Abstract
The introduced Nile perch, Lates niloticus Linnaeus 1758 in Lake Victoria is considered to be a major contributor to the decline of haplochromine species. In the 1990s, the abundance of Nile perch declined and the recovery of some haplochromine species, mainly detritivores and zooplanktivores was observed. However, the resurgence of detritivores group was much slower than that of zooplanktivores. Differential Nile perch predation is thought to be the main cause for the differential recovery of these two groups. To test this, the frequency of occurrence (FOO) of the detritivores and zooplanktivores in the stomachs of the Nile perch and their FOO in the lake were compared. On their vulnerability to predation, body shape of the two groups was measured and compared with Nile perch mouth gape. The FOO for haplochromines in Nile perch stomach contents were 13.7 % detritivores and 86.3 % zooplanktivores, whereas in trawl catches they were 24.3 and 75.7 % respectively. The FOO of detritivores in the Nile perch diet was significantly lower than in the environment. The same holds for the FOO of juvenile haplochromines (13 % in the diet and 45 % in the environment). Body depth of detritivores (0.8–1.9 cm) in the diet of Nile perches was significantly larger than that of the zooplanktivores (0.6–1.7 cm). However, based on Nile perch mouth gape (2.0–5.3 cm), only Nile perches <13 cm TL (<1.9 m mouth gape) would not be able to swallow the largest detritivores. Thus, selective predation by Nile perch cannot explain the relatively slow resurgence of detritivores.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the late 1950s and early 1960s, the predatory Nile perch, Lates niloticus (Linnaeus, 1758) was introduced into the Lake Victoria basin (Pringle 2005) to improve the declining fishery (Ogutu-Ohwayo 1990). In the 1980s, the Nile perch suddenly increased in Lake Victoria and, concomitantly, the haplochromines in the sub-littoral and off-shore areas vanished almost completely (Barel et al. 1985; Ogutu-Ohwayo 1990; Witte et al. 1992a). A lake-wide survey in 1969–1970 revealed that 80 % of the demersal fish mass consisted of haplochromine cichlids (Kudhongania and Cordone 1974). The dramatic decline of haplochromines cichlids in Lake Victoria triggered many studies and debates about the possible causes and consequences of their decline (Barel et al. 1985; Acere 1988; Witte et al. 1992a, b; Goldschmidt et al. 1993; Hecky 1993; Seehausen et al. 1997a, b). Although environmental changes and overfishing played a role in the decline of the haplochromines (Witte et al. 1992a, b; Hecky 1993; Hecky et al. 1994, 2010; Seehausen et al. 1997a; Balirwa et al. 2003), Nile perch predation is thought to have been a major cause of the faunal collapse in Lake Victoria Hughes 1986; Witte et al. 1992a).
In a course of 1990s, the Nile perch population in the lake had declined (Pitcher and Bundy 1995; Mkumbo 2002; Mkumbo et al. 2007) and a concomitant resurgence of some haplochromine species was observed (Seehausen et al. 1997b; Witte et al. 2000, 2007; Balirwa et al. 2003; Getabu et al. 2003; Kishe-Machumu 2012). However, the rate at which the trophic groups recovered differed. In the Mwanza Gulf, detritivores and zooplanktivores that previously dominated the ichthyomass were more successful in recovering than other trophic groups (Witte et al. 2007; Kishe-Machumu 2012). Prior to the Nile perch upsurge, detritivores in this gulf made up ca. 75–85 % of the total number of haplochromines in the bottom trawl catches and zooplanktivores contributed between 10 and 20 % (Goldschmidt et al. 1993; Witte et al. 2007; Kishe-Machumu 2012). Between 1990 and 2006, zooplanktivore numbers were higher than those of detritivores (Witte et al. 2007; Kishe-Machumu 2012). In 2006 for instance, detritivores comprised about 21 % of the number of haplochromines and zooplanktivores made up about 71 %, but in 2008, the former was the dominant group again with abundance of 52 % (Kishe-Machumu 2012). However, the difference in abundance of detritivores between 2006 and 2008 was not significant (Kishe-Machumu 2012); thus, a reverse may just be a matter of chance.
xSome papers discussed the possible factors of the differential recovery and shift in dominance between these two groups (Witte et al. 2007; Kishe-Machumu et al. 2008). Kishe-Machumu et al. (2008) showed that the slower recovery of detritivores is unlikely to be a result of their diet as they switched to one that was similar to that of the zooplanktivores. In the 2000s, abundance of Nile perch had further decreased (Matsuishi et al. 2006; Kayanda et al. 2009), however it was still more abundant than in the 1970s (Goudswaard et al. 2008) and haplochromines once again became a considerable part of its diet after their resurgence (Kishe-Machumu et al. 2012). It was suggested that differential predation by Nile perch might cause differences in resurgence rate between haplochromine trophic groups (Goudswaard et al. 2004; Witte et al. 2007, 2012). To test this, we compared the ratio of detritivorous and zooplanktivorous haplochromines in the environment to their ratio in stomach contents of Nile perch. Further, the body shape and length for these two groups were measured for examining the maximum prey size and their vulnerability to Nile perch predation.
