Abstract
Biogeographical transitional areas are often characterised by fluctuating environmental conditions and high variability in species composition and abundance. A 10-year summer sampling programme was undertaken in the permanently open Breede Estuary, situated between the winter and bimodal rainfall regions and in the cool/warm-temperate biogeographical transition zone. This allowed for the collection of data highlighting inter-annual changes in the fish community of a transition zone estuary and relating these changes to selected environmental variables (salinity, temperature and flow). Although the abundance of individual species varied on an inter-annual basis, the ranking of fish taxa in the Breede Estuary varied little over the 10-year period, indicating a fairly stable species composition. Multivariate analysis showed that the fish communities sampled in summer within each reach (upper, middle, lower) remained similar, regardless of year, and this was largely driven by salinity patterns which showed little inter-annual variation during the low-flow summer period. The Breede Estuary is, however, subject to regular winter and spring flooding, where up to 50% of the annual rainfall may occur over a few days. Although the magnitude of flooding prior to sampling had little effect on species abundance, changes in the frequency or return-time of events did; such that when two floods occurred in succession species abundance and richness declined.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The interaction of fresh and marine waters in estuaries gives rise to highly dynamic systems, which are characterised by large fluctuations in environmental conditions (Flint 1985). Estuaries provide a variety of benefits to fish, including an abundance of food for juveniles, sheltered nursery habitats and reduced predation risk in shallow turbid waters (Claridge et al. 1986; Maes et al. 1998). Numerous environmental factors affect the utilisation of estuaries by fish, including water temperature, salinity and freshwater flow (e.g., Thiel et al. 1995; Marshall and Elliot 1998; Harrison and Whitfield 2006; Costa et al. 2007). Freshwater flow is a particularly important driver of estuarine ecology in southern Africa, as rivers here and in eastern Australia exhibit amongst the most variable flows in the world (Gillson 2011). Environmental conditions in estuaries change on an hourly, daily, weekly, monthly, annual and decadal basis (Flint 1985) and, as such, a long-term time series of data on both the environment and the fish community is required to establish a sound understanding of temporal changes in fish communities within these systems (Cuesta et al. 2006). This is even more important in estuaries situated in transition zones where, due to the environmental unpredictability in such zones, high variability in species composition and abundance can be expected (Brodeur et al. 2003).
The Breede Estuary is an important nursery area for many coastal fish species and contains a high proportion of endemic taxa (Harrison 1999; Lamberth et al. 2008). It is also the largest warm-temperate estuary along the South African coastline (Turpie et al. 2002) and is in close proximity to the biogeographic boundary between the warm-temperate and cool-temperate regions, which occurs at Cape Agulhas (Harrison 2002). Therefore, the Breede Estuary falls within the warm-temperate and cool-temperate transition-zone, a zone that is characterised by species overlap between two biogeographical regions (Konar et al. 2010). Species occurring in these transition-zones are often near their range limit and typically show more variability in abundance between years (Kling et al. 2003).
The ichthyofauna of the Breede Estuary has been well studied over time scales that have varied from once-off (Carter 1983; Harrison 1999) to seasonal studies over a period of less than a year (Montoya-Maya and Strydom 2009) and short-term studies covering up to five years (Coetzee and Pool 1991; Hutchings and Lamberth 2003; Lamberth et al. 2008). However, the relationship between inter-annual changes in the fish community and climatic or environmental variables (including episodic events) in the Breede Estuary is largely unknown.
The primary aim of this study was to assess changes in the fish assemblage structure over a 10 year period and to relate these changes to climate or environmental variability. It was hypothesized that the Breede Estuary fish assemblage undergoes inter-annual changes in composition, diversity and abundance as a result of variability in key climatic variables, particularly water temperature, salinity and river flow.
Materials and methods
Study site
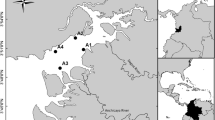
The Breede Estuary (34° 24′ 21.6″S, 20° 51′ 08.2″E) is a permanently open estuary located approximately 7 km north of Cape Infanta (Fig. 1) and is fed by a river 322 km in length. The estuary has a total surface area of 455 ha and a mean depth of 4.6 m, with 17 m deep scour holes occurring in the middle and upper reaches (Lamberth et al. 2008). The estuarine bed consists of three distinct zones: an estuarine sand zone that extends from the mouth to 5.5 km upstream, an estuarine mud zone that ranges from 5.5 km to 18.5 km upstream and a fluvial sand zone that extends from 18.5 km to 35 km upstream (Carter 1983). The gentle gradient of the coastal plain allows the tidal effects to penetrate 50 km upstream, as far as the town of Malgas (Day 1981).
