Abstract
During inshore migration, glass eels experience great environmental changes they must adapt to, but there have been few studies on the behavior of juvenile Japanese eels, Anguilla japonica, entering estuaries. To investigate the relative effects of periodic environmental changes on their inshore migration and ascending behavior from the bottom to swim with flood tidal currents, year-round collections were carried out from December 2003 to December 2005 inside Hamana Lake, Japan, where strong tidal flows occur. Glass eel collections (N = 543) near the inlet to the lake indicated that recruitment occurred mainly in winter from November to May. Time-series autocorrelation analysis of average daily catch rates found negative autocorrelation coefficients at 16–20 days periodicities and positive ones at 30–32 days periodicities in the 2004–2005 season, suggesting that the amount of recruiting glass eels fluctuates with a lunar cycle. Abundances of glass eels showed a diel rhythm and a tidal rhythm with eels appearing only at night and catches during flood tide being higher than during ebb tide, indicating ‘Selective Tidal Stream Transport’ that eels utilize flood tidal-streams selectively at night to migrate inshore. The generalized additive models (GAM) for the abundance suggested that relatively long timescale factors such as ‘recruitment season’ and ‘lunar age’ could affect the abundance of glass eels. The GAM for glass eel presence/absence indicated that the timing of glass eel ascending into the water column during flood tide at night was at 150–180 min after low tide, which tended to synchronize with the rise of water temperature caused by the inflow of warm water from the Pacific coast outside the lake. Results suggested that a temperature increase at the beginning of flood tide may be a cue for the ascending behavior, or at least could be a zeitgeber of the circatidal rhythm regulating the ascending behavior.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recruitment of glass eels into continental habitats occurs in two distinct phases: a migration from the sea into estuaries, called inshore migration, and a migration from estuaries into rivers, called upstream migration (Jellyman 1977). During inshore migration, glass eels experience various environmental changes of great magnitude that they must adapt to. The aquatic environment in coastal areas has various solar and lunar related rhythms with periodicities at scales such as annual, lunar, semilunar, diel and semi-diel. Periodic phenomena in fish behavior and the adaptation to these rhythms have been examined in various fish species (e.g. Gibson 1992; Leatherland et al. 1992), and are also known to occur in recruiting anguillid glass eels (Harrison et al. 2014).
The anguillid eels have a catadromous life history during which they migrate between their oceanic spawning areas and continental growth habitats. The Japanese eel, Anguilla japonica, spawns near the West Mariana ridge (Tsukamoto 1992, 2006) and their leptocephali are transported towards their recruitment areas by the North Equatorial Current and the Kuroshio Current (Kimura et al. 1994). Leptocephali metamorphose into glass eels in the Kuroshio (Otake et al. 2006) and then they migrate towards land. The inshore migration of glass eels is one of the most important events in their life history, because this is the final stage of their recruitment process determining the success of the transition from oceanic to estuarine and freshwater habitats.
Serious declines of the Japanese eel, A, japonica, have been documented (Tsukamoto et al. 2009; Jacoby and Gollock 2014), so effective resource management based on accurate ecological information is urgently needed (Yokouchi et al. 2008). Ecological information about Japanese eels during their growth phase in continental waters is still limited compared to that about European eels and other temperate anguillid species (Tsukamoto et al. 2009). Investigating the yearly glass eel recruitment abundance would be a useful way to evaluate if eel resource management works effectively, and understanding their migration patterns is necessary to design effective scientific monitoring methods.
The inshore and upstream migrations of anguillid glass eels and elvers are affected by many periodic changes as well as other environmental factors unrelated to periodicity such as rain fall, river flow, coastal current speed, temperature or turbidity (Gandolfi et al. 1984; White and Knights 1997; Laffaille et al. 2007; Zompola et al. 2008; Hwang et al. 2014; Barry et al. 2015). Recruitment of temperate eels to estuaries is seasonal and occurs mainly in winter to spring in the Japanese eel (Tzeng 1985; Yamamoto et al. 2001; Shinoda 2004; Han 2011) and other temperate species (A. anguilla: Tesch 2003; Zompola et al. 2008; A. rostrata: Haro and Krueger 1988; Powles and Warlen 2002; A. australis, A dieffenbachii: Jellyman 1977; Jellyman et al. 1999), whereas recruitment of some tropical eels such as A. marmorata and A. bicolor occurs to some degree throughout the year (Arai et al. 1999; Sugeha et al. 2001; Aoyama et al. 2015). Although it’s also known that semilunar or lunar cycles affect the upstream migration of anguillid glass eels and elvers (A. japonica: Tzeng 1985; Hwang et al. 2014; A. anguilla: Ciccotti et al. 1995; Zompola et al. 2008; A. rostrata: Sorensen and Bianchini 1986; Sullivan et al. 2006; A. australis, A dieffenbachii: Jellyman 1979; Jellyman and Lambert 2003), different temporal patterns were often seen among localities or years even in the same species (Tzeng 1985; Jellyman and Lambert 2003; Zompola et al. 2008). At short time scales, tidal cycles usually have semidiurnal periodicity and they influence the migration of glass eels and elvers because they utilize the upstream flows during flood tides to migrate into river systems, which is called ‘Selective Tidal Stream Transport’ (STST) (Creutzberg 1959, 1961; Gascuel 1986; McCleave and Kleckner 1982; Wippelhauser and McCleave 1987).
Various periodic environmental changes with short to long timescale have influences on glass eel recruitment that produce a complex recruitment wave, but relative effects of each periodic factor have not been clarified. In addition, ascending behavior associated with STST was hypothesised to be under the control of endogenous rhythms (biological clock), rather than exogenous cues, with the result that glass eels often ascend from the bottom when the tide is still ebbing (Wippelhauser and McCleave 1987). However, the mechanisms of this behavior are unclear, given that tidal strength and duration varies both spatially and temporally. Therefore it seems plausible to assume that glass eels, at least in the later stages of development, must also use exogenous cues to control the timing and duration of vertical migrations (Harrison et al. 2014). Understanding the mechanisms from field studies has not been progressed since the report about A. rostrata by Wippelhauser and McCleave (1987) and has never been studied in Japanese eels.
