish disturbance calls: implications for the mechanism of sound generation. J Exp Biol 203:1503–1512
Connaughton MA, Fine ML, Taylor MH (2002) Weakfish sonic muscle: influence of size, temperature and season. J Exp Biol 205:2183–2188
Cousseau MB (1985) Los peces del Río de la Plata y de su Frente Marítimo. In: Yañez-Arancibia A (ed) In Fish community ecology in estuaries and coastal lagoons. Towards an ecosystem integration. UNAM Press, Mexico, pp 515–534
Fine ML, Parmentier E (2015) Mechanisms of fish sound production. In: Ladich F (ed) Sound communication in fishes, animal signals and communication 4. Springer, New York, pp 77–126. https://doi.org/10.1007/978-3-7091-1846-7-3
Fine ML, Winn HE, Olla B (1977) Communication in fishes. In: Sebeok T (ed) How animals communicate. Indiana University Press, Bloomington, pp 472–518
Fine ML, Malloy KL, King CB, Mitchell SL, Cameron TM (2001) Movement and sound generation by the toadfish swimbladder. J Comp Physiol A 187:371–379
Fine ML, Schrinel J, Cameron TM (2004) The effect of loading on disturbance sounds of the Atlantic croaker Micropogonius undulatus: air versus water. J Acoust Soc Am 116:1271–1275
Fish MP, Mowbray WH (1970) Sound of the Western North Atlantic fishes. Johns Hopkins Press, Baltimore
Fish JF, Cummings WC (1972) A 50-dB increase in sustained ambient noise from fish (Cynoscion xanthulus). J Acoust Soc Am 52(4B):1266–1270
Haimovici M, Vieira PC (1986) Captura e esforço na pesca de arrasto de fundo no litoral sul do Brasil, no período 1975–1984. Anais do IV Congresso Brasileiro de Engenharia de Pesca 2:15–234
Hammer Ǿ, Harper DAT, Ryan PD (2001) PAST: paleontological statistic software package for education and data analysis. Palaeontol Electron 4(1):1–9
Hawkins AD, Rasmussen KJ (1978) The calls of gadoid fishes. JMBA 58:891–911
Hill AV (1950) The dimensions of animals and their muscular dynamics. Sci Prog 38:209–230
Hill GL, Fine ML, Musick JA (1987) Ontogeny of the sexually dimorphic sonic muscle in three sciaenid species. Copeia 1987:708–713
Holt SA (2008) Distribution of red drum spawning sites identified by a towed hydrophone array. Trans Am Fish Soc 137(2):551–561
Holt J, Godbout R, Arnold CR (1981) Effects of temperature and salinity on egg hatching and larval survival of red drum Sciaenops ocellata. United States National Marine Fisheries Service Fishery Bulletin 79:569–573
Jaureguizar AJ, Milessi AC (2008) Assessing the sources of the fishing down marine food web process in the Argentinean-Uruguayan common fishing zone. Sci Mar 72:25–36
Ladich F (1997) Agonistic behaviour and significance of sounds in vocalizing fish. Mar Freshw Behav Physiol 29:87–108
Ladich F, Fine ML (2006) Sound-generating mechanisms in fishes: a unique diversity in vertebrates. In: Ladich F et al (eds) Communication in fishes. Science, Enfield, pp 3–43
Ladich F, Collin SP, Moller P, Kapoor BG (eds) (2006) Communication in fishes. Science, Enfield
Lagardère JP, Mariani A (2006) Spawning sounds in meager Argyrosomus regius in the Gironde estuary, France. J Fish Biol 69:1697–1708. https://doi.org/10.1111/j.1095-8649.2006.01237.x
Lin YC, Mok HK, Huang BQ (2007) Sound characteristics of big-snout croaker, Johnius macrorhynus (Sciaenidae). JASA 121:586–593. https://doi.org/10.1121/1.2384844
Lobel PS (2002) Diversity of fish spawning sounds and the application of passive acoustic monitoring. Bioacoustics 12:286–289
Lobel PS, Mann DA (1995) Spawning sounds of the damselfish, Dascyllus albisella (Pomacentridae), and relationship to male size. Bioacoustics 6:187–198
Locascio JV, Mann DA (2008) Diel periodicity of fish sound production in Charlotte Harbor, Florida. Trans Am Fish Soc 137:606–615. https://doi.org/10.1577/T06-069.1
Locascio JV, Mann DA (2011) Localization and source level estimates of black drum (Pogonias cromis) calls. J Acoust Soc Am 130:1868–1879. https://doi.org/10.1121/1.3621514
Luczkovich JJ, Sprague MW, Johnson SE, Paullinger RC (1999) Delimiting spawning areas of weakfish, Cynoscion regalis (family Sciaenidae) in Pamlico sound, North Carolina using passive hydroacoustic surveys. Bioacoustics 10:143–160
