Abstract
Transient ecosystem-level disturbances such as oxygen depletion (hypoxia) in aquatic systems modulate species distributions and interactions. In highly eutrophic systems, hypoxic areas (“dead zones”) have expanded around the world, temporarily preventing many demersal predators from accessing their food resources. Here, we investigate how yellow perch (Perca flavescens), an exploited, cool-water mesopredator, interact with their dominant invertebrate prey in benthic habitat–non-biting midge (chironomid) larvae–as bottom-water hypoxia develops in central Lake Erie (United States–Canada) during summer. We apply linear mixed-effects models to individual-level data from basin-wide field surveys on size-based interactions between perch and midge larvae under varying habitat conditions and resource attributes. We test if 1) midge populations (larval body size and density) differ among habitat states (unstratified normoxia, stratified normoxia, and stratified hypoxia); and 2) size-based perch–midge interactions (predator–prey mass ratio or PPMR) differ among habitat states with varying temperature and midge density. Midge populations remained highly abundant after bottom-water oxygen depletion. Despite their high densities, midge larvae also maintained their body size in hypoxic water. In contrast, perch on average consumed relatively smaller (by up to ~64%) midges (higher PPMR) in warmer and hypoxic water, while prey size ingested by perch shrunk less in areas with higher midge density. Our analysis shows that hypoxia-tolerant midges largely allow perch to maintain their consumer–resource relationships in contracted habitats through modified size-mediated interactions in dead zones during summer, revealing plasticity of their trophic coupling in the chronically perturbed ecosystem.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ecosystem state modulates energy fluxes that fuel primary and secondary production through trophic interactions in aquatic systems (Carpenter et al. 1985; Benndorf et al. 2002). However, ecosystem-level perturbations by human activities such as nutrient enrichment, pollution, and habitat destruction have disrupted species performance, populations, species assemblages, and food webs in many systems (Lotze et al. 2011; Lotze and Milewski 2004). In particular, areas with low dissolved oxygen (DO) concentration (hypoxia–defined hereafter as ≤2 mg O2 l−1) or “dead zones” induced by excessive nutrient loading have expanded (Diaz and Rosenberg 2008) and become of ecological importance worldwide, modifying species distributions and interactions (e.g., Mesa et al. 2005; Taylor et al. 2007). Although eutrophication may promote some warm-water herbivore (e.g., invasive carps) production (Djemali et al. 2017), further expansion of dead zones may have implications for ecosystem services such as fisheries production and distribution of cool- and cold- water species in these perturbed systems (Breitburg 2002; Breitburg et al. 2009).
Spatial and temporal variation in ecosystem properties such as temperature can drive individual-level processes of trophic interactions among ectotherms through prey accessibility and predator behavior (bottom-up control, Persson 1986; Diehl 1992; Abrahams et al. 2007). Moreover, natural and human-induced disturbances such as surface water warming and droughts may further constrain aquatic predator–prey interactions (Abrahams et al. 2007; Domenici et al. 2007). In eutrophic systems, when thermally stratified, hypoxia often develops as DO concentration declines to stressful levels for organisms below the pycnocline or thermocline. Hypoxia effects on predator–prey interactions thus likely depend on the tolerances (and resulting spatial redistributions) of the predator and the prey to hypoxia (Kolar and Rahel 1993; Abrahams et al. 2007). In some cases, spatial overlap between predators and prey may increase as their habitat sizes shrink as DO concentration declines (Costantini et al. 2008; Brandt et al. 2011). In bottom waters, resilient predators may experience higher prey availability as sediment-dwelling organisms are forced by hypoxia (or anoxia) to surface and become vulnerable to predation (Pihl et al. 1992; Roberts et al. 2012). By contrast, sensitive predators may miss foraging opportunities in benthic habitat and switch to alternative prey resources (e.g., from zoobenthos to zooplankton, Pothoven et al. 2009) as they escape into shallow oxygenated nearshore areas or into oxygenated surface waters (Magnuson et al. 1985; Zhang et al. 2009).
Stability and strength of predator–prey interactions also depend on species traits (Emmerson and Raffaelli 2004). Body size is such a trait that may filter environmental variability through physiological responses (e.g., temperature-dependent metabolism) and in turn govern ecological processes (e.g., size-dependent prey capture) in trophic interactions and energy transfer (Barnes et al. 2010; Brose et al. 2006; Woodward and Warren 2007; Goto and Wallace 2011). In hypoxic bottom-waters of thermally stratified systems, because of limited time for searching and handling prey, some predators may consume prey differently (Brante and Hughes 2001; Shin et al. 2005). Further, because of hypoxia-induced shifts in prey spatial distribution, these predators may also experience modified accessibility to size-structured resources (Pihl et al. 1992). Variability in predator–prey body mass ratio may thus reflect integrated processes in organisms and the environment modified by transient ecosystem disturbances (Woodward and Warren 2007; Nakazawa et al. 2011).