Our study was designed to examine the relative effects of various periodic factors on glass eel recruitment and their ascending timing during flood tides. The sampling station in this study was located approximately 2 km inside the entrance of the Hamana Lake where a boundary line between warm seawater of the pacific coast and cold seawater of the Lake was seen in winter, which tidally goes in and out. The location is an effective place to observe how A. japonica glass eels utilize flood tidal currents. Our surveys were conducted during two recruitment seasons to facilitate understanding of the effects of various periodicities on glass eel recruitment and the way they may synchronize their activity with tidal cycles.
Materials and methods
Study site and temperature measurement
Hamana Lake is located in the middle region of Japan (34°45’N, 137°35’E) and is connected to the Pacific Ocean through a 200 m wide channel (Fig. 1). The narrow channel causes powerful tidal-streams both into and out of the lake, which continue to flow through the second set of constrictions a few kilometers from the lake where sampling occurred (St. B, Fig. 1). In winter, the water temperature is different between inside and outside the lake because the winter atmosphere makes the lake water colder (Uemura and Fushimi 1979). The difference in water temperature is approximately 5 °C in winter. Therefore, temperature can be used to evaluate the movement of sea water into the lake that can potentially transport glass eels as they enter the lake.
To observe horizontal temperature fluctuations by tidal currents from the opening to the center of the Lake, time-series temperature was measured at seven stations (St. A to St. G, Fig. 1). Seven temperature data loggers (Stowaway TidbiT, Onset Computer Corporation, USA) were set at each station to measure temperatures at 5–15 min. intervals from 18 Jan. to 28 Feb. 2004 during which period clear differences in water temperature between inside and outside the lake were seen. Each logger was tied at a depth of 1.5 m to a rope extended from the bottom weight to a surface buoy. The horizontal fluctuation of temperatures was visualized using the Ocean Data View software (Schlitzer 2009). To monitor the year-round temperature, measurements were continued at St. B and St. G from 9 April 2004 to 9 September 2005 except for 7 August 2004 to 12 October 2004.
Glass eel collection
Glass eel collection was carried out at St. B under a bridge crossing the lake, which is located approximately 2 km inside the narrow channel entering the lake (Fig. 1). To monitor various possible timescales of glass eels moving through the lake after entering the opening, sampling for glass eels was carried out from December 2003 to December 2005. A ring net (1 mm mesh, 0.56 m diameter, 3 m long) deployed from the bridge was used as fishing gear (Fig. 2). The net was attached to a rope with a large bottom weight (30 kg) at the end, and it sampled at a fixed position depth of 0–1 m as tidal currents flowed past it. The net sampling was conducted only near the surface because of the shallow water at the sampling site (approximately 2–3 m) and the difficulty in keeping the net near the bottom while in the strong tidal current. The net was always oriented to face toward the current, so the fishing efficiency was the same during ebb tide and flood tide.
Diagrams of the plankton net and how it was deployed to collect glass eels at St. B. A rope formed a triangle through three pulleys was attached to the railing of the bridge and a 30 kg anchor. A plankton net with a flow meter was attached to the rope that was movable from the bridge to water, which enabled rapid deployment and retrieval
To investigate the seasonality of glass eel recruitment, the net was deployed during flood tide at night around the new moon of every month except for May, June, July and Sep. in 2004, and July in 2005. To analyze whether glass eel catches fluctuate in a lunar cycle (new moon - full moon cycle) or semilunar cycle (neap - spring cycle), sampling during flood tide at night was conducted every 2 days during the glass eel recruitment season ranging from 30 Dec 2003 to 11 Mar 2004 (2003–2004 season) and from 13 Dec 2004 to 7 Mar 2005 (2004–2005 season). The length of time of each tow was 5–60 min (mostly 10–20 min) and the interval between tows was 10–80 min.
Additional sampling was conducted to investigate the influence of diel and tidal cycle on glass eel recruitment. To compare the catch rates of glass eels between flood tide and ebb tide, surveys during ebb tide were carried out on 16 nights. The interval between tows during ebb tide was 25–110 min. To compare the catch rates between day and night, surveys during flood tide were carried out from 1 to 3 h before dusk or after dawn. Twenty four hour surveys were also carried out on 9 Feb. and 2 Dec in 2005.
At the beginning of each tow, temperature was measured using digital thermometer (Japan Pet Drugs Co., Ltd., Japan) during the 2003–2004 season and temperature and salinity were measured using a handy meter (YSI Model 85, YSI/Nanotech Inc., Japan) during the 2004–2005 season. The water volume filtered during each tow was measured using a flow meter (Rigo Co., Ltd., Japan) attached to the net. Current speed was calculated from the flow meter as meters per minute. When current speed was slow, the flow meter did not perform accurately, so the tows with less than 2 cycle/s (approximately 20 cm/s) of the flow meter were excluded from data analysis. The change of water level in the lake was delayed according to the distance from the opening of the lake (Matsuda 1999). The time lag between the back part of the lake and the site of the tide gage near the opening of the lake was 2.5–3 h (Matsuda 1999). In this study, the time of low tide at the sampling site was defined as 2 h after the time of low tide at the tide gage (data acquired from the Japan Metrological Agency, http://www.jma.go.jp). Glass eels were preserved as frozen samples. Afterward, their total length, preanal length, predorsal length (to the nearest 0.1 mm) and wet body mass (to the nearest 0.001 g) were measured, and their pigmentation stages were classified based on the stages defined by Fukuda et al. (2013).