Luczkovich JJ, Mann DA, Rountree RA (2008) Passive acoustics as a tool in fisheries science. Trans Am Fish Soc 137:533–541. https://doi.org/10.1577/T06-258.1
Malavasi S, Collatuzzo S, Torricelli P (2008) Interspecific variation of acoustic signals in Mediterranean gobies (Perciformes, Gobiidae) comparative analysis and evolutionary outlook. Biol J Linn Soc 93:763–778
Mann DA, Lobel PS (1997) Propagation of damselfish (Pomacentridae) courtship sounds. JASA 101:3783–3379
Mazzoni D (2006) AUDACITY 1.2.6. Available at http://audacity.sourceforge.net/
Merriner JV (1976) Aspects of the reproductive biology of the weakfish, Cynoscion regalis (Sciaenidae), in North Carolina. US Fish Bull 74:18–26
Militelli MI, Macchi GJ (2004) Spawning and fecundity of king weakfish, Macrodon ancylodon, in the Rio de la Plata estuary, Argentina-Uruguay. JMBA 84:443–447
Militelli MI, Macchi GJ, Rodrigues K (2013) Comparative reproductive biology of Sciaenidae family species in the Río de La Plata and Buenos Aires coastal zone, Argentina. JMBA 93(2):413–423
Millot S, Vandewalle P, Parmentier E (2011) Sound production in red-bellied piranhas (Pygocentrus nattereri, Kner): an acoustical, behavioural and morphofunctional study. J Exp Biol 214(21):3613–3618
Mok HK, Gilmore RG (1983) Analysis of sound production in estuarine aggregations of Pogonias cromis, Bairdiella chrysoura, and Cynoscion nebulosus (Sciaenidae). Bull Inst Zool Acad Sin 22:157–186
Mok HK, Yu HY, Ueng JP, Wei RC (2009) Characterization of sounds of the Blackspotted croaker Protonibea diacanthus (Sciaenidae) and localization of its spawning sites in estuarine coastal waters of Taiwan. Zool Stud 48:325–333
Montie EW, Kehrer C, Yost J, Brenkert K (2016) Long-term monitoring of captive red drum Sciaenops ocellatus reveals that calling incidence and structure correlate with egg deposition. J Fish Biol 88:1776–1795
Montie EW, Hoover M, Kehrer C, Yost J, Brenkert K, O'Donnell T, Denson MR (2017) Acoustic monitoring indicates a correlation between calling and spawning in captive spotted seatrout (Cynoscion nebulosus). PeerJ 5:e2944. https://doi.org/10.7717/peerj.2944
Myrberg AA (1981) Sound communication and interception in fishes. In: Tavolga WN, Popper AN, Fay RR (eds) Hearing and sound communiction in fishes. Springer-Verlag, New York, pp 395–428
Nelson JS (2006) Fishes of the world. 4th ed. Hoboken (New Jersey, USA): John Wiley & Sons. Xix+601 p
Ono RD, Poss SG (1981) Structure and innervation of the swim bladder musculature in the weakfish, Cynoscion regalis (Teleostei: Sciaenidae). Can J Zool 60:1955–1967
Parmentier E, Fine ML (2016) Fish sound production. In: Suthers RA, Fitch T (eds) Handbook of auditory research. Springer, New York, pp 19–49
Parmentier E, Lecchini D, Frederich B, Brie C, Mann D (2009) Sound production in four damselfish (Dascyllus) species: phyletic relationships? Biol J Linn Soc 97:928–940. https://doi.org/10.1111/j.1095-8312.2009.01260.x
Parmentier E, Tock J, Falguière JC, Beauchaud M (2014) Sound production in Sciaenops ocellatus: preliminary study for the development of acoustic cues in aquaculture. Aquaculture 432:204–211
Phillips CT, Johnston CE (2009) Evolution of acoustic signals in Cyprinella: degree of similarity in sister species. J Fish Biol 74:120–132
Picculin M, Calcagno G, Sebastianutto L, Bonacito C, Codarin A, Costantini M, Ferrero EA (2012) Diagnostics of nocturnal calls of Sciaena umbra (L., fam. Sciaenidae) in a nearshore Mediterranean marine reserve. Bioacoustics 212:1–12
Picculin M, Bolgan M, Corò AB, Calcagno G, Malavasi S (2016) Sound production by the shi drum Umbrina cirrosa and comparison with the brown meagre Sciaena umbra: a passive acoustic monitoring perspective. J Fish Biol 88:1655–1660
Ramcharitar J, Gannon DP, Popper AN (2006) Bioacoustics of the family Sciaenidae (croakers and drumfishes). Trans Am Fish Soc 135:1409–1431
Rodrigues R, Santos S, Haimovici M, Saint-Paul U, Sampaio I, Schneider H (2014) Mitochondrial DNA reveals population structuring in Macrodon atricauda (Perciformes: Sciaenidae): a study covering the whole geographic distribution of the species in the southwestern Atlantic. Mitochondrial DNA 25(2):150–156