The Breede River is located within the transition zone between the Western Cape bimodal and winter rainfall regions, but most of the 12,600 km2 catchment falls within the winter rainfall area (Carter 1983). The Breede Estuary thus experiences greater flow variability than adjacent systems. Approximately 80% of the catchment rainfall falls between April and September for the Breede River (Steynor et al. 2009) and the estuary is situated within an area that receives 400–500 mm per annum (DWAF 2004). The mean annual run-off (MAR) is 1785 × 106 m3 per annum (van Niekerk et al. 2012) but this has been reduced due to abstraction and off-channel and in-stream impoundments on the river and its tributaries respectively (Taljaard et al. 2001; Lamberth et al. 2008).
Salinity in the estuary varies with river flow and tidal state, with estuarine conditions (> 10) extending 30–50 km from the mouth during low river flow (Lamberth et al. 2008). During heavy winter floods the estuary may be fresh from the surface to the bottom at low tide (Lamberth et al. 2008).
River flow data
Hydrological data (flow rates) for the Breede River were obtained from the South African Department of Water Affairs (DWA) (www.dwaf.gov.za/Hydrology/). The verified mean monthly flow rate data (January 1968 – March 2012) were obtained from two stations that contribute to the overall Breede River flow, namely the Swellendam H7H006 (34°04′03″S, 20°24′20.16″E) and Eenzaamheid Buffelsjas H7H013 (34°01′9.1″S, 20°34′58.08″E) stations. Mean monthly flow rates were compared to historical mean monthly values (1970–2010) to determine higher than average flow rates (above historical average) and lower than average flow rates (below historical average).
Field sampling
The Breede Estuary has been sampled up to four times a year since 1997 as part of a long-term monitoring programme (S.J. Lamberth, unpubl. data). The summer data (January – March) collected between February 2002 and February 2012 from this monitoring programme were utilised in this study. Autumn, winter and spring data were excluded from the analysis as these seasons were not consistently sampled each year. The Breede Estuary was sampled at 24 sites on each occasion (Fig. 1), unless impractical due to factors such as site flooding. At each site water temperature (°C) and salinity (expressed according to the practical salinity scale), were recorded with a YSI multi-parameter probe.
A seine net (30 m × 2 m × 15 mm bar mesh seine net with a 5 mm bar mesh in the middle 10 m including the cod-end) was used to sample littoral habitats at the 24 sites during daylight hours. The seine net was deployed using a row boat and placed in a semi-circle formation and subsequently hauled to shore by four people. Fish collected in the seine nets were placed into buckets of estuarine water and then sorted to the lowest taxonomic level (usually species). Once the fish were identified they were measured (mm TL) and returned alive to the system.
Data analysis
Richness and diversity
Species were allocated one of five estuary-association categories, according to the classification system of Whitfield (1994):
-
Estuarine resident species (Category I) which breed in estuaries
-
Euryhaline marine species (Category II) which usually breed at sea and whose juveniles show varying degrees of dependence on estuaries
-
Marine species (Category III) which occur in estuaries in small numbers but are not dependant on them
-
Euryhaline freshwater species (Category IV) whose penetration into estuaries is determined primarily by salinity tolerance
-
Catadromous species (Category V) which use estuaries as transit routes between the marine and freshwater environments.
Spatial and temporal trends
Catch per unit effort (CPUE) was used as the index of relative abundance and was given as fish-per-haul. Catch-per-unit-effort values per sampling occasion and estuary reach for each species were calculated by dividing the number of individuals by the total number of seine hauls. The percentage contribution of individual species to the overall summer fish community was calculated by dividing each species CPUE by the total species CPUE. To study changes in species diversity, multivariate diversity indices based on taxonomic distinctness were calculated (Δ* and Δ+). Taxonomic diversity measures have been found to be more sensitive to environmental change and less sensitive to sample size or sampling effort than traditional diversity indices (e.g. Simpsons, Shannon-Weiner and Pielou) (Warwick and Clarke 1998). Taxonomic distinctness is expected to decrease with increasing disturbance (Collie et al. 2004). Stability of the estuarine fish assemblage was calculated using the non-parametric Kendall’s coefficient of concordance (W), which tested for significant rank differences based on the relative abundance of species in the ten years of sampling. Values ranged from 0 (no agreement) to 1 (complete agreement) (Kendall 1962). Spearman rank correlation coefficients determined the similarity of the fish assemblages between years. A Bonferroni correction was applied to the level of significance in order to account for multiple comparisons (corrected P < 0.001).
The association between summer fish assemblage structure and environmental conditions in the Breede Estuary were analysed using canonical correspondence analysis (CCA) from the CANOCO package (version 4.5). In CCA the ordination axes are restricted to linear combinations of environmental variables, which allow a direct relationship between community variation and environmental variation (ter Braak 1986). The following environmental variables were used in the CCA: mean summer (January–March) estuarine temperature and salinity per reach, mean monthly flow (six months before sampling) and year. Annual summer fish abundance data were used to calculate CPUE in the lower, middle and upper reaches of the estuary. The data were log transformed (CPUE +1) to reduce the influence of abundant species on the analysis and rare species (species with a mean CPUE <0.03 fish/haul) were excluded from the analysis. Two-way crossed analysis of similarity (ANOSIM) was carried out in PRIMER v 6.1.6 (Plymouth Routines in Multivariate Ecological Research) to determine if there were significant differences between estuary reach and year.