Statistical analyses
The difference of total length and body weight among months in both recruitment seasons was tested using analysis of variance (ANOVA). If there was a statistically significant difference among months, a Dunn-Bonferroni multiple comparison test was conducted. The difference in the pigmentation stages among months was tested using Kruskal-Wallis tests. Given the significance among months, a Steel Dwass multiple comparison test was conducted.
Catch rates (ind./100 m3 of water filtered) were calculated from the data of each tow and used for various time-series analyses. Daily catch rate was calculated as mean catch rate of all tows during a flood tide in one night. To detect lunar/semilunar periodicity in the 2003–2004 and 2004–2005 seasons, autocorrelation analysis was conducted using a time series dataset of daily catch rate. To compare catch rates between ebb tide and flood tide periods, a Mann Whitney U-test was used.
To clarify the timing of glass eel migration during flood tides at night, the effects of temporal factors on the presence/absence and the abundance of glass eels were examined by generalized additive models (GAMs). GAMs are able to deal with non-linear relationships between a response variable and multiple explanatory variables in the same model, and are nonparametric generalizations of multiple linear regression that are less restrictive in assumptions about the underlying distribution of data (Hastie and Tibshirani 1990). Data from 357 tows during flood tide at night in the 2003–2004 and 2004–2005 recruitment season were used as a dataset because they contained data of all the candidate factors. Statistical analysis of GAMs was carried out using the R software with the mgcv package (R Development Core Team 2009).
Due to the high frequency (197/357 tows, 55 %) of zero values in the data set, a Delta-X error-model was employed, which makes it possible to treat separately the question of whether an abundance rate is zero or not, and the size of a rate given that it is non-zero (Vignaux 1996). For the purposes of this analysis, the value 0 was assigned to tows with no glass eels and the value 1 was assigned to positive ones. The GAM for glass eel presence/absence was fitted with a binomial distribution and a logistic link function as follows,
where P is the probability of glass eels presence in a tow, s() is a smoothing cubic spline, a is a constant. Automatic selection of the degrees of freedom for the regression splines were performed using the generalized cross validation method based on minimizing the jackknifed squared prediction error. ‘Recruitment season’ and ‘Month’ were dealt with as categorized factors. The water volume (m3) of each tow was used as an offset to eliminate the effect of the volume on the catchability. Subsequently, the GAM for glass eel abundance was fitted using a dataset with non-zero catches. In the GAM, catch, which is the number of glass eels in each tow, was a response variable with a Poisson distribution accompanied by a log link function. The same explanatory variables and offset in presence/absence model were considered for inclusion in the model.
In all GAMs, variable selection proceeded by a stepwise forward entry and the Akaike Information Criterion (AIC) was used to detect the relative importance of each explanatory variable in explaining variations and determine the order of those that should be included in the final model. The AIC statistic accounts simultaneously for the degrees of freedom used and the goodness of fit, whereas a smaller AIC statistic corresponds to a better model in the sense of smaller residual deviance penalized by the number of parameters that are estimated in fitting the model. A stepwise forward entry adopted an explanatory variable that reduce AIC the most. A final model was obtained from the continuous stepwise forward entry until no variables have reduced AIC or have significant p-values (<0.05). To evaluate the significance of each additional factor, p-values based on an ANOVA F-ratio test between the model and the previous model were used. Additionally, to evaluate the non-linear contribution of a non-parametric term, a Chi-square test was used.
Results
Temperature changes with tidal flow in the lake
The movement of seawater into the lake that can potentially transport glass eels as they enter the lake was monitored by water temperature. The horizontal distribution of water temperature at 1.5 m depth was observed to change in a semidiurnal cycle (Fig. 3) along the central region of the lake. This was due to the exchange of oceanic water (warm) and lake water (cold) with distinctly different temperatures in winter. The strength of the inflow during flood tide changed according to a semilunar cycle. During 1 to 9 Feb. in 2004, the oceanic water with higher temperatures flowed into the lake as far as 6.5 km during spring and intermediate tides and 5 km during neap tides (Fig. 3).
During sampling in the 2003–2004 and 2004–2005 seasons, the inflow of oceanic water with high temperature and slightly high salinity was observed during flood tide. The water temperature rapidly increased about 120–180 min after low tide during winter surveys (Fig. 4). Slight salinity increases (by a salinity up to about five) were synchronized with the temperature changes during the winter surveys in 2004–2005 season (Fig. 4).
Time series fluctuations of water temperature during the 2003–2004 and 2004–2005 seasons and salinity during the 2004–2005 season at the glass eel sampling site (St. B in Fig. 1)
Total length, body weight and pigmentation stage of glass eels
A total of 543 glass eels (total length: 57.5 ± 2.4 mm) were caught in 197 of the 574 net tows during all periods of the surveys (Table 1). The total length was not significantly different among months in the 2003–2004 and 2004–2005 seasons, except for a significant difference between November and January in the 2004–2005 season (Dunn-Bonfferoni, p < 0.05). The body weight was not significantly different among months in both seasons (ANOVA, p > 0.05, Table 1). The pigmentation was not significantly different among months in the 2003–2004 (Kruskal Wallis, p > 0.05, Table 1), but was significantly different in the 2004–2005 season (Kruskal Wallis, p < 0.05, Table 1). In the 2004–2005 season, the pigmentation in February was significantly more advanced than that in January that was more advanced than in November (Steel Dwass, p < 0.05, Table 1).