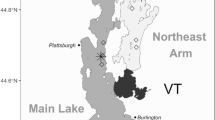
Here, we investigate consequences of summer bottom-water oxygen depletion for trophic coupling by yellow perch (Perca flavescens) in benthic habitats of a large, eutrophic system, Lake Erie (United States–Canada; Fig. 1). Its largest (central) basin (~11,000 km2) of Lake Erie has been experiencing increasingly widespread seasonal (late summer/early fall) hypoxia during recent decades because of human-driven environmental changes (Rucinski et al. 2010; Scavia et al. 2014). Recent ecosystem state shifts likely have induced a series of extreme events such as massive harmful algal blooms (Michalak et al. 2013), which may modify food web balance and threaten cool- and cold- water fisheries production (Scavia et al. 2014). Lake Erie yellow perch are a productive, cool-water mesopredator that supports one of largest capture fisheries in North American freshwaters (Roseman et al. 2008), with annual lake-wide total (commercial and recreational) harvest ranging from over 2700 to 5000 metric tons (~95% from Ontario and Ohio waters) during 2000–2015 (Belore et al. 2016). A benthic stage of perch bridges the gap between the planktivorous larval stage and the piscivorous adult stage, allowing them to grow out of size-dependent predation risk. Unlike many other predators, some perch continue to ingest zoobenthos such as chironomid (non-biting midge) larvae and pupae in hypoxic bottom waters (Roberts et al. 2009, 2012). We use individual-level data sets from large-scale field observations to examine variability in a size-mediated interaction between perch and midge larvae–a dominant benthic invertebrate–under varying habitat conditions and resource attributes to test the following questions: 1) do the quality and quantity of resources–midge populations (larval body size and density)–differ among bottom-water habitat states (stratification and oxygen depletion)?; and 2) do size-mediated consumer–resource links (perch–midge body size ratio) differ among habitat states with varying temperature and food density gradients?
Materials and methods
Central Lake Erie field surveys
We collected yellow perch (juveniles and adults) and non-biting midge larvae as part of large-scale scientific surveys, International Field Years of Lake Erie (IFYLE; www.glerl.noaa.gov/res/projects/ifyle/), conducted at 13 stations in the central basin of Lake Erie across two years (2005 and 2007). We sampled at three to five stations on June 17–21, August 15–18, September 17–19, and October 13–14 of 2005, and August 27–30 and September 17–20 of 2007 (Table 1 and Fig. 1). Detailed sample collection and processing methods were documented previously in Pothoven et al. (2009) and Roberts et al. (2009). Briefly, we conducted bottom (13 mm mesh lining) and mid-water (6.4 mm mesh lining) trawling every four hours over a 24-h sampling period along a five km transect at each station on each sampling date. Once collected, we identified, counted, and immediately stored fish samples at −20 °C until processing for diets in the laboratory. In the same cruises, we also collected zoobenthos once during each 24-h period in triplicate at the middle and endpoints of each site transect using a Ponar grab sampler (250 μm mesh; sampling area = 0.046 m−2; n = 9 per station and date). Depth-specific water temperature and dissolved oxygen data were recorded using a CTD (Conductivity, Temperature, and Depth), fluorometer and dissolved oxygen sensor (Sea-Bird Electronics, Bellevue, WA, USA) every 24 h at each east–west five-km station transect (n = 3 per station). We identified seven thermally unstratified normoxia (>2 mg O2 l−1), eight stratified normoxia, and six stratified hypoxia (≤ 2 mg O2 l−1) events during 2005 and 2007 surveys (Table 1).
In the laboratory, we measured the total mass (g wet) of each perch; in total, we processed 1126 fish from the 2005 and 2007 surveys [see Roberts et al. (2012, 2009) for detailed diet analysis]. We identified, counted, and measured (nearest 0.0001 mm) the body length (n = 5375 individuals, at least 20 individuals per fish sample when available) of midges in 433 perch stomachs (Table 2). Similarly, we identified, counted, and measured the body length (n = 3935 individuals, at least 20 individuals per sample when available) of midges in Ponar grab samples (Table 2). When parts of the body were missing (and fewer than 20 individuals were available), we measured the head capsule width (mm) and estimated the total body length (mm) using the following regression derived from the survey samples: ln(total body length) = 1.48 × ln(head width) + 3.37 (R 2 = 0.99). We then converted body length to mass using the following power function derived from Lake Erie midges from the 2005 survey: Body mass = 0.0013 × (total body length)2.69 × 10−3 (T. Nalepa, NOAA-GLERL, personal communication).
Statistical modeling
We tested for effects of 1) bottom-water habitat conditions on attributes of prey populations (midge larva body mass and biomass density), and 2) habitat conditions and prey attributes on predator–prey (yellow perch–midge larvae) mass ratio. For predator–prey mass ratio (PPMR), we used ‘individual-link PPMRs’–mass of an individual perch divided by mass of an individual midge in a perch stomach–to minimize potential bias associated with averaging of prey mass (Woodward and Warren 2007; Nakazawa et al. 2011). Further, we used a linear mixed-effects model to account for 1) the unbalanced, hierarchical nature (non-independence) of our field surveys (multiple prey items ingested by individual fish collected on different survey dates at the same station) (Zuur et al. 2009; Barnes et al. 2010), and 2) interannual and spatial variability in the onset of thermal stratification and bottom-water oxygen depletion (Table 1).
Midge larvae in the sediment
We tested for significance of fixed effects, bottom-water habitat state (nominal: unstratified normoxia, stratified normoxia, or stratified hypoxia) and mean temperature in the hypolimnion (°C; for unstratified stations, mean temperatures of the entire water column were calculated), on midge body mass (mg) and biomass density (g m−2) as a response variable with survey station (n = 13) as a random intercept. Midge biomass density was also added as a fixed effect for the models with body mass as a response variable to test for density dependence.
Perch–midge interactions
We tested if habitat conditions and prey attributes contribute to variation in individual-level perch–midge mass ratio; we tested for significance of four survey-level fixed effects; 1) bottom-water habitat state, 2) temperature, 3) midge density, and 4) perch biomass. We evaluated the following full model for perch k at station j (with survey station and perch as random intercepts):
where βs are fixed effect coefficients; PPMR jk is perch: midge mass ratio for perch k at station j, state j , temp j , dens j , and biom j are bottom-water state, temperature, midge density in the sediment, and perch CPUE from the bottom trawl surveys (kg hr.−1) at site j, b j S and b k P are normally distributed random effect intercepts (jth station and kth fish ID, respectively) with mean of zero and variance of σ 2, and ɛ jk is a normally distributed error term with mean of zero and variance of σ 2. PPMRs may vary with the time of the day (e.g., diel activities) or predator size (e.g., size-dependent foraging efficiency). However, our preliminary exploratory analysis showed that adding the terms of sampling time of the day or perch mass was not supported by our data and did not improve the model fit (based on a difference in Deviance Information Criterion or ΔDIC ≥7, Spiegelhalter et al. (2002); ΔDIC = 2.7 and 5.3, respectively); we thus did not test further for these terms in model evaluation.