The significance of the relationships between species and individual environmental variables were analysed using t-value bi-plots with Van Dobben circles (ter Braak and Looman 1994). These ordination diagrams are based on t-values of canonical co-efficients from CCA and are used to evaluate significant positive and negative correlations between species and environmental variables. Species with arrows-heads ending inside the Van Dobben circles have statistically significant associations with the explanatory variable (t-value < −2 or > +2) (as in the methods outlined in Garcia et al. 2012).
Results
Environmental variability
The combined monthly river flow rate (Swellendam and Buffelsjags) for 1970–2010 showed obvious seasonal patterns, with peak flow occurring in winter and spring (peak flow = 91 m3 s−1) and low flow rates in autumn and summer (low flow <7 m3 s−1). Flooding was recorded in winter or spring preceding sampling in August (142 m3 s−1) and October 2001 (140 m3 s−1), September 2002 (140 m3 s−1), September 2006 (281 m3 s−1), August (130 m3 s−1) and October 2008 (307 m3 s−1) and July 2011 (141 m3 s−1) (Fig. 2).
Summer sampling always occurred during low flow periods (flow <7 m3 s−1) and as a result salinity during summer sampling showed little inter-annual variation. In most years, monthly flow during summer sampling ranged between 2 to 6 m3 s−1 and the water column ranged from well mixed to stratified, with estuarine conditions (30–10) recorded up to 20 km from the mouth (Fig. 3). The lowest monthly flow rate during summer sampling was recorded in January 2004 (1.7 m3 s−1) and in this month the estuary was marine dominated, with marine conditions (>30) extending for 11 km from the mouth and estuarine salinities recorded up to 30 km from the mouth.
Water temperature showed a spatial pattern of variation, with mean summer values showing a trend of increasing temperature from the lower (23.8 °C) to the upper reaches (25.1 °C). Similar to salinity there was little inter-annual summer temperature variation and, in most years, mean water temperature in the estuary was approximately 24.0 °C. The warmest temperatures (mean ± 26.0 °C) were recorded in March 2003, February 2006 and March 2007 (Fig. 4).
Community change
A total of 41 species were documented in summer during the period 2002–2012. The total number of species recorded each year varied between 18 (2002, 2009, 2012) and 35 (2011) (Table 1). The species assemblage was fairly similar each year, with six species consistently caught; these were Caffrogobius spp. (6.8–58.4%), Galeichthys feliceps (Valenciennes, 1840) (0.6–62.5%), Gilchristella aestuaria (Gilchrist, 1913) (0.5–36.0%), Liza richardsonii (Smith, 1846) (7.1–42.7%), Liza dumerili (Steindachner, 1870) (0.7–6.9%) and Solea turbynei (Gilchrist, 1904) (1.3–12.9%). These species together comprised more than 76% of the total catch in all years (Table 2).
Caffrogobius spp. were not identified to species level in the field but can assumed to be in the same proportions determined from otolith identification of a subsample, which comprised 74% C. gilchristi, 15% C. nudiceps, 8% C. natalensis and 3% C. saldanha and C. agulhensis (Lamberth et al. 2008). Other dominant species included Monodactylus falciformis (Lacepéde, 1801), which comprised between 0% and 11.7% of the catch and Rhabdosargus holubi (Steindachner, 1881), which comprised between 0.4% and 10.8% of the catch (Table 2). Redigobius dewaali (Weber, 1897), a subtropical estuarine species previously not recorded along the southwest coast, was recorded in catches almost every year after 2004 in the upper reaches (Table 1).
In terms of estuarine association categories, estuarine resident species (Category I) contributed between 23.7% (2004) and 72.4% (2003) of the catch and dominated the catch in 2003, 2005 and 2011. Estuary-associated marine migrants (Category II) contributed between 27.1% (2003) and 76.2% (2004) and dominated the catch in the other six years (Table 2).
Kendall’s coefficient of concordance (W = 0.74) indicated that there was little change in the rank order of abundance of individual species within the fish assemblage between years (χ2, r = 265, P < 0.05). The Spearman rank correlations showed a similar result with no significant differences between years, which indicates a similar rank order of abundance for the different species each year. Taxonomic distinctness (Δ*) (r2 = 0.258, P = 0.17) and average taxonomic distinctness based on presence/absence data (Δ+) (r2 = 0.183, P = 0.25) showed no significant (P < 0.05) linear trends over time.
Total CPUE varied considerably between years and ranged from 89 fish per seine net haul in 2009 to 367 fish per haul in 2004, which mirrors the considerable annual variation in the CPUE of individual species (Table 1).