Relationship between glass eel catch and periodicities
The results of the year-round collections showed that recruitment of glass eels had seasonality and occurred from November to May (Table 1). Daily catch of glass eels indicated peaks around the last quarter moon of January during both recruitment seasons (Fig. 5). The catch rate during both recruitment seasons tended to increase around new moon and decrease around full moon, except for a smaller pulse of recruitment in February 2004 just before full moon (Fig. 5). The autocorrelation of daily catch rate in the 2004–2005 season had a significant negative correlation at 16–20 days periodicities and a positive correlation at 30–32 days periodicities, suggesting that the daily catch rate fluctuated not with semilunar cycle, but with lunar cycle (p < 0.05, Fig. 6a). Similarly, the autocorrelation of daily catch rate in the 2003–2004 season had the highest negative correlation at a 16 days periodicity and the highest positive correlation at a 32 days periodicity (Fig. 6b), though it was not significant.
Time series of daily catch rates of glass eels (ind./100 m3 water) in the 2003–2004 and 2004–2005 seasons at the sampling site (St. B in Fig. 1). New moon (●), full moon (○) periods are shown. The dashed lines indicate minimum water temperature during flood tide (arround low tide) and the solid lines indicate maximam water temperature during flood tide (arround high tide)
Correlograms of daily catch rates of glass eels in 2003–2004 and 2004–2005 seasons at the sampling site (St. B in Fig. 1). The dashed line indicates the significance level (p = 0.05)
Glass eels were only caught at night and primarily during flood tides. Despite 63 tows being made during the day, no glass eels were caught in those tows. At night, the average abundance of glass eels during flood tide (2003–2004: avg. 1.62 ind./100 m2, 2004–2005: 0.36 ind./100 m2) was higher than that in ebb tide (2003–2004: 0.01 ind./100 m2, 2004–2005: 0.04 ind./100 m2) (Mann-Whitney U-test, p < 0.05, Fig. 7). The ratio of the abundance during ebb tides to that of flood tides ranged 0–46 % and was 5.5 % on average.
Generalized additive model
In the GAM analysis, the presence/absence during flood tide at night and the abundance when it was non-zero catches were explained by different explanatory variables in each final model (Table 2). The GAM for presence/absence selected the variables of ‘Minutes after low tide’, ‘Recruitment season’, ‘Lunar age’, ‘Water temperature’ and ‘Time of day’ in order of relative importance (Table. 2). The variables of ‘Minutes after low tide’, ‘Lunar age’ and ‘Recruitment season’ contributed to 91.7 % of explained deviance in the best model. The curves in Fig. 8a show the effects of significant variables on the probability of glass eel presence in a tow, where positive and negative values indicate increasing and decreasing influences on the probability. The effect of ‘Minutes after low tide’ and ‘Lunar age’ on the probability of glass eel presence was maximum around 150–180 min after low tide and 23 and 0 days in a lunar cycle (Fig. 8a). The 2004–2005 season contributed negatively to the probability of glass eel presence in a tow compared to 2003–2004 season (Fig. 8a).
Results of generalized additive models (GAM) to (a) the occurrence probability of glass eels (left row) and (b) the abundance in non-zero catches cases (right row). Y-axis is effect of significant explanatory variables (Table 2) on logit(occurrence probability) in presence/absence model and log(catches) in the abundance model. Dashed lines (or upper and lower brackets) indicate 95 % confidence bands. Tick marks on the x-axes indicate data presence
The GAM for glass eel abundance selected the variables of ‘Recruitment season’, ‘Lunar age’, ‘Flow speed’, ‘Minutes after low tide’ and ‘Time of day’ in order of relative importance (Table 2). The variables of ‘Recruitment season’ and ‘Lunar age’ contributed to about 70 % of explained deviance in the best model. The 2004–2005 season contributed negatively to the glass eel abundance compared to 2003–2004 season (Fig. 8b). The effect of ‘Lunar age’ on the abundance was high around 21 and 0 days in a lunar cycle (Fig. 8b).
Discussion
Seasonality of glass eel migration
In the present study of two recruitment seasons from 2003 to 2005, A. japonica glass eels were observed to be entering Hamana Lake from November to May with a peak in January. This is basically consistent with the historical study by Inaba and Yamamoto (1938) that reported the recruitment into the lake occurred from November to April. The recruitment season of A. japonica into estuaries has been generally recognized to be October to May and was earlier at the lower latitudes or in locations closer to the Kuroshio Current (Tzeng 1985; Tsukamoto 1990; Shinoda 2004; Han 2011). The recruitment season in Hamana Lake appears to correspond to its location in the middle-northern latitudinal part of the species’ range and along the Kuroshio Current.
The recent study by Aoyama et al. (2012) reported that a recruitment peak of A. japonica glass eels in the Sagami River estuary occurred in the early summer during 2009–2011, which was inconsistent with the present study and other past studies. That late arrival of glass eels was thought to be because the Japanese eel was experiencing unusual phenology, which may be a possible response to recent global climate change (Aoyama et al. 2012). Late arrivals to estuaries may be disadvantageous because more predators may be actively feeding in the summer and not having a full early growth season before temperatures drop in autumn may reduce survival of the young eel recruits. A drastic change of the recruitment seasonality could have impacts on not only their biology, but the Japanese eel aquaculture industry. Thus long-term monitoring of their recruitment at various locations will be necessary to verify whether this tendency will be sustained or only occurred exceptionally in the Sagami River during 2009–2011.
Lunar and semilunar effects on glass eels migration
The autocorrelation analysis in this study suggested glass eel migration fluctuated not in a semilunar cycle but in a lunar cycle. Results of the GAM showed that the probability of occurrence and the abundance during flood tide at night increased around lunar age 21–23 (last quarter moon) and 0 days (new moon). This is generally consistent with the study of Nakagawa and Nonaka (1976) that reported commercial catch at Hamana Lake in 1974 increased around the last quarter moon to new moon.