We followed the two-step protocol of Zuur et al. (2009) to develop the linear mixed-effects models; we first evaluated the model structure of random effects (intercepts); station (n = 13) and individual fish ID (n = 433) as random intercepts separately and in combination (non-hierarchically and hierarchically structured) with ΔDIC ≥7 as a threshold (Spiegelhalter et al. 2002). The model with the selected random effects was subsequently evaluated for fixed effects. We evaluated the model convergence for random effects and fixed effects using restricted maximum likelihood and maximum likelihood, respectively (Zuur et al. 2009). The variance structures were evaluated visually for homogeneity and normality with plots of residuals and covariates. All the models were fit using a function, lmer, of an R package, lme4 (ver. 1.1–12). As reference, we also estimated pseudo-R 2 for a model with varying intercepts using a function, r.squaredGLMM, from an R package, MuMIn (Nakagawa and Schielzeth 2013; Johnson 2014). All the response variables were log10-transformed; temperature and prey covariates were log10-transformed and centered.
Results
Biomass density and body mass of midge larvae in the sediment
Biomass density of midge larvae in the sediment varied from 0.01 to 3.96 g m−2 and differed among bottom-water habitat states. Under normoxia, mean density was 2.5-fold greater when stratified than when unstratified (0.25 vs. 0.10 g m−2, respectively; Fig. 2a, Table 3). Further, mean density was 1.9-fold greater under hypoxia than stratified normoxia (0.47 vs. 0.25 g m−2, respectively; Fig. 2a, Table 3). Mean bottom-water temperature in central Lake Erie varied from 8.7 to 20.5 °C when stratified and from 9.0 to 23.9 °C when unstratified during summers of 2005 and 2007 (Table 1). However, including the temperature term did not improve the model fit (Table 3).
Biomass density (a) and body mass (b) of non-biting midge (chironomid) larvae in the sediment under varying bottom-water habitat states (unstratified normoxia, stratified normoxia, and stratified hypoxia) in central Lake Erie. Circles and error bars indicate mean ± 95% confidence intervals estimated by the selected linear mixed-effects models with habitat state as a fixed effect and station as a random intercept for biomass density as a response variable or with habitat state and temperature as fixed effects and station as a random intercept for body mass as a response variable (Table 3). In (b), the temperature effect is controlled by fixing at mean values across all surveys
Individual body mass of midge larvae in the sediment varied from 1.6 to 29.4 mg and differed among bottom-water habitat states. Under normoxia, mean body mass was 1.2-fold greater when unstratified than when stratified (10.5 vs. 8.8 mg, respectively; Fig. 2b, Table 3). Further, when stratified, mean body mass was 1.4-fold greater under hypoxia than normoxia (12.1 vs. 8.8 mg, respectively; Fig. 2b, Table 3). Mean body mass was negatively correlated with bottom-water temperature (ΔDIC = 13.3; Table 4). Moreover, including the interaction term, habitat state × temperature, improved the model fit (ΔDIC = 20.7; Table 3), revealing that the linear correlation between mean body mass and temperature was stronger when stratified. Including the midge density term did not improve the model fit (ΔDIC = 3.8; Table 3).
Perch–midge body mass ratios
Individual body mass of midge larvae in yellow perch stomachs varied from 2.0 to 14.2 mg with 29.9% of total variation found within individual fish and 28.6% among fish. Midge body mass in stomachs increased with perch body mass (ΔDIC = 23.1) and differed among bottom-water habitat states (ΔDIC = 11.4; Fig. 3a–c, Table 4). Under normoxia, realized perch–midge mass ratios were on average 1.3-fold greater (perch consuming smaller midges) when unstratified than when stratified (3836:1 vs. 2894:1, respectively; Fig. 4a, Table 4). Further, when stratified, realized perch–midge mass ratios were 1.6-fold greater under hypoxia than normoxia (4754:1 vs. 2894:1, respectively; Fig. 4a, Table 4). Perch–midge mass ratios were positively correlated with bottom-water temperature (ΔDIC = 44.7, Fig. 4b, Table 4); including the interaction term, habitat state × temperature, did not improve the model fit (ΔDIC = 4.8). Perch–midge mass ratios were also positively correlated with midge density in the sediment (Table 5). Further, including the interaction term, habitat state × midge density, improved the model fit (ΔDIC = 22.1; Table 4), revealing that mean mass ratios were negatively correlated with midge density under hypoxia, whereas mean mass ratios were positively correlated with midge density under normoxia (Fig. 4c). Including the perch biomass term did not improve the model fit (ΔDIC = 0.01, Table 5).