Environmental influences on species composition
The estuarine fish assemblage separated into three distinct estuary reaches, regardless of year, except for fish assemblages sampled in 2002 and 2009 after two successive high flow events (Fig. 5). Results from a two-way crossed ANOSIM revealed similar trends, with highly significant differences among reaches (R = 0.76, P < 0.01) and differences between years being less important (R = 0.32). The most dissimilarity occurred between the upper and lower reaches (Table 3). Salinity was the dominant environmental variable structuring the fish assemblage in each reach, with the Monte-Carlo test indicating that although there was a significant (P < 0.001) relationship between the measured environmental factors and species abundance, only salinity was considered significant (P < 0.05).
Fish assemblages from sites in the upper reaches were associated with low salinity and higher temperatures and were grouped together on the right hand side of the ordination (Fig. 5). There was a significant negative correlation between salinity and abundance for L. richardsonii, L. dumerili, Hyporhamphus capensis (Thominot, 1886), Myxus capensis, G. aestuaria, P. knysnaensis and M. falciformis (Fig. 6) as these species were more abundant in the upper reaches (Fig. 5). The fish assemblages from sites that were recorded in the middle reaches were largely limited to the middle of the ordination. The lower reaches sites were clustered mainly at the left of the ordination, with high salinity and lower temperatures. Amblyrhynchotes honckenii (Bloch, 1785), Sarpa salpa (Linnaeus, 1758), Diplodus capensis (Smith, 1844), Heteromycteris capensis (Kaup, 1858), G. feliceps, Clinus superciliosus (Linnaeus, 1758), Rhabdosargus holubi, Liza tricuspidens (Smith, 1935) and Syngnathus temminckii (Kaup, 1856), were more abundant in the lower and middle reaches (Fig. 5), with their abundance significantly correlated with salinity (Fig. 6). The abundance of Amblyrhynchotes honckenii and S. temmickii was also significantly correlated with low temperatures (Fig. 6).
Bi-plots of t-values with Van Dobben circles showing pairwise relationships between fish species and environmental variables. The species arrows ending with their tips within a circle are predicted to respond significantly to the particular environmental variable. Grey circles represent positive relationships and white circles negative relationships
When flood events are viewed against the total CPUE (Fig. 7), there is no obvious relationship between high flow rates and abundance except after two floods occurred within two months of each other in the winter and spring of 2001 and in the winter and spring of 2008, the second flood in 2008 was a massive flood event, with flow exceeding 300 m3 s−1 (Fig. 7). The lowest total CPUE was recorded in February 2002 (98 fish/haul) and February 2009 (89 fish/haul), with these years also separating out in the CCA ordination (Fig. 5). This was as a result of low catches of most estuarine resident species, with the exception of G. aestuaria (Table 1). The average size of both Caffrogobius spp. (38 mm TL) and G. aestuaria (31 mm TL) was also lowest in 2002 and 2009 (Table 4). The severe flooding in 2001 and 2008, also affected some marine species, with the lowest abundance of G. feliceps recorded in 2002 and 2009 (Table 1) and the average size of L. richardsonii decreasing in 2002 and 2009 (Table 4). Interestingly, the single massive flood event (281 m3 s−1) recorded in September 2006 (late winter/early spring) had no obvious effect on fish abundance or size during the subsequent summer.
Discussion
Although aquatic communities in biogeographic transition zones are often characterised by high variability in species composition and abundance (Brodeur et al. 2003) the Breede Estuary summer fish assemblage was relatively stable in terms of annual taxonomic distinctness and annual and spatial species composition, with a similar assemblage recorded in each reach per year. The spatial distribution observed each year was largely driven by the salinity profile in the estuary, which differs little during the low rainfall summer period.
Estuaries are characteristically dominated by a few species (e.g., Jackson and Jones 1999; Garcia et al. 2003; Paperno and Brodie 2004) and the Breede conforms to that pattern (Harrison 1999; Lamberth et al. 2008). The majority of the Breede Estuary fish assemblage comprised six species that were consistently caught throughout the study period, and that are common in most permanently open South African warm-temperate estuaries (Harrison 2005). Assemblage stability indicates resilience to disturbances through persistence of species and relationships within a system (Holling 1973; Dugan and Livingston 1982). This was measured using Kendall’s coefficient of concordance (W) and taxonomic distinctness indices (Δ* and Δ+). Kendall’s coefficient of concordance (W) compared the ranking among species per year and a consistent rank abundance amongst years indicates a natural persistence of species in a system (Dugan and Livingston 1982). The coefficient of concordance in the Breede Estuary (0.74) indicates a 74% concordance of the rankings of the species caught each year, which implies stability and a persistence of species in the Breede Estuary fish assemblage structure over the study period, irrespective of the year-to-year variation in relative abundance and species richness.