A semilunar periodicity of glass eel catches was reported in A. anguilla (Ciccotti et al. 1995), A. rostrata (Sorensen and Bianchini 1986; Sullivan et al. 2006) and Anguilla spp. including A. dieffenbachii and A. australis (Jellyman 1979; Jellyman and Lambert 2003; Jellyman et al. 2009), whereas lunar periodicity was reported in tropical eels such as A. celebesensis and A. marmorata (Sugeha et al. 2001) and temperate eels of A. dieffenbachii and A. australis (Jellyman and Lambert 2003). The semilunar periodicity was explained by the glass eels behavior corresponding to tidal amplitude (Jellyman 1979; Tzeng 1985). Since anguillid glass eels selectively swim with flood tide (Creutzberg 1959, 1961; McCleave and Kleckner 1982; Gascuel 1986), high activity during spring tide might also make them to go further landward or upstream. The lunar periodicity of catch has been explained to likely be a result of moonlight intensity affecting their behavior in addition to the effect of tidal amplitude (Tzeng 1985; Sugeha et al. 2001; Jellyman and Lambert 2003). High moonlight intensity and low turbidity induced A. anguilla glass eels to mainly reside close to the bottom, whereas low moonlight intensity and/or high turbidity induced dispersion of glass eels throughout the water column (De Casamajor et al. 1999).
Not only environmental conditions, but also the development stage represented by pigmentation categories might affect their behavior in relation to light levels. Tzeng (1985) reported that the periodicity in catch of A. japonica glass eels was different between coastal waters (lunar periodicity with a peak around new moon) and a river (semilunar periodicity with a peak around full moon and new moon). The pigmentation stages in the river were advanced compared to those in coastal waters (Tzeng 1985). In laboratory experiments, pigmented anguillid juveniles were less influenced by light compared to unpigmented ones (Matsuoka 1971; Bardonnet et al. 2005), so eels with advanced pigmentation stage might sometimes be active even during full moon. The causes of behavioral differences between pigmentation stages were unclear but there is a possibility that the development of pigmentation on the skull could weaken sensitivity of glass eels toward light because the pineal body in the skull of fish has a function of photoreception (Helfman et al. 2009).
The lunar periodicity of glass eel recruitment in the present study could be due to the environmental conditions and the pigmentation stages. The depth at the sampling site is 3 m and the water was usually clear as the substrate was clearly seen from the surface in daytime. These conditions suggest that glass eels are likely to perceive the influence of moonlight. Glass eels were mainly at the early pigmentation VB1 stage, therefore they would still have sensitivity towards light. The shallow clear water and the early pigmentation stage of the glass eels may result in the lunar periodicity of their recruitment into Hamana Lake, which decreases around full moon.
Diurnal and semi-diurnal effects on glass eel migration
The present study did not collect any glass eels during the day, which was consistent with many studies on other anguillid eels (Deelder 1952; Creutzberg 1961; Jellyman 1977; Sugeha et al. 2001; Jellyman and Lambert 2003). Glass eels may generally avoid high light conditions, and A. japonica glass eels tended to hide in the sand in aquaria in the laboratory during the day (Dou and Tsukamoto 2003). As mentioned above, moonlight would suppress their activity, so they are even more inhibited from moving during daylight conditions.
Migration of A. japonica glass eels into the lake appears to be related to selective tidal stream transport (STST) as seen in other anguillid glass eels. At night, more glass eels in the present study were also caught during flood tides than ebb tides. In one tidal cycle, 17–39 % of water in the lake can be exchanged by outside water (Matsuda 1999), which means that more than 60 % of the water volume entering the lake during flood tide outflows in the next ebb tide. This indicated that more than 60 % of glass eels migrating into the lake with flood tides would return to outside the lake if they continued to swim in the water column. The estimated abundance during ebb tide in this study was on average 5.5 % of that during flood tide on the same day, suggesting most glass eels migrating into the lake with flood tidal stream would remain in the lake. As mentioned above, regular sampling was conducted only near the surface, however, glass eels were also absent in 3 preliminary surveys near the bottom after sampling near the surface (Fukuda, personal observation). So it is possible that glass eels did not go back near bottom during ebb tides in this study. Glass eels of A. anguilla and A. rostrata were found to remain on or near the bottom during ebb tide and move into the water column on flood tide (Creutzberg 1959, 1961; McCleave and Kleckner 1982; Gascuel 1986), so the same behavior is likely utilized by A. japonica as well.
Timing of migration during flood tide
In the GAM models for glass eels presence/absence and abundance in non-zero catches, different explanatory variables were selected in each best fitting model. The probability of glass eels presence was influenced the most by ‘minutes after low tide’, which is a factor included equally in every flood tide. Thus ‘minutes after low tide’ could explain the timing of glass eels migration during flood tide. On the other hand, the abundance was influenced by ‘recruitment season’ and ‘lunar age’, which were relatively long time-scale variables. This suggests that not short but long timescale factors could affect the abundance of glass eels when they migrate during flood tide.
The probability of glass eels presence in catches increased around 150–180 min in‘minutes after low tide’. This time corresponded to the rise of water temperature, which was caused by the inflow of warm water from outside the lake. This shows that glass eels recruited with the head of inflow from outside the lake. Considering that horizontal temperature fluctuation in the lake indicated the water from the sea flows at least 5 km into the lake, glass eels could migrate up to 5 km into the lake with one flood tidal stream. Beaulaton and Castelnaud (2005) estimated the ratio of the observed migration speed to the potential speed (flood tide current speed and swimming speed), as 0.15 and 0.19 for A. angulla glass eel in the Gironde basin, suggesting that the migration speeds seem to be low compared to the flood tide current. Considering the possibility that glass eels in the present study do not use the full flood tides as well, further study is needed to investigate their migration speeds in the lake by setting additional sampling sites.