Relationships between log10-transformed body mass of central Lake Erie yellow perch (Perca flavescens) and non-biting midge (chironomid) larvae consumed by perch. Triangles, circles, and diamonds indicate observed mean midge mass ± standard deviation in each fish stomach under different bottom-water habitat states. Dash-dotted, solid, and dashed lines indicate the selected linear mixed-effects model with habitat state (unstratified normoxia, stratified normoxia, and stratified hypoxia, respectively) as a fixed effect and station and fish ID as random intercepts: (a) y = 0.76× – 2.36; (b) y = 0.76× – 2.25; and (c) y = 0.76× – 2.44
Body mass ratios (log10-transformed) of central Lake Erie yellow perch (Perca flavescens): non-biting midge (chironomid) larvae ingested by perch under varying bottom-water habitat states (unstratified normoxia, stratified normoxia, and stratified hypoxia), water temperature and midge biomass density. In a, circles and error bars indicate mean ± 95% confidence intervals estimated by the selected linear mixed-effects model. In (b) and (c), triangles, circles, and diamonds indicate observed mean ± standard deviation for survey-averaged fish under varying bottom-water habitat states. In (b) and (c), dash-dotted black, solid blue, and dashed red lines indicate the selected linear mixed-effects model with habitat state (unstratified normoxia, stratified normoxia, and stratified hypoxia, respectively), water temperature, and midge biomass density as fixed effects and station and fish ID as random intercepts (Table 4). In (b), the midge density effect in the model is controlled by fixing at mean values across all surveys; and in (c), the temperature effect is controlled by fixing at mean values across all surveys
Discussion
Our study based on individual-level field observations revealed that Lake Erie yellow perch largely maintained their trophic relationships with non-biting midge larvae in contracted benthic habitats through modified size-mediated interactions after bottom-water dissolved oxygen depletion during summer (Table 6). Bottom-water hypoxia may directly and indirectly enhance or restrict foraging by demersal predators (Pihl et al. 1992; Rahel and Nutzman 1994; Taylor and Eggleston 2000), modulating trophic interactions and transfer efficiencies in lake ecosystems (Schindler and Scheuerell 2002). In Lake Erie, midge populations remained productive in thermally stratified, hypoxic waters, becoming a dominant prey base (midge larvae comprised up to ~70% of zoobenthos biomass) for perch. Despite their high densities, midge larvae also maintained their body size in hypoxic water. In contrast, perch on average ingested relatively smaller midges (but in higher numbers) in hypoxic water, while prey size ingested by perch shrunk less in areas with higher midge density. These findings indicate that higher hypoxia tolerance of midge larvae may allow them to maintain an abundant food supply to perch, sustaining the perch–midge size-based link (albeit temporarily modified) in thermally stratified, hypoxic water during summer.
 ) indicate an increase in a response variable; down triangles (
) indicate an increase in a response variable; down triangles ( ) indicate a decrease in a response variable; and right triangles (
) indicate a decrease in a response variable; and right triangles ( ) indicate no effect on a response variable
) indicate no effect on a response variableBody size variation of Lake Erie midge larvae in the sediment may be more strongly regulated by size-based predation (indirectly) by demersal fishes including yellow perch responding to variable bottom water habitat conditions. When bottom water was well-oxygenated, midge larvae in the sediment attained larger body size in unstratified (warmer) water than stratified (cooler) water, independent of their density; however, their body size variation was negatively correlated with thermal variation in bottom water (Table 6). These seemingly contradictory patterns may have resulted from an indirect temperature effect, size-based predation by perch and likely other demersal predators, on midge body size. Top-down regulation of prey body size structure can occur in systems with high size-mediated predation rates, as demonstrated in a whole-lake experiment by Blumenshine et al. (2000). In Lake Erie, perch ingested larger midge larvae (lower PPMRs) in stratified, normoxic water than unstratified water (Table 6). Further, PPMR variation was also positively correlated with thermal variation in bottom water. Thermal stratification may allow cool-water (and perhaps cold-water) predators such as yellow perch to conserve energy (lower metabolic cost) and forage more efficiently in bottom water (Levy 1990; Mehner 2012), ultimately promoting size-biased predation effects on zoobenthos (Blumenshine et al. 2000).
The size-based perch–midge link appears to persist in oxygen-depleted bottom water. Despite severe hypoxia, perch continued to consume large amounts (~85% of the diet) of midges, likely through brief, frequent diving (a ‘foraging foray’) into bottom waters documented in hydroacoustic surveys (Roberts et al. 2012). However, relative prey size ingested by perch was up to ~64% smaller (higher PPMRs) in stratified, hypoxic water than stratified, normoxic water, whereas body size of midge larvae in the sediment was 37% larger in hypoxic water (Table 6). These reversed patterns may indicate less efficient foraging by perch (Brante and Hughes 2001; Shin et al. 2005) such as brief forays into hypoxic water, allowing them to capture only prey near the sediment surface. Severe hypoxia can restrict behaviors including foraging (Kramer 1987; Rahel and Nutzman 1994). Possible mechanisms involved in modified foraging by diving predators in hypoxic water may include; 1) reduced foraging duration–hypoxia can reduce the time spent by predators (with increased swimming speeds) in bottom water (Taylor et al. 2007; Neuenfeldt et al. 2009); and 2) reduced foraging efficiency–hypoxia may force predators to forage less selectively (e.g., lower net energy gain, Taylor et al. 2007). Such alternative foraging behaviors also have been documented for benthic and pelagic predators in other aquatic systems (Rahel and Nutzman 1994; Mistri 2004; Seibel 2011). Predatory marine crabs (Carcinus maenas and Thalamita danae), for example, spent more time in handling and selected smaller bivalve prey when temporarily exposed to hypoxia (Brante and Hughes 2001; Shin et al. 2005). Further studies on explicit mechanisms underlying foraging in hypoxic water (e.g., the number of and duration of dives by diving predators) would help understand energetic tradeoffs in foraging in food-rich, but potentially lethal waters (Scarbro 2014).