Dugan and Livingston (1982) recorded a coefficient of concordance of 0.65 among the ranking of the macroinvertebrate species in the Econfina Estuary in Apalachee Bay (USA), which indicated resilience to natural disturbances. Similarly, stability in the fish assemblage structure of the temporarily open/closed East Kleinemonde Estuary (South Africa) (James et al. 2008) and Cedar Fork Creek (USA) (Meffe and Berra 1988) occurred with coefficient of concordance values of 0.57. Taxonomic distinctness indices can determine different aspects of biodiversity, which can then be used to interpret assemblage structure (Rogers et al. 1999). No significant temporal changes occurred in the taxonomic distinctness indices during this study despite several large flood events recorded during the study period. This implies a high degree of biotic resilience within the Breede Estuary fish assemblage. Similarly, over a four year period Whitfield (1986) recorded major changes in the biotic and abiotic environment in Swartvlei, a warm-temperate estuarine lake, but relatively minor changes in fish species composition and abundance. This was attributed to the resilience of southern African estuary-associated fish species and their ability to tolerate a wide range of conditions so that only severe changes over long time periods produce significant effects.
The fish communities in the lower, middle and upper reaches were consistent irrespective of time, which again demonstrates a fairly stable spatial and temporal trend within the fish assemblage structure. In the Breede Estuary, salinity recorded at the time of sampling strongly influenced the spatial structure of the fish assemblage, with the remaining environmental variables (temperature and winter and spring flow) being less important. There was very little inter-annual variation in summer salinity over the 10-year study period and this may explain the stable pattern observed. In the sub-tropical Patos Lagoon, Garcia et al. (2012) found relatively low spatial variation in the fish community and inter-annual patterns of fluctuation. In the Patos Lagoon, salinity showed strong inter-annual variation, with different patterns observed in different years. In South African estuaries, although most species are tolerant of a wide range of salinities (Whitfield et al. 2006), the abundance of 15 species has been positively correlated with salinity while several tropical species showed a negative correlation with salinity, occurring in lower salinities in subtropical estuaries (Harrison and Whitfield 2006).
The fact that high winter and spring flow had little impact on the overall fish assemblage was surprising. The Breede Estuary is subject to regular winter and spring flooding, where up to 50% of the annual rainfall may occur over a few days (Fig. 2). Episodic flood events play an important role in maintaining and enhancing biological productivity in estuarine systems (Gillson 2011) and often play a driving role in structuring fish communities. Wingate and Secor (2008) sampled the summer-autumn fish assemblage in the Patuxent Estuary (USA) and found that environmental conditions at the time of sampling had little influence on the fish assemblage and it was mean winter flow preceding sampling that affected the fish assemblage structure. High flow was associated with high abundances of some species, while the abundance of others were associated with low flow conditions. (Wingate and Secor 2008).
In the Breede Estuary, the mean monthly flow preceding summer sampling showed no observable correlation with the abundance of species, except in 2001 and 2008 when two anomalous floods with a 1/50 year return period, followed quickly in succession. These successive floods affected both abundance and diversity, with CPUE and the number of species recorded being lowest in the summer following these events. The first flood cued good recruitment but the second appeared to flush the new recruits out of the system. This result indicates that these estuary-associated fish species are adapted to respond positively to one event but not two in quick succession. This was also picked up in the bottleneck test for Argyrosomus japonicus (Temminck and Schlegel, 1843), where the population bottleneck coincided with these anomalous paired events (Mirimin et al. 2016).
The species most adversely affected by frequent river flooding were estuary resident species, with catches of Caffrogobius spp. lowest in 2002 and 2009. The average length of G. aestuaria (31 mm TL) and Caffrogobius spp. (38 mm TL) was also lowest in these years. These lengths correspond to fish less than one year old (Talbot 1982; Bennett 1989; Whitfield 1998) and suggest that estuarine resident species were flushed out of the system during the floods, with catches in the subsequent summer dominated by recently recruited 0+ juveniles. Strydom et al. (2002) found that G. aestuaria larvae were flushed out of the Great Fish River (South Africa) during periods of high flow. Boucek and Rehage (2014) suggested that flood events are likely to affect the abundance of individual species depending on the resilience of the species, rather than altering the overall species assemblage and this appears to be the case in the Breede Estuary.
Floods can temporarily reduce species diversity and abundance in estuaries (Gillson 2011), through the physical removal of animals and plants and through changes in factors such as salinity and turbidity (Marais 1982). Habitat diversity can, however, reduce the negative effects of a flood on fish species (Pearsons et al. 1992). Pearsons et al. (1992) found that fish assemblages in streams with complex habitats were more resilient to floods than those in simple streams. They attributed this to complex habitats dissipating the power of high discharges and complex habitats providing refuges for fishes during flooding. The Great Fish and Thukela estuaries (South Africa) contain limited habitat diversity (Strydom et al. 2002; Van Niekerk et al. 2012) and have shown a temporary decline in the abundance of fish species following high river flow conditions (Ter Morshuizen et al. 1996; Whitfield and Harrison 2003). The diverse range of habitats present within the Breede Estuary (Carter 1983) could explain why only successive floods had an effect on the fish community. The fish species occurring in the Breede may also be well adapted to variable flow because of the regular occurrence of floods in the estuary.