The ascending behavior of A. anguilla and A. rostrata into the water column at flood tide has been suggested to be regulated by exogenous or endogenous factors. Creutzberg (1961) demonstrated experimentally that A. anguilla glass eels showed a preference for natural inland water and the change of odour according to the tidal cycle triggered their ascending behavior into the water column. The changes in behavior pattern during the tidal cycle might possibly be partly the result of an internal rhythm, because all the factors that might be responsible for releasing behavioral changes (such as the influence of freshwater, changes in turbulence, and overcast condition) do not always coincide with high or low tides (from Tesch 2003). Subsequently, glass eels in the laboratory exhibited rhythmic activity with circatidal periodicities, suggesting that they had an endogenous circatidal rhythm (A. rostrata; Wippelhauser and McCleave 1988, A. anguilla; Bolliet et al. 2007). The results of the present study could not verify which of these two mechanisms of ascending behavior were being used, but environmental cues are likely to be indispensable at least for A. japonica glass eels moving in Hamana Lake.
In terms of exogenous cues, glass eels might detect the ambient changes and ascend into water column when the water from outside the lake flowed into the lake during flood tide. Swimming behavior might synchronise to several environmental cues related to tide because the rhythmic activity in A. anguilla was weakly sustained in the constant conditions (Bolliet et al. 2007). Laboratory experiments showed that activity of A. japonica glass eels increased at higher temperatures (Dou et al. 2003) and A. anguilla glass eels preferred an acclimated temperature rather than a different temperature in short term experiments (Tongiorgi et al. 1986; Tosi et al. 1988), so the rapid rise of temperature could be a trigger of ascending behavior of anguillid glass eels. A. anguilla glass eels could use the low salinity (Tosi et al. 1988, 1990; Edeline et al. 2005, 2006) and the odour from inland water as orientational cues (Creutzberg 1961: Tosi et al. 1990; Sola and Tosi 1993; Sola and Tongiorgi 1996) as did A. rostrata glass eels (Sorensen 1986). The inflow of the water from outside the lake during flood tide should change the salinity, the odour and current direction in the water column. These changes also could be triggers of ascending behavior by A. japonica glass eels, but the rise of water temperature was plausible in the present study because of the high probability of its occurrence.
In terms of endogenous cues, the simultaneous occurrence of A. japonica glass eels with increased probability of presence around 150–180 min at ‘minutes after low tide’ could be explained by their migration being linked to a circa-tidal rhythm. Generally, the time of flood tide and ebb tide is varied among their recruiting locations, so anguillid glass eels would need to adjust their endogenous rhythm to the local tidal situations as they move further into estuaries. Environmental factors may act as zeitgeber and provide the necessary synchronization (Tesch 2003). A behavioral experiment also showed that synchronisation of tidal stream transport is weak under constant flow conditions and therefore glass eels might necessitate several environmental cues related with flood tides to remain synchronised (Bolliet et al. 2007). Because the recruitment was synchronized with the rapid change of temperature during flood tide into the lake that type of environmental change might be used to synchronize the circa-tidal rhythm.
References
Aoyama J, Shinoda A, Yoshinaga T, Tsukamoto K (2012) Late arrival of Anguilla japonica glass eels at the Sagami River estuary in two recent consecutive year classes: ecology and socio-economic impacts. Fish Sci 78(6):1195–1204
Aoyama J, Yoshinaga T, Shinoda A, Shirotori F, Yambot AV, Han Y-S (2015) Seasonal changes in species composition of glass Eels of the Genus Anguilla (teleostei: anguillidae) recruiting to the Cagayan River, Luzon Island, the Philippines 1. Pac Sci 69(2):263–270
Arai T, Aoyama J, Limbong D, Tsukamoto K (1999) Species composition and inshore migration of the tropical eels Anguilla spp. recruiting to the estuary of the Poigar River, Sulawesi Island. Mar Ecol: Prog Ser 188:299–303
Bardonnet A, Bolliet V, Belon V (2005) Recruitment abundance estimation: role of glass eel (Anguilla anguilla L.) responset to light. J Exp Mar Biol Ecol 321:181–190
Barry J, McHarg K, Dodd JA, Adams CE (2015) Local scale, coastal currents influence recruitment to freshwater populations in the European eel Anguilla anguilla: a case study from the Isle of Man. J Fish Biol 86(6):1873–1880
Beaulaton L, Castelnaud G (2005) The efficiency of selective tidal stream transport in glass eel entering the Gironde (France). Bull Fr Pêche Piscic 378-379:5–21
Bolliet V, Lambert P, Rives J, Bardonnet A (2007) Rhythmic swimming activity in Anguilla anguilla glass eels: synchronisation to water current reversal under laboratory conditions. J Exp Mar Biol Ecol 344(1):54–66
Ciccotti E, Ricci T, Scardi M, Fresi E, Cataudella S (1995) Intraseasonal characterization of glass eel migration in the River Tiber: space and time dynamics. J Fish Biol 47:248–255
Creutzberg F (1959) Discrimination between ebb and flood tide in migrating elvers (Anguilla vulgaris Turt) by means of olfactory perception. Nature 184(4703):1961–1962