Materials and methods
Study area and fish sampling
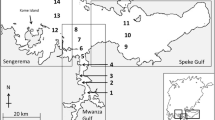
Fish samples were collected at six stations (E, F, G, H, I and J) along the research transect between Butimba and Kissenda Bays (Fig. 1) in the Mwanza Gulf from January to December in 2006. A small bottom trawler powered by a 25 hp outboard engine towing a bottom trawl net with a 4.6 m head-rope and a 5 mm cod-end mesh was used. As this trawler caught only small-sized Nile perch (up to ca. 30 cm TL), larger individuals were collected from the same area with a large trawl net (24 m head-rope, 50 mm cod-end mesh), mounted on the Research Vessel (R.V.) Lake Victoria Explorer (250 hp). Haplochromines were only collected with the small trawler because they were too small to be caught in the wider cod-end meshes of the large trawl net.
In each haul, haplochromines were sorted into trophic groups and species. The total length (TL, to the nearest cm) of each Nile perch was recorded, the gut was opened and the gut contents were visually identified. Haplochromines obtained from the Nile perch stomachs were preserved in a 5 % formaldehyde solution and later transferred to 70 % alcohol for identification. With the small trawler, a trawl-shot was made at each station once per month, whereas the large trawler covered all stations and was used in January, March, May and December only due to its availability. Sampling was mostly conducted in morning hours between 06:30 and 11:00 h and trawl shots lasted for 10 and 30 min for the small and large trawlers respectively.
Identification of zooplanktivorous and detritivorous haplochromines
Detritivores and zooplanktivores individuals from the Nile perch stomachs were identified according to their morphological differences. Of these two groups, zooplanktivores are generally more slender-bodied than detritivores (Witte and van Oijen 1990), so we used depth/length ratios to distinguish the two groups. The body depth/standard length (BD/SL) ratio in detritivores is >33 and <33 % in zooplanktivores (Witte and van Oijen 1990).
Since most of haplochromine individuals from Nile perch stomachs were often partly digested with part of the head or the tail missing, it was not always possible to measure SL or BD. Therefore, alternative morphometric ratios were established and used to discriminate between the two groups. To do so, representative individuals of the two groups (27 detritivores and 30 zooplanktivores; Table 1) caught in 2006 on the research transect with the small trawler were used and collectively were referred as the ‘control group’. It should be noted that, the species and trophic groups for fishes that were used in the ‘control group’ were known. However, the alternative ratios had to meet two main criteria: (1) they should be significantly different in detritivores and zooplanktivores, and (2) they should be applicable to most haplochromines obtained from Nile perch stomachs.
From a ‘control group’’ (Table 1), BD/SL and 17 alternative depth/length (D/L) ratios fulfilling the above two criteria were selected as reference measures for trophic group identification of haplochromines from Nile perch stomachs (Table 2).
It should be noted that the definitions of BD and head length (HL) (Fig. 2) differ from those given in Barel et al. (1977), because it was not always possible to find the exact landmarks used by the authors in fish taken from Nile perch stomachs.
Positions of landmarks used to measure the lengths of haplochromine. Abbreviations are as follows: SL standard length (snout to origin of caudal fin), HL head length (snout to base of pectoral fin), DPL rostral insertion of dorsal fin to dorsal insertion of pectoral fin, BD body depth (rostral insertion of dorsal fin to rostral insertion of pelvic fin), DAL rostral insertion of dorsal fin to rostral insertion of anal fin, DCL rostral insertion dorsal fin to origin of caudal fin, SDAL base of last spinous dorsal fin ray to rostral insertion of anal fin, PVCL rostral insertion of pelvic fin to origin of caudal fin and PCL dorsal insertion pectoral fin to origin of caudal fin
The haplochromines from the Nile perch stomachs were placed on graph paper with a ruler as reference measure next to it. Seven landmarks that were least affected by Nile perch digestion (Fig. 2) were put on the left side of the fish with a permanent marker pen. Digital photographs were taken with a Nikon Coolpix E990 camera and distances between landmarks were measured with ImageJ. The D/L ratios (Table 2) were used to determine to which of the two trophic groups the individuals belonged.