Marais (1982) compared the effects of floods on the marine fish communities of the Swartkops Estuary (South Africa), which has saltmarsh and extensive mud and sand flats, and the neighbouring Sundays Estuary, which has no saltmarshes and a narrow intertidal zone. They found that flooding decreased fish numbers, particularly mullet, in the Sundays Estuary, whereas flooding resulted in an increase in the abundance of mullet in the Swartkops Estuary. In an estuary, such as the Sundays which has limited habitat diversity, the decrease in abundance of mullet was attributed to the physical removal of silt, clay and benthic detrital material. In contrast, river flooding deposited silt in the Swartkops Estuary and after a lag period came food for mullet, which feed on diatoms associated with these rich deposits. Even in the Sundays Estuary, however, catches normalised within six months after the flood.
Although the fish assemblage in the Breede Estuary has been shown to be relatively resilient to extreme events (e.g., single river flooding events), global climate change will result in both altered freshwater flow and sea-level rise, thus influencing salinity which is the most important environmental variable governing structuring of the Breede Estuary fish community. Such change is likely to have profound effects on the species diversity and composition of the Breede Estuary fish assemblage. Future scenarios include an increase in the incidence and intensity of heavy precipitation events, as well as an increase in the frequency and duration of drought periods and ultimately changes in flow and salinity (Fauchereau et al. 2003; James et al. 2013). The Western Cape region that incorporates the Breede Estuary is projected to have a reduction of up to 10% in annual precipitation by the end of the twenty-first century as a result of climate change (De Wit and Stankiewicz 2006; Engelbrecht et al. 2009).
In addition, Lamberth et al. (2008) predicted that, under various reductions in freshwater flow scenarios, the Breede Estuary would shift from a freshwater rich to a marine dominated system. These authors suggested that estuarine-resident, catadromous and freshwater species would become less abundant and that estuary-dependent marine species such as Argyrosomus japonicus and Pomadasys commersonnii (Lacepéde, 1801) could collapse by 50% of historical numbers in association with an extreme river flow reduction of 64%. However, overall fish abundance would increase as marine opportunistic species, such as Liza richardsonii, took advantage of changed conditions and visual feeders become more prolific as turbidity decreases in the system. In turn, an increase in overall fish diversity is predicted for the Breede Estuary as some warm-temperate and subtropical estuarine species become established and some stenohaline marine fish species overwinter in the lower reaches of the estuary if seawater conditions prevail for extended periods in this region with the end result being range expansion.
Conclusions
The current medium-term study indicates that the Breede Estuary fish assemblage remained relatively stable between 2002 and 2012. Salinity was identified as the most important environmental variable responsible for spatial variability in the summer fish assemblages, and climate change is likely to directly and indirectly influence this variable in the future. Further, changes in the frequency of flood events may have a profound negative effect on fish recruitment, diversity and abundance whereas increases in the magnitude of single floods will not.
References
Bennett BA (1989) A comparison of the fish communities in nearby permanently open, seasonally open and normally closed estuaries in the south-western cape, South Africa. S Afr J Mar Sci 8:43–55
Boucek RE, Rehage JS (2014) Climate extremes drive changes in functional community structure. Glob Chang Biol 20:1821–1831
Brodeur RD, Pearcy WG, Ralston S (2003) Abundance and distribution patterns of nekton and micronekton in the northern California current transition zone. J Oceanogr 59:515–535
Carter RA (1983) Report No 21: Breë (CSW22). In: Heydorn AEF, Grindley JR (eds) Estuaries of the Cape. Part 2, Synopses of available information on individual systems. CSIR research report No. 420, Stellenbosch, pp 58
Claridge PN, Potter IC, Hardisty MW (1986) Seasonal changes in movements, abundance, size composition and diversity of the fish fauna of the Severn estuary. J Mar Biol Assoc UK 66:229–258
Coetzee DJ, Pool RC (1991) Diets of the larger fish species in the Breede River estuary, with emphasis on the prey species Palaemon capensis. Bontebok 7:27–35
Collie JS, Richardson K, Steele JH (2004) Regime shifts: can theory illuminate the mechanisms? Prog Oceanogr 60:281–302