Creutzberg F (1961) On the orientation of migrating elvers (Anguilla vulgaris Turt.) in a tidal area. Neth J Sea Res 1:257–338
De Casamajor MN, Bru N, Prouzet P (1999) Influence of night brightness and turbidity on the vertical migratory behaviour of glass-eels (Anguilla anguilla L.) in the Adour estuary. Bull Fr Pêche Piscic 72(355):327–347
Deelder CL (1952) On the migration of the elver (Anguilla vulgaris Turt.) at sea. J Cons 20:187–218
Dou S, Tsukamoto K (2003) Observations on the nocturnal activity and feeding behabior of Anguilla japonica glass eels under laboratory conditions. Environ Biol Fish 67:389–395
Dou S, Miller MJ, Tsukamoto K (2003) Growth, pigmentation and activity of juvenile Japanese eels in relation to temperature and fish size. J Fish Biol 63(1):152–165
Edeline E, Dufour S, Elie P (2005) Role of glass eel salinity preference in the control of habitat selection and growth plasticity in Anguilla anguilla. Mar Ecol: Prog Ser 304:191–199
Edeline E, Lambert P, Rigaud C, Elie P (2006) Effects of body condition and water temperature on Anguilla anguilla glass eel migratory behavior. J Exp Mar Biol Ecol 331(2):217–225
Fukuda N, Miller MJ, Aoyama J, Shinoda A, Tsukamoto K (2013) Evaluation of the pigmentation stages and body proportions from the glass eel to yellow eel in Anguilla japonica. Fish Sci 79(3):425–438
Gandolfi G, Pesaro M, Tongiorgi P (1984) Environmental factors affecting the ascent of elvers, Anguilla anguilla (L.), into the Arno River. s 10:17–35
Gascuel D (1986) Flow carried and swimming migration of the glass eel (Anguilla anguilla) in the tidal area of a small estuary on the French Atlantic coast. Helgol Meeresunters 40:321–326
Gibson R (1992) Tidally-synchronised behaviour in marine fishes. In: Rhythms in fishes. NATO ASI Series A: Life Sciences vol. 236. Springer, New York, pp 63–81
Han YS (2011) Temperature-dependent recruitment delay of the Japanese glass eel Anguilla japonica in East Asia. Mar Biol 158(10):2349–2358
Haro AJ, Krueger WH (1988) Pigmentation, size, and migration of Elvers (Anguilla rostrata (Lesueur)) in a coastal Rhode-Island Stream. Can J Zool 66(11):2528–2533
Harrison AJ, Walker AM, Pinder AC, Briand C, Aprahamian MW (2014) A review of glass eel migratory behaviour, sampling techniques and abundance estimates in estuaries: implications for assessing recruitment, local production and exploitation. Rev Fish Biol Fish 24(4):967–983
Hastie T, Tibshirani R (1990) Exploring the nature of covariate effects in the proportional hazards model. Biometrics 46(4):1005–1016
Helfman G, Collette BB, Facey DE, Bowen BW (2009) The diversity of fishes: biology, evolution, and ecology, 2nd edn. Chichester, John Wiley & Sons
Hwang SD, Lee TW, Choi IS, Hwang SW (2014) Environmental factors affecting the daily catch levels of Anguilla japonica glass eels in the Geum River Estuary, South Korea. J Coastal Res 30(5):954–960
Inaba S, Yamamoto S (1938) Glass eels and its aquaculture (in Japanese). Month Rep Fish Res Agen Shizuoka Pref 47:19–32
Jacoby D, Gollock M (2014) Anguilla japonica. The IUCN Red List of Threatened Species. Version 2015.2. <www.iucnredlist.org>. Downloaded on 22 July 2015
Jellyman DJ (1977) Invation of a New Zealand freshwater stream by glass-eels of two Anguilla spp. N Z J Mar Freshwater Res 11(2):193–209
Jellyman DJ (1979) Upstream migration of glass eel (Anguilla spp.) in the Waikato river. N Z J Mar Freshwater Res 13:13–22
Jellyman DJ, Lambert PW (2003) Factors affecting recruitment of glass eels into the Grey River, New Zealand. J Fish Biol 63(5):1067–1079
Jellyman DJ, Chisnall BL, Bonnett ML, Sykes JRE (1999) Seasonal arrival patterns of juvenile freshwater eels (Anguilla spp.) in New Zealand. N Z J Mar Freshwater Res 33:249–261
Jellyman DJ, Booker D, Watene E (2009) Recruitment of Anguilla spp. glass eels in the Waikato River, New Zealand. Evidence of declining migrations? J Fish Biol 74(9):2014–2033
Kimura S, Tsukamoto K, Sugimoto T (1994) Model for the larval migration of the Japanese eel: roles of the trade winds and salinity front. Mar Biol 119:185–190
Laffaille P, Caraguel J-M, Legault A (2007) Temporal patterns in the upstream migration of European glass eels (Anguilla anguilla) at the Couesnon estuarine dam. Estuar Coast Shel S 73(1):81–90
Leatherland JF, Farbridge KJ, Boujard T (1992). Lunar and semi-lunar rhythms in fishes. In: Rhythms in Fishes. Springer: New York, vol 236, pp 83–107
Matsuda Y (1999) Hamanakosui no fushigi (in Japanese). Shizuoka Shimbun, Shizuoka
Matsuoka T (1971) An experiment about choice of shelters by glass eels (in Japanese). Month Rep Fish Res Agen Shizuoka Pref 44(1):51–60
McCleave DJ, Kleckner RC (1982) Selective tidal stream transport in the estuarine migration of glass eels of the American eel (Anguilla rostrata). J Cons 40:262–271
Nakagawa M, Nonaka T (1976) A little information about catch of glass eels in Hamana lake (in Japanese). Month Rep Fish Res Agen Shizuoka Pref 10:77–82
Otake T, Miller MJ, Inagaki T, Minagawa G, Shinoda A, Kimura Y, Sasai S, Oya M, Tasumi S, Suzuki Y, Uchida M, Tsukamoto K (2006) Evidence for migration of metamorphosing larvae of Anguilla japonica in the Kuroshio. Coast Mar Sci 30:453–458
Powles PM, Warlen SM (2002) Recruitment season, size, and age of young American eels (Anguilla rostrata) entering an estuary near Beaufort, North Carolina. Fish Bull 100(2):299–306
R Development Core Team (2009) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org.