Density dependence may have played an indirect role in a size-based perch–midge interaction in stratified, hypoxic water. Although body size of midges in the sediment varied independently of their density, body size of midges ingested by perch varied with midge density in the sediment (Table 6). Further, this prey density-dependent predation by perch in hypoxic water differed from perch in normoxic water; body size of midges ingested by perch was positively correlated with midge density in hypoxic water, whereas ingested midge body size was negatively correlated with midge density in normoxic water. These contrasting patterns in size-mediated trophic interactions may be shaped by spatial distributions of size-structured prey populations under variable habitat conditions (Macciusi and Baker 1991; Pihl et al. 1992; Kornijów 1997). Transient hypoxia may promote production of resilient zoobenthos such as midges by releasing them from resource competition and predation in eutrophic systems (Brodersen and Quinlan 2006), allowing them to grow, survive, and reproduce at higher rates (a ~ 10–20-fold increase in biomass density in central Lake Erie). However, an experimental study also has demonstrated that hypoxia can induce upward movement within the sediment and heightened activities at the sediment–water column interface by larger midge larvae (Irving et al. 2004), which normally reside in deeper layers of the sediment (Panis et al. 1996). Although our sampling method prevented us from examining midge vertical distribution in the sediment (and thus midge size ranges encountered by perch), a positive density dependence in size-based predation of midges by perch indicate greater density (crowding) may have ‘promoted’ upward migration of larger midges in hypoxic water. Such prey aggregation forced by habitat contraction could increase their detectability by predators and reduce search time in a stressful environment (Rahel and Nutzman 1994; Aday et al. 2000). Prey behavioral responses to hypoxia may thus partially compensate for compromised foraging opportunities of predators by migrating between microhabitats (Rahel and Kolar 1990; Kolar and Rahel 1993). In Lake Erie, increased vulnerability of productive midge populations to perch predation (via vertical shifts) may have maintained a predator–prey link in stratified, hypoxic water, revealing plasticity of this predator–prey system.
A hypoxia-driven increase in vulnerability to predation is shared by other zoobenthos (Pihl et al. 1992; Kolar and Rahel 1993), zooplankton (Taylor and Rand 2003), and larval fish (Keister et al. 2000). Because of limited mobility and obligate benthic life history stages, zoobenthos often remain in hypoxic areas (Pihl et al. 1992; Rabalais et al. 2002; Craig et al. 2005) and become more vulnerable to predation (Pihl et al. 1992; Aday et al. 2000). Marine bivalves, for example, respond to hypoxia by protruding their siphon above the sediment surface (Seitz et al. 2003), increasing consumption of their siphons by predators. Although pelagic prey such as zooplankton and larval fish may escape from hypoxia as dissolved oxygen concentration progressively declines (Eby and Crowder 2002; Ludsin et al. 2009), some may become more exposed to pelagic predators (Costantini et al. 2008; Brandt et al. 2011). Because bottom-water hypoxia forces sensitive prey out of their dark bottom-water refugia into more illuminated and oxygenated waters (Goto et al. 2012), pelagic predators (piscivores and planktivores) that feed by sight may experience a short-term increase in prey encounter rates and thus consumption rates (Taylor and Rand 2003; Vanderploeg et al. 2009).
Amplified predator–prey interactions in contracted habitats also have been documented for other sources of natural and human-induced disturbances in aquatic ecosystems (Holt 1977; Domenici et al. 2007), including dry seasons in tropical river floodplains (Willis et al. 2005), droughts in temperate streams (Dewson et al. 2007; Goto et al. 2015), and fragmentation in tidal creeks (Layman et al. 2007). Under such disturbances, predators, prey, or both may modify their behaviors to minimize the risk of exposure to stressors (e.g., excessive heat), which can ultimately increase predator–prey encounter rates (Dewson et al. 2007; Domenici et al. 2007). Resulting changes in their interactions may thus modulate top-down or bottom-up effects in food webs and ultimately how an ecosystem operates (Ives et al. 2005; Heithaus et al. 2008; Frank et al. 2011).
Continued access to zoobenthos, abundant and energy-rich food sources, during summer is vital for growth and survival of demersal predators (Wu and Culver 1992; Gopalan et al. 1998; Goto and Wallace 2010). Resilient trophic coupling in the perch–midge system may further suggest perch’s critical role as a benthic–pelagic coupler (Schindler and Scheuerell 2002; Vander Zanden and Vadeboncoeur 2002), sustaining productive fisheries in frequently perturbed systems such as Lake Erie (Kraus et al. 2015). Modified benthic–pelagic coupling may however have additional management implications. Altered trait-mediated predator–prey interactions can cascade through food webs, potentially effecting further consequences (e.g., alternative energy pathways) in perturbed ecosystems (Schmitz et al. 2004). Many trophic interactions disrupted by transient disturbances such as summer bottom-water hypoxia may modify nutrient recycling and alter ecosystem productivity regimes (Österblom et al. 2007; Diaz and Rosenberg 2008; Scavia et al. 2014). With large-scale environmental changes such as climate warming and excessive land use, we may expect further increases in spatial and temporal variation in bottom-water oxygen depletion that may ultimately reshape benthic–pelagic coupling (Baird et al. 2004; Long and Seitz 2008). Monitoring transient size-mediated predator–prey interactions may therefore help the assessment of long-term implications for ecosystem productivity and resource sustainability in nutrient-enriched systems.
Change history
23 October 2017
In the original article lacks one institution of Daisuke Goto and incorrectly list the institution of Tomas O. Höök.