Nile perch and prey size
To test if the whole size range of Nile perch analyzed was able to swallow the available zooplanktivores and detritivores, the maximum body depth (BD) of these two groups (from the Nile perch stomachs) was compared to the mouth gape (MG) of the Nile perch. The mouth gape of Nile perch was related to its total length by MG = 0.112*TL + 0.43 (both MG and TL in cm; Ligtvoet and Mkumbo 1990). In addition, we compared the maximum TL of detritivores and zooplanktivores with the TL of the Nile perch. In Lake Victoria, Nile perch can feed on prey up to 25–30 % of its own length (Hamblyn 1966; Gee 1969; Ogutu-Ohwayo 1985), although Hopson (1972) suggested that in Lake Chad, “Nile perch of all lengths are capable of eating fish up to approximately half their own length”. In this study, the TL of many haplochromines from Nile perch stomachs could not be measured because the caudal fin was damaged. However, for those specimens of which SL could be measured, TL could be determined since TL = SL*5/4, as the caudal fin length is generally 25 % of the SL in both trophic groups (de Zeeuw et al. 2010). Both haplochromine groups mature at about 4 cm SL (Witte et al. 2007), so the percentage of haplochromine prey smaller than 4 cm SL was calculated to establish the frequency of juveniles in the diet.
Prey selectivity
Prey selectivity of Nile perch was determined by comparing the frequency of occurrence (FOO) of haplochromines in the environment with their FOO in the stomachs of Nile perch. The FOO of haplochromine trophic groups in the environment was calculated from every small trawl catch of 10 min at the research transect from which the Nile perch were used and the haplochromines could be identified in their stomachs. For those Nile perch, which were collected with the large trawl, all catches at all six stations made with the small trawl on that particular month were used. This is because we did not make representative haplochromines catches with the large trawler and the large trawl catches covered all stations.
Data analysis
Depth/Length ratios of detritivores and zooplanktivores in the ‘control group’ were not normally distributed (Shapiro Wilk test); therefore, the non-parametric Mann-Whitney U-test was used to test for differences between the D/L ratios of detritivores and zooplanktivores.
A discriminant analysis was used to determine to which trophic group each haplochromine obtained from the Nile perch stomachs belonged. Ungrouped fish from Nile perch stomachs (out-group) were compared to the grouping variable, the trophic groups (detritivores and zooplanktivores) defined in the ‘control group’. It was rarely possible to obtain all 18 ratios for each individual haplochromine and because a discriminant analysis cannot analyse cells with missing values, it was not possible to run the analysis for all individuals together. Therefore, individuals having the same kind of ratios were analyzed together.
Univariate general linear models (GLM) with haplochromine trophic group (detritivores versus zooplanktivores) as factor and Nile perch length (TL) as covariant were used to test for differences in BD and TL of the detritivores and zooplanktivores eaten by Nile perch.
A Chi-square test was performed to test if the frequencies of occurrence of detritivorous and zooplanktivorous haplochromines in Nile perch stomachs were significantly different from the frequencies that were found in the trawl catches. The statistical packages SPSS 16.0 for Windows were used for all tests.
Results
Control group
In the ‘control group’, 18 D/L ratios were significantly different between detritivores and zooplanktivores (Mann Whitney U-test, p < 0.001 in all cases). The ranges of ten ratios however overlapped in which the minimum value(s) for detritivores were lower than the maximum value(s) for zooplanktivores (Table 2).
Frequency of haplochromine trophic groups in Nile perch stomachs
A total of 344 haplochromines were obtained from the Nile perch stomachs of which 105 (31 %) were intact enough to be measured. Of 95 (28 %) of these haplochromines their trophic group could be identified with a probability ≥90 % in which 82 specimens were identified as zooplanktivores and 13 as detritivores. Eight of the remaining 10 individuals were identified as zooplanktivores and two detritivores, but the probability of correct identification was <90 %, so they were not included in the ratio of detritivores and zooplanktivores in the Nile perch stomachs. The haplochromines were obtained from 60 Nile perches of size range from 13.6 to 43.2 cm TL.