Schlitzer R (2009) Ocean data view http://odv.awi.de
Shinoda A (2004) The ecology of inshore migration of the Japanese eel, Anguilla japonica (in Japanese). The University of Tokyo, Tokyo, PhD dissertation
Sola C, Tongiorgi P (1996) The effect of salinity on the chemotaxis of glass eels, Anguilla anguilla, to organic earthy and green odorants. Environ Biol Fish 47(2):213–218
Sola C, Tosi L (1993) Role of geosmin, a typical inland water odor, in guiding glass eel Anguilla anguilla (L) migration. Ethology 95(3):177–185
Sorensen PW (1986) Origins of the fresh-Water attractant(S) of migrating Elvers of the American Eel, Anguilla rostrata. Environ Biol Fish 17(3):185–200
Sorensen PW, Bianchini ML (1986) Environmental correlates of the freshwater migration of elvers of the American eel in a Rhode Island brook. Trans Am Fish Soc 115:258–268
Sugeha H, Arai T, Miller MJ, Limbong D, Tsukamoto K (2001) Inshore migration of the tropical eels Anguilla spp. recruiting to the Poigar River estuary on North Sulawesi Island. Mar Ecol: Prog Ser 221:233–243
Sullivan MC, Able KW, Hare JA, Walsh HJ (2006) Anguilla rostrata glass eel ingress into two, US east coast estuaries: patterns, processes and implications for adult abundance. J Fish Biol 69(4):1081–1101
Tesch F-W (2003) The eel, 3rd edn. London, Blackwell Publishing
Tongiorgi P, Tosi L, Balsamo M (1986) Thermal preferences in upstream migrating glass eels of Anguilla anguilla (L). J Fish Biol 28(4):501–510
Tosi L, Sala L, Sola C, Spampanato A, Tongiorgi P (1988) Experimental analysis of the thermal and salinity preferences of glass-eels, Anguilla anguilla (L.), before and during the upstream migration. J Fish Biol 33:721–733
Tosi L, Spampanato A, Sola C, Tongiorgi P (1990) Relation of water odor, salinity and temperature to ascent of glass eels, Anguilla anguilla (L) - a laboratory study. J Fish Biol 36(3):327–340
Tsukamoto K (1990) Recruitment mechanism of the eel, Anguilla japonica, to the Japanese coast. J Fish Biol 36:659–671
Tsukamoto K (1992) Discovery of the spawning area for the Japanese eel. Nature 356:789–791
Tsukamoto K (2006) Spawning of eels near a seamount. Nature 439(7079):929–929
Tsukamoto K, Aoyama J, Miller, MJ (2009) Present status of the Japanese Eel: Resources and Recent Research. In: Eels at the Edge: Science, Status and Conservation concerns. American Fisheries Society, Symposium 58, Bethesda, Maryland, pp 21–35
Tzeng WN (1985) Immigration timing and activity rhythms of the Eel, Anguilla japonica, elvers in the estuary of Northern Taiwan, with emphasis on environmental influences. BULL Japn Soc Fish Oceanogr 47(48):11–27
Uemura N, Fushimi H (1979) Oceanographical reserch on property of Hamana Lake. the water temperature and it's seasonality (in Japanese). Month Rep Fish Res Agen Shizuoka Pref 13:21–41
Vignaux M (1996) Analysis of spatial structure in fish distribution using commercial catch and effort data from the New Zealand hoki fishery. Can J Fish Aquat Sci 53(5):963–973
White EM, Knights B (1997) Environmental factors affecting migration of the European eel in the Rivers Severn and Avon, England. J Fish Biol 50:1104–1116
Wippelhauser GS, McCleave JD (1987) Precision of begavior of migrating juvenile American eels (Anguilla rostrata) utilizing selective tidal stream transport. J Cons 44:80–89
Wippelhauser GS, McCleave JD (1988) Rhythmic activity of migrating juvenile American eels Anguilla rostrata. J Mar Biol Assoc U K 68(1):81–91
Yamamoto T, Mochioka N, Nakazono A (2001) Seasonal occurrence of anguillid glass eels at Yakushima Island, Japan. Fish Sci 67(3):530–532
Yokouchi K, Aoyama J, Oka HP, Tsukamoto K (2008) Variation in the demographic characteristics of yellow-phase Japanese eels in different habitats of the Hamana Lake system, Japan. Ecol Freshw Fish 17(4):639–652
Zompola S, Katselis G, Koutsikopoulos C, Cladas Y (2008) Temporal patterns of glass eel migration (Anguilla anguilla L. 1758) in relation to environmental factors in the Western Greek inland waters. Estuar Coast Shelf S 80(3):330–338
Acknowledgments
We thank the staff and students of Behavior and Ecology Laboratory of Ocean Research Institute, the University of Tokyo, the Fisheries Cooperatives of Hamana Lake and the Fisheries Division of Shizuoka Prefecture for cooperation in many aspects of the study. We also thank R. Sudo for field survey assistance and M.J. Miller for helping to improve the manuscript. This study was supported by a Grant-in-aid for Scientific Research (Nos.10460081, 11691177) from the Ministry of Education, Culture, Sports, Science and Technology of Japan and “Research for the Future“ Program No.JSPS-RFTF 97 L00901 from the Japan Society for the Promotion of Science and Fisheries Research Agency. KT was supported by the Research foundation from Touwa Shouhin Shinkoukai and the Eel Research Foundation from Nobori-kai.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fukuda, N., Aoyama, J., Yokouchi, K. et al. Periodicities of inshore migration and selective tidal stream transport of glass eels, Anguilla japonica, in Hamana Lake, Japan. Environ Biol Fish 99, 309–323 (2016). https://doi.org/10.1007/s10641-016-0475-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-016-0475-z


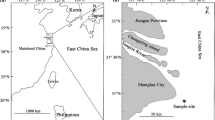

 , St. B) and locations of temperature data loggers (
, St. B) and locations of temperature data loggers ( , ●, St. A-G) in the Hamana Lake in Japan. Bridges are represented by dotted lines
, ●, St. A-G) in the Hamana Lake in Japan. Bridges are represented by dotted lines