References
Abrahams MV, Mangel M, Hedges K (2007) Predator–prey interactions and changing environments: who benefits? Philos Trans R Soc, B 362(1487):2095–2104
Aday DD, Rutherford DA, Kelso WE (2000) Field and laboratory determinations of hypoxic effects on RNA-DNA ratios of bluegill. Am Midl Nat 143(2):433–442
Baird D, Christian RR, Peterson CH, Johnson GA (2004) Consequences of hypoxia on estuarine ecosystem function: energy diversion from consumers to microbes. Ecol Appl 14(3):805–822
Barnes C, Maxwell D, Reuman DC, Jennings S (2010) Global patterns in predator–prey size relationships reveal size dependency of trophic transfer efficiency. Ecology 91(1):222–232
Belore M, et al. (2016) Report of the Lake Erie Yellow Perch Task Group http://www.glfc.org/pubs/lake_committees/erie/YPTG.htm#pub
Benndorf J, Böing W, Koop J, Neubauer I (2002) Top-down control of phytoplankton: the role of time scale, lake depth and trophic state. Freshw Biol 47(12):2282–2295
Blumenshine SC, Lodge DM, Hodgson JR (2000) Gradient of fish predation alters body size distributions of lake benthos. Ecology 81(2):374–386
Brandt SB et al (2011) Does hypoxia reduce habitat quality for Lake Erie walleye (Sander vitreus)? A bioenergetics perspective. Can J Fish Aquat Sci 68(5):857–879
Brante A, Hughes RN (2001) Effect of hypoxia on the prey-handling behaviour of Carcinus maenas feeding on Mytilus edulis. Mar Ecol Prog Ser 209:301–305
Breitburg D (2002) Effects of hypoxia, and the balance between hypoxia and enrichment, on coastal fishes and fisheries. Estuaries 25(4):767–781
Breitburg D et al (2009) Nutrient enrichment and fisheries exploitation: interactive effects on estuarine living resources and their management. Hydrobiologia 629(1):31–47
Brodersen KP, Quinlan R (2006) Midges as palaeoindicators of lake productivity, eutrophication and hypolimnetic oxygen. Quat Sci Rev 25(15):1995–2012
Brose U et al (2006) Consumer–resource body-size relationships in natural food webs. Ecology 87(10):2411–2417
Carpenter SR, Kitchell JF, Hodgson JR (1985) Cascading trophic interactions and lake productivity. Bioscience 35(10):634–639
Costantini M, Ludsin SA, Mason DM, Zhang X, Boicourt WC, Brandt SB (2008) Effect of hypoxia on habitat quality of striped bass (Morone saxatilis) in Chesapeake Bay. Can J Fish Aquat Sci 65(5):989–1002
Craig JK, Crowder LB, Henwood TA (2005) Spatial distribution of brown shrimp (Farfantepenaeus aztecus) on the northwestern Gulf of Mexico shelf: effects of abundance and hypoxia. Can J Fish Aquat Sci 62(6):1295–1308
Dewson ZS, James AB, Death RG (2007) A review of the consequences of decreased flow for instream habitat and macroinvertebrates. J N Am Benthol Soc 26(3):401–415
Diaz RJ, Rosenberg R (2008) Spreading dead zones and consequences for marine ecosystems. Science 321(5891):926–929
Diehl S (1992) Fish predation and benthic community structure: the role of omnivory and habitat complexity. Ecology 73(5):1646–1661
Djemali I, Guillard J, Yule DL (2017) Seasonal and diel effects on acoustic fish biomass estimates: application to a shallow reservoir with untargeted common carp (Cyprinus carpio). Mar Freshw Res 68(3):528–537
Domenici P, Claireaux G, McKenzie DJ (2007) Environmental constraints upon locomotion and predator–prey interactions in aquatic organisms: an introduction. Phil Trans R Soc B 362:1929–1936
Eby LA, Crowder LB (2002) Hypoxia-based habitat compression in the Neuse River estuary: context-dependent shifts in behavioral avoidance thresholds. Can J Fish Aquat Sci 59(6):952–965
Emmerson MC, Raffaelli D (2004) Predator–prey body size, interaction strength and the stability of a real food web. J Anim Ecol 73(3):399–409
Frank KT, Petrie B, Fisher JA, Leggett WC (2011) Transient dynamics of an altered large marine ecosystem. Nature 477(7362):86–89
Gopalan G, Culver DA, Wu L, Trauben BK (1998) Effects of recent ecosystem changes on the recruitment of young-of-the-year fish in western Lake Erie. Can J Fish Aquat Sci 55(12):2572–2579. https://doi.org/10.1139/cjfas-55-12-2572
Goto D, Wallace WG (2010) Bioenergetic responses of a benthic forage fish (Fundulus heteroclitus) to habitat degradation and altered prey community in polluted salt marshes. Can J Fish Aquat Sci 67(10):1566–1584
Goto D, Wallace WG (2011) Altered feeding habits and strategies of a benthic forage fish (Fundulus heteroclitus) in chronically polluted tidal salt marshes. Mar Environ Res 72(1):75–88
Goto D, Hamel MJ, Hammen JJ, Rugg ML, Pegg MA, Forbes VE (2015) Spatiotemporal variation in flow-dependent recruitment of long-lived riverine fish: Model development and evaluation. Ecological Modelling 296:79–92
Goto D, Lindelof K, Fanslow DL, Ludsin SA, Pothoven SA, Roberts JJ, Vanderploeg HA, Wilson AE, Höök TO (2012) Indirect consequences of hypolimnetic hypoxia on zooplankton growth in a large eutrophic lake. Aquatic Biology 16 (3):217–227
Heithaus MR, Frid A, Wirsing AJ, Worm B (2008) Predicting ecological consequences of marine top predator declines. Trends Ecol Evol 23(4):202–210
Holt RD (1977) Predation, apparent competition, and the structure of prey communities. Theor Popul Biol 12(2):197–229