Rountree RA, Gilmore RG, Goudey CA, Hawkins AD, Luczkovich JJ, Mann DA (2006) Listening to fish: applications of passive acoustics to fisheries science. Fisheries 31:433–446
Santos S, Hrbek T, Farias IP, Schneider H, Sampaio I (2006) Population genetic structuring of the king weakfish, Macrodon ancylodon (Sciaenidae), in Atlantic coastal waters of South America: deep genetic divergence without morphological change. Mol Ecol 15:4361–4373
Saucier MH, Baltz DM (1993) Spawning site selection by spotted seatrout, Cynoscion nebulosus, and black drum, Pogonias cromis, in Luisiana. Environ Biol Fish 36:257–272
Schneider VR, Hasler AD (1960) Laute und Lauterzeugung bein Suesswassertrommler Aplodinotus grunniens Rafinesque (Sciaenidae, Pisces). Z vergl Physiol 43:499–517
Shao KT (2005) Taiwan fish database World Wide Web electronic publication version 2005/5. Available at http:// fishdb.sinica.edu.tw
Smith HM (1905) The drumming of the drum-fishes (Sciaenidae). Science 22:376–378
Sokal RR, Rohlf FJ (1995) Biometry: the principles and practice of statistics in biological research. W.H. Freeman and Company, New York, 850 p
Spanier E (1979) Aspects of species recognition by sounds in four species of damselfishes, genus Eupomacentrus (Pisces: Pomacentridae). Z Tierpsychol 51:301–316
Tavolga WN (1964) Sonic characteristics and mechanisms in marine fishes. In: Tavolga WN (ed) Marine bio-acoustics, vol 1. Pergamon, New York, pp 195–211
Tavolga WN (1980) Hearing and sound communication in fishes in relation to fisheries management. In: Bardach JE, Magnuson J, May RC, Reinhart JM (eds) Fish behavior and its use in the capture and culture of fishes (ICLARM Conference Proceedings 5, Manilla), pp 102–123
Tellechea SJ, Norbis W (2012) Sexual dimorphism in sound production and relationship between fish size and call characteristics in the striped weakfish Cynoscion guatucupa. Zool Stud 51:946–955
Tellechea SJ, Martinez C, Fine ML, Norbis W (2010a) Sound production in whitemouth croaker (Micropogonias furnieri – Sciaenidae) and relationship between fish size and disturbance call parameters. Environ Biol Fish 89:163–172
Tellechea SJ, Olsson D, Norbis W, Fine ML (2010b) Calls of the black drum (Pogonias cromis–Sciaenidae): geographical differences in sound production between northern and southern hemisphere populations. J Exp Zool A 315:48–55
Tellechea SJ, Fine ML, Norbis W (2017) Passive acoustic monitoring, development of disturbance calls and differentiation of disturbance and advertisement calls in the argentine croaker Umbrina canosai (Sciaenidae). J Fish Biol 90(4):1631–1643. https://doi.org/10.1111/jfb.13257
Ueng JP, Huang BQ, Mok HK (2007) Sexual differences in spawning sounds of the Japanese croaker Argyrosomus japonicus (Sciaenidae). Zool Stud 46:103–110
Vizziano D, Berois N (1990) Histologia del ovario de Macrodon ancylodon (Bloch y Schneider, 1801) Teleostei: Sciaenidae. Ovoge.nesis. Folículos post-ovulatorios. Atresia. Rev Bras Biol 50:523–536
Wainwright PC, Barton RC (1995) Scaling in the feeding mechanism of the largemouth bass (Micropterus salmoides): motor pattern. J Exp Biol 198:1161–1171
Winn HE (ed) (1964) The biological significance of fish sounds. Marine Bio-acoustics. Pergamon, New York
Winn HE (1972) Acoustic discrimination by the toadfish with comments on signal systems. In: Winn HE, Olla BL (eds) Behavior of Marine Animals: Current Perspectives in Research, Vol. 2. Vertebrates. Plenum Press, New York, pp 361–385
Acknowledgments
I would like to thank to MSc Daniela Olsson for her help in the fish handling and transport to the tanks on the boat and also the for their suggestions and improvement to the English text. Also to ANII (Agencia Nacional de Investigación e Innovación) through the National Research System (ANII–SIN–Uruguay) for support. This manuscript been approved by an Animal Care Committee of Uruguay: Comisión Honoraria de Experimentation Animal (CHEA), Universidad de la República, Montevideo, Uruguay.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tellechea, J.S. The acoustic behavior of Southern King Weakfish (Macrodon atricauda-Sciaenidae). Environ Biol Fish 102, 1253–1264 (2019). https://doi.org/10.1007/s10641-019-00902-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-019-00902-6