Costa MJ, Vasconcelos R, Costa JL, Cabral HN (2007) River flow influence on the fish community of the Tagus estuary (Portugal). Hydrobiologia 587:113–123
Cuesta JA, Gonzáles-Ortegón E, Rodrĩguez A, Baldó F, Vilas C, Drake P (2006) The decapod crustacean community of the Guadalquivir estuary (SW Spain): seasonal and inter-year changes in community structure. Hydrobiologia 557:85–95
Day JH (1981) Summaries of current knowledge of 43 estuaries in southern Africa. In: Day JH (ed) Estuarine Ecology with particular reference to southern Africa. A.A. Balkema, Cape Town, pp 251–330
De Wit M, Stankiewicz J (2006) Changes in surface water supply across Africa with predicted climate change. Science 311:1917–1921
Department of Water Affairs and Forestry (2004) Breede water management area: internal strategic perspective. DWAF Report No. In: P WMA 18/000/00/0304. National water resource planning, South Africa
Dugan PJ, Livingston RJ (1982) Long-term variation of macroinvertebrate assemblages in Apalachee Bay, Florida. Estuar Coast Shelf Sci 14:391–403
Engelbrecht FA, McGregor JL, Engelbrecht CJ (2009) Dynamics of the conformal-cubic atmospheric model projected climate-change signal over southern Africa. Int J Climatol 29:1013–1033
Fauchereau N, Trzaska S, Rouault M, Richard Y (2003) Rainfall variability and changes in southern Africa during the 20th century in the global warming context. Nat Hazards 29:139–154
Flint RW (1985) Long-term estuarine variability and associated biological response. Estuaries 8(2):158–169
Garcia AM, Viera JP, Winemiller KO (2003) Effects of 1997-1998 el Niño on the dynamics of the shallow-water fish assemblage of the Patos lagoon estuary (Brazil). Estuar Coast Shelf Sci 57:489–500
Garcia AM, Viera JP, Winemiller KO, Moraes LE, Paes ET (2012) Factoring scales of spatial and temporal variation in fish abundance in a subtropical estuary. Mar Ecol Prog Ser 461:121–135
Gillson J (2011) Freshwater flow and fisheries production in estuarine and coastal systems: where a drop of rain is not lost. Rev Fish Sci 19:168–186
Harrison TD (1999) A preliminary survey of the estuaries on the south coast of South Africa, Cape Agulhas-cape St Blaize, Mossel Bay, with particular reference to the fish fauna. T Roy Soc South Africa 54:285–301
Harrison TD (2002) Preliminary assessment of the biogeography of fishes in south African estuaries. Mar Freshw Res 53:479–490
Harrison TD (2005) Ichthyofauna of south African estuaries in relation to the zoogeography of the region. Smithiana Bulletin 6:1–27
Harrison TD, Whitfield AK (2006) Temperature and salinity as primary determinants influencing the biogeography of fishes in south African estuaries. Estuar Coast Shelf Sci 66:335–345
Holling CS (1973) Resilience and stability of ecological systems. Annu Rev Ecol Syst 4:1–23
Hutchings K, Lamberth SJ (2003) The likely impacts of an eastward expansion of the inshore gill-net fishery in the western cape, South Africa: implications for management. Mar Freshw Res 54:39–56
Jackson G, Jones GK (1999) Spatial and temporal variation in nearshore fish and macroinvertebrate assemblages from a temperate Australian estuary over a decade. Mar Ecol Prog Ser 182:253–268
James NC, Whitfield AK, Cowley PD (2008) Long-term stability of the fish assemblages in a warm-temperate south African estuary. Estuar Coast Shelf Sci 76:723–738
James NC, van Niekerk L, Whitfield AK, Potts WM, Götz A, Paterson AW (2013) Effects of climate change on south African estuaries and associated fish species. Clim Res 57:233–248
Kendall MG (1962) Rank correlation methods, 3rd edn. Charles Griffin, London
Kling GW, Hayhoe K, Johnson LB, Magnuson JJ, Polasky S, Robinson SK, Shuter BJ, Wander MM, Wuebbles DJ, Zak DR, Lindroth RL, Moser SC, Wilson ML (2003) Confronting climate change in the great lakes region: impacts on our communities and ecosystems. Union of Concerned Scientists, Cambridge, Massachusetts, and Ecological Society of America, Washington
Konar B, Iken K, Pohle G, Miloslavich P, Cruz-Motta JJ, Benedetti-Cecchi L, Kimani E, Knowlton A, Trott T, Iseto T, Shirayama (2010) Surveying nearshore biodiversity. In: McIntyre AD (ed) Life in the world’s oceans diversity, distribution, and abundance. Blackwell, London, pp 27–41
Lamberth SJ, Van Niekerk L, Hutchings K (2008) Comparison of, and the effects of altered freshwater inflow on, fish assemblages of two contrasting south African estuaries: cool-temperate Olifants and the warm-temperate Breede. Afr J Mar Sci 30:311–336
Maes J, Taillieu A, Van Damme PA, Cottenie K, Ollevier F (1998) Seasonal patterns in the fish and crustacean community of a turbid temperate estuary (Zeeschelde estuary, Belgium). Estuar Coast Shelf Sci 47:143–151
Marais JFK (1982) The effects of river flooding on the fish populations of two eastern cape estuaries. S Afr J Zool 17:96–104