Irving EC, Liber K, Culp JM (2004) Lethal and sublethal effects of low dissolved oxygen condition on two aquatic invertebrates, Chironomus tentans and Hyalella azteca. Environ Toxicol Chem 23(6):1561–1566
Ives AR, Cardinale BJ, Snyder WE (2005) A synthesis of subdisciplines: predator–prey interactions, and biodiversity and ecosystem functioning. Ecol Lett 8(1):102–116
Johnson PC (2014) Extension of Nakagawa & Schielzeth's R2GLMM to random slopes models. Methods Ecol Evol 5(9):944–946
Keister JE, Houde ED, Breitburg DL (2000) Effects of bottom-layer hypoxia on abundances and depth distributions of organisms in Patuxent River, Chesapeake Bay. Mar Ecol Prog Ser 205:43–59
Kolar CS, Rahel FJ (1993) Interaction of a biotic factor (predator presence) and an abiotic factor (low oxygen) as an influence on benthic invertebrate communities. Oecologia 95(2):210–219
Kornijów R (1997) The impact of predation by perch on the size-structure of Chironomus larvae—the role of vertical distribution of the prey in the bottom sediments, and habitat complexity Shallow Lakes’ 95. Springer, p 207–213
Kramer DL (1987) Dissolved oxygen and fish behavior. Environ Biol Fish 18(2):81–92
Kraus RT et al (2015) Dynamic hypoxic zones in Lake Erie compress fish habitat, altering vulnerability to fishing gears 1. Can J Fish Aquat Sci 72(6):797–806
Layman CA, Quattrochi JP, Peyer CM, Allgeier JE (2007) Niche width collapse in a resilient top predator following ecosystem fragmentation. Ecol Lett 10(10):937–944
Levy DA (1990) Sensory mechanism and selective advantage for diel vertical migration in juvenile sockeye salmon, Oncorhynchus nerka. Can J Fish Aquat Sci 47(9):1796–1802
Long WC, Seitz RD (2008) Trophic interactions under stress: hypoxia enhances foraging in an estuarine food web. Mar Ecol Prog Ser 362:59–68
Lotze HK, Milewski I (2004) Two centuries of multiple human impacts and successive changes in a North Atlantic food web. Ecol Appl 14(5):1428–1447
Lotze HK, Coll M, Dunne JA (2011) Historical changes in marine resources, food-web structure and ecosystem functioning in the Adriatic Sea, Mediterranean. Ecosystems 14(2):198–222
Ludsin SA et al (2009) Hypoxia-avoidance by planktivorous fish in Chesapeake Bay: implications for food web interactions and fish recruitment. J Exp Mar Biol Ecol 381:S121–S131
Macciusi F, Baker RL (1991) Prey behaviour and size-selective predation by fish. Freshw Biol 25(3):533–538
Magnuson JJ, Beckel AL, Mills K, Brandt SB (1985) Surviving winter hypoxia: behavioral adaptations of fishes in a northern Wisconsin winterkill lake. Environ Biol Fish 14(4):241–250. https://doi.org/10.1007/bf00002627
Mehner T (2012) Diel vertical migration of freshwater fishes–proximate triggers, ultimate causes and research perspectives. Freshw Biol 57(7):1342–1359
Mesa MG, Poe TP, Gadomski DM, Petersen JH (2005) Are all prey created equal? A review and synthesis of differential predation on prey in substandard condition. J Fish Biol 45(sA):81–96
Michalak AM et al (2013) Record-setting algal bloom in Lake Erie caused by agricultural and meteorological trends consistent with expected future conditions. Proc Natl Acad Sci 110(16):6448–6452
Mistri M (2004) Predatory behavior and preference of a successful invader, the mud crab Dyspanopeus sayi (Panopeidae), on its bivalve prey. J Exp Mar Biol Ecol 312(2):385–398. https://doi.org/10.1016/j.jembe.2004.07.012
Nakagawa S, Schielzeth H (2013) A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol 4(2):133–142
Nakazawa T, Ushio M, Kondoh M (2011) Scale dependence of predator–prey mass ratio: determinants and applications. Adv Ecol Res 45:269–302
Neuenfeldt S, Andersen KH, Hinrichsen HH (2009) Some Atlantic cod Gadus morhua in the Baltic Sea visit hypoxic water briefly but often. J Fish Biol 75(1):290–294
Österblom H et al (2007) Human-induced trophic cascades and ecological regime shifts in the Baltic Sea. Ecosystems 10(6):877–889
Panis L, Goddeeris B, Verheyen R (1996) On the relationship between vertical microdistribution and adaptations to oxygen stress in littoral Chironomidae (Diptera). Hydrobiologia 318(1–3):61–67. https://doi.org/10.1007/bf00014132
Persson L (1986) Temperature-induced shift in foraging ability in two fish species, roach (Rutilus rutilus) and perch (Perca fluviatilis): implications for coexistence between poikilotherms. J Anim Ecol 55:829–839
Pihl L, Baden SP, Diaz RJ, Schaffner LC (1992) Hypoxia-induced structural changes in the diet of bottom-feeding fish and crustacea. Mar Biol 112(3):349–361
Pothoven SA, Vanderploeg HA, Ludsin SA, Höök TO, Brandt SB (2009) Feeding ecology of emerald shiners and rainbow smelt in central Lake Erie. J Great Lakes Res 35(2):190–198
Rabalais NN, Turner RE, Wiseman WJJ (2002) Gulf of Mexico hypoxia, AKA The Dead Zone. Annu Rev Ecol Syst 33:235–263
Rahel FJ, Kolar CS (1990) Trade-offs in the response of mayflies to low oxygen and fish predation. Oecologia 84(1):39–44
Rahel FJ, Nutzman JW (1994) Foraging in a lethal environment: fish predation in hypoxic waters of a stratified lake. Ecology 75:1246–1253
Roberts JJ, Höök TO, Ludsin SA, Pothoven SA, Vanderploeg HA, Brandt SB (2009) Effects of hypolimnetic hypoxia on foraging and distributions of Lake Erie yellow perch. J Exp Mar Biol Ecol 381(S1):S132–S142