Marshall S, Elliot M (1998) Environmental influences on the fish assemblages of the Humber estuary, U.K. Estuar Coast Shelf Sci 46:175–184
Meffe GK, Berra TM (1988) Temporal characteristics of fish assemblage structure in an Ohio stream. Copeia 3:684–691
Mirimin L, Macey B, Kerwath S, Lamberth S, Bester-van der Merwe A, Cowley P, Bloomer P, Roodt-Wilding R (2016) Genetic analyses reveal declining trends and low effective population size in an overfished south African sciaenid species, the dusky kob (Argyrosomus japonicus). Mar Freshw Res 67:266–276
Montoya-Maya PH, Strydom NA (2009) Description of larval composition, abundance and distribution in nine south and west coast estuaries of South Africa. Afr Zool 44:75–92
Paperno R, Brodie RB (2004) Effects of environmental variables upon the spatial and temporal structure of a fish community in a small, freshwater tributary of the Indian River lagoon, Florida. Estuar Coast Shelf Sci 61:229–241
Pearsons TN, Li HW, Lamberti GA (1992) Influence of habitat complexity on resistance to flooding and resilience of stream fish assemblages. Trans Am Fish Soc 121:427–436
Rogers SI, Clarke KR, Reynolds JD (1999) The taxonomic distinctness of coastal bottom-dwelling fish communities of the north-east Atlantic. J Anim Ecol 4:769–782
Steynor AC, Hewitson BC, Tadross MA (2009) Projected future runoff of the Breede River under climate change. Water SA 35:433–440
Strydom NA, Whitfield AK, Paterson AW (2002) Influence of altered freshwater flow regimes on abundance of larval and juvenile Gilchristella aestuaria (Pisces: Clupeidae) in the upper reaches of two south African estuaries. Mar Freshw Res 53:431–438
Talbot MMJF (1982) Aspects of the ecology and biology of Gilchristella aestuarius (G & T) (Pisces: Clupeidae) in the Swartkops estuary. MSc thesis, University of Port Elizabeth, Port Elizabeth
Taljaard S, van Niekerk L, Huizinga P (2001) Breede river estuary ERF/RDM study. Specialist report on physical dynamics and water quality. CSIR report No. ENV-S-C 2001. Stellenbosch: Council for Scientific and Industrial Research
Ter Braak CJF (1986) Canonical correspondence analysis: a new eigenvector technique for multivariate direct gradient analysis. Ecology 67:1167–1179
Ter Braak CJF, Looman CWN (1994) Biplots in reduced-rank regression. Biom J 36:983–1003
Ter Morshuizen LD, Whitfield AK, Paterson AW (1996) Influence of freshwater flow regime on fish assemblages in the great fish river and estuary. S Afr J Aquat Sci 22:52–61
Thiel R, Sepúlveda A, Kafeman R, Nellen W (1995) Environmental factors as forces structuring the fish communities of the Elbe estuary. J Fish Biol 46:47–69
Turpie JK, Adams JB, Joubert AR, Harrison TD, Colloty BM, Maree RC, Whitfield AK, Wooldridge TH, Lamberth SJ, Taljaard S, Van Niekerk L (2002) Assessment of the conservation priority status of south African estuaries for use in management and water allocation. Water SA 28:191–206
Van Niekerk L, Lamberth SJ, Adams JB, McCord ME, Childs A-R (2012) Ecosystem services. In: Van Niekerk L, Turpie JK (eds), South African National Biodiversity Assessment 2011:Techical report. Volume 3: Estuary component. CSIR report No. CSIR/NRE/ECOS/ER/2011/0045/B. Stellenbosch: Council for Scientific and Industrial Research
Warwick RM, Clarke KR (1998) Taxonomic distinctness and environmental assessment. J Appl Ecol 35:532–543
Whitfield AK (1986) Fish community structure response to major habitat changes within the littoral zone of an estuarine lake. Environ Biol Fish 17:41–51
Whitfield AK (1994) An estuary-association for fishes of southern Africa. S Afr J Sci 90:411–417
Whitfield AK (1998) Biology and ecology of fishes in southern African estuaries. Icthyological monograph of the J.L.B. Smith Institute of Ichthyology 2:1–223
Whitfield AK, Harrison TD (2003) River flow and fish abundance in a south African estuary. J Fish Biol 62:1467–1472
Whitfield AK, Taylor RH, Fox C, Cyrus DP (2006) Fishes and salinities in the St Lucia estuarine system—a review. Rev Fish Biol 16:1–20
Wingate RL, Secor DH (2008) Effects of winter temperature and flow on the summer-fall nursery fish assemblage in the Chesapeake Bay, Maryland. Trans Am Fish Soc 137:1147–1156
Acknowledgements
Funding provided by the Department of Agriculture, Fish and Fisheries (DAFF) and National Research Foundation (NRF) is gratefully acknowledged. This project was reviewed by the Animal Ethics Committee of the South African Institute for Aquatic Biodiversity.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
James, N.C., Lamberth, S.J., Midgley, C. et al. Resilience of fish assemblages in the Breede Estuary, South Africa, to environmental perturbations. Environ Biol Fish 101, 109–126 (2018). https://doi.org/10.1007/s10641-017-0685-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-017-0685-z