Roberts JJ, Grecay PA, Ludsin SA, Pothoven SA, Vanderploeg HA, HööK TO (2012) Evidence of hypoxic foraging forays by yellow perch (Perca flavescens) and potential consequences for prey consumption. Freshw Biol 57(5):922–937. https://doi.org/10.1111/j.1365-2427.2012.02753.x
Roseman EF, et al. (2008) Ecology and international governance of Lake Erie’s percid fisheries. International governance of fisheries ecosystems: learning from the past, finding solutions for the future American Fisheries Society, Bethesda Maryland:145–169
Rucinski DK, Beletsky D, DePinto JV, Schwab DJ, Scavia D (2010) A simple 1-dimensional, climate based dissolved oxygen model for the central basin of Lake Erie. J Great Lakes Res 36(3):465–476
Scarbro BLB (2014) The physiological and behavioral responses of yellow perch to hypoxia. PhD Dissertation. The University of Toledo
Scavia D et al (2014) Assessing and addressing the re-eutrophication of Lake Erie: central basin hypoxia. J Great Lakes Res 40(2):226–246
Schindler DE, Scheuerell MD (2002) Habitat coupling in lake ecosystems. Oikos 98(2):177–189
Schmitz OJ, Krivan V, Ovadia O (2004) Trophic cascades: the primacy of trait-mediated indirect interactions. Ecol Lett 7(2):153–163
Seibel BA (2011) Critical oxygen levels and metabolic suppression in oceanic oxygen minimum zones. J Exp Biol 214(2):326–336
Seitz RD, Marshall L, Hines A, Clark K (2003) Effects of hypoxia on predator-prey dynamics of the blue crab Callinectes sapidus and the Baltic clam Macoma balthica in Chesapeake Bay. Mar Ecol Prog Ser 257:179–188
Shin P, Cheung P, Yang F, Cheung S (2005) Intermittent exposure to reduced oxygen levels affects prey size selection and consumption in swimming crab Thalamita danae Stimpson. Mar Pollut Bull 51(8):1003–1009
Spiegelhalter DJ, Best NG, Carlin BP, Van Der Linde A (2002) Bayesian measures of model complexity and fit. J R Stat Soc Ser B (Stat Methodol) 64(4):583–639
Taylor DL, Eggleston DB (2000) Effects of hypoxia on an estuarine predator-prey interaction: foraging behavior and mutual interference in the blue crab Callinectes sapidus and the infaunal clam prey Mya arenaria. Mar Ecol Prog Ser 196:221–237
Taylor JC, Rand PS (2003) Spatial overlap and distribution of anchovies (Anchoa spp.) and copepods in a shallow stratified estuary. Aquat Living Resour 16(3):191–196
Taylor JC, Rand P, Jenkins J (2007) Swimming behavior of juvenile anchovies (Anchoa spp.) in an episodically hypoxic estuary: implications for individual energetics and trophic dynamics. Mar Biol 152(4):939–957. https://doi.org/10.1007/s00227-007-0745-6
Vander Zanden MJ, Vadeboncoeur Y (2002) Fishes as integrators of benthic and pelagic food webs in lakes. Ecology 83(8):2152–2161
Vanderploeg HA et al (2009) Hypoxic zones as habitat for zooplankton in Lake Erie: refuges from predation or exclusion zones? J Exp Mar Biol Ecol 381:S108–S120
Willis S, Winemiller K, Lopez-Fernandez H (2005) Habitat structural complexity and morphological diversity of fish assemblages in a Neotropical floodplain river. Oecologia 142(2):284–295
Woodward G, Warren P (2007) Body size and predatory interactions in freshwaters: scaling from individuals to communities. In: Hildrew AG, Raffaelli DG, Ronni Edmonds-Brown R (eds) Body Size - TheStructure and Function of Aquatic Ecosystems, 1st edn. Cambridge University Press, Cambridge, pp 98–117
Wu L, Culver DA (1992) Ontogenetic diet shift in Lake Erie age-0 yellow perch (Perca flavescens): a size-related response to zooplankton density. Can J Fish Aquat Sci 49(9):1932–1937
Zhang H et al (2009) Hypoxia-driven changes in the behavior and spatial distribution of pelagic fish and mesozooplankton in the northern Gulf of Mexico. J Exp Mar Biol Ecol 381:S80–S91
Zuur A, Ieno EN, Walker N, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer
Acknowledgements
We thank all those who provided lab, field, or data management support, including the captains and crew of the R/V Laurentian and R/V Lake Guardian, Anne Clites, Marco Constantini, Hal Gunder, Darryl Hondorp, Sean Sisler, Joann Calvaletto, Theodore Bambakidis, Anna Belyeava, Grace Milanowski, Megan Miner, and Chris Rae. This work was supported by the NOAA Center for Sponsored Coastal Ocean Research grant (No. NA07OAR432000) with samples and experiments conducted as part of the International Field Years on Lake Erie (IFYLE) program, supported primarily by NOAA-GLERL, the US EPA Great Lakes National Program Office, and the National Sea Grant College Program. This manuscript is NOAA-GLERL contribution # xxxx and EcoFore Lake Erie publication No. 1863.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Human participants and animal study
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study formal consent is not required.
Additional information
A correction to this article is available online at https://doi.org/10.1007/s10641-017-0683-1.
Rights and permissions
About this article
Cite this article
Goto, D., Roberts, J.J., Pothoven, S.A. et al. Size-mediated control of perch–midge coupling in Lake Erie transient dead zones. Environ Biol Fish 100, 1587–1600 (2017). https://doi.org/10.1007/s10641-017-0667-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-017-0667-1