Nile perch size and haplochromine body depth
The body depth of detritivores from the Nile perch stomachs ranged from 0.8 to 1.9 cm (n = 11) and those of zooplanktivores from 0.6 to 1.7 cm (n = 50). The GLM for BD of the zooplanktivores and detritivores in Nile perch stomachs, showed that there was a slight increase in prey BD with increasing Nile perch size (BD = 1.296 + 0.009 TL for detritivores and BD = 0.97 + 0.009 TL for zooplanktivores; p = 0.042). Body depth of the detritivores in the stomachs was significantly larger than that of the zooplanktivores (estimated marginal means: 1.53 ± 0.08 versus 1.23 ± 0.04 respectively; p = 0.001). The smallest Nile perch in the present study (TL = 13.6 cm, MG = 2.0 cm) swallowed a detritivore of 1.3 cm BD (Fig. 3a). The detritivore with the largest body depth (1.9 cm) had been eaten by a relatively small Nile perch of 20.2 cm TL, with a MG of 2.7 cm (Fig. 3a).
Relationships between Nile perch size and its prey size: a Body depth (BD) of haplochromines against total length (TL) of Nile perch b TL of haplochromines against TL of Nile perch. Filled circles represent detritivores and open circles zooplanktivores. In case of a significant effect of the TL of Nile perch, regression lines as estimated by the general linear model are depicted. Shaded areas represent Nile perch features that restrict prey sizes: a haplochromine BD > Nile perch mouth gape (MG); b haplochromine TL > 30–50 % Nile perch TL. Vertical dotted lines indicate smallest Nile perch that had eaten a haplochromine; horizontal dotted lines indicate measure of largest prey in this study. The corner of the dark shaded area enclosed by the dotted lines indicates the Nile perch size range that (theoretically) cannot eat the maximum prey size of haplochromines (for further explanation see text)
Nile perch size and haplochromine length
The size of the detritivores eaten by Nile perch ranged from 5.3 to 7.2 cm TL (4.2–5.8 cm SL, n = 5) while zooplanktivores ranged from 3.4 to 7.7 cm TL (2.7–6.1 cm SL, n = 18; Fig. 3b). The GLM for total length of the detritivores and zooplanktivores in the Nile perch diet revealed no significant increase in length of detritivores and zooplanktivores in relation to Nile perch size. There was also no significant difference in total length between the two groups. None of the detritivores and only three of the zooplanktivores had a SL < 4 cm, which implies that the number of juvenile haplochromines eaten was only 13 % of all the juveniles collected in the samples. The longest haplochromine (7.7 cm TL) in the stomach contents was a zooplanktivore eaten by the largest Nile perch (43.2 cm TL). The smallest Nile perch that had eaten a haplochromine in the present study had a TL of 6.8 cm, but the trophic group and size of this prey could not be identified. The TL of the two relatively largest prey in the present study were 37.8 % and 35.6 % of the Nile perch length that swallowed them; a zooplanktivore of 5.6 cm in a Nile perch of 14.8 cm and a detritivore of 7.2 cm in a Nile perch of 20.2 cm respectively (Fig. 3b).
Prey selectivity
The total numbers of detritivores and zooplanktivores in 36 bottom trawl catches of 10 min in 2006 (from the same days and stations as the Nile perches with identifiable haplochromine prey) were 7,108 and 22,148 respectively. By comparison, detritivores accounted for 24.3 % of haplochromines in the catches and zooplanktivores 75.7 % and in the Nile perch stomachs 13.7 and 86.3 %, respectively. There was a significant difference between the FOO of detritivores and zooplanktivores in the environment (indicated in their proportion in trawl catches; p < 0.025). The detritivore : zooplanktivore ratio was about two times lower in Nile perch stomachs than in the environment meaning that detritivores occurred less frequently than expected in the diet of Nile perch while zooplanktivores were more frequent.
The total number of adult detritivorous and zooplanktivorous individuals in the trawl catches was 29,256 and they made up 95.1 % of the total number of adult haplochromines (30,758). The total number of juvenile haplochromines in the same catches was 23,757. Assuming that for juveniles, the percentage of zooplanktivores and detritivores was about the same as for adults, this would result in 0.95x23,757 = 22,569 juvenile zooplanktivores and detritivores. Thus, in the environment the percentage of juveniles (<4 cm SL) was about 45 %. The FOO of juvenile haplochromines in the Nile perch stomachs (13 %) was significantly lower than in the trawl catches (p < 0.005). In the Nile perch stomachs, the juveniles : adult detritivores + zooplanktivores ratio was five times lower in Nile perch stomachs than in the environment meaning that juvenile haplochromines occurred less frequently in the diet of Nile perch.
Discussion
Body shape differences
As the body depth of detritivorous haplochromines was significantly greater than that of zooplanktivores of the same size (Table 2; Witte and van Oijen 1990), they were to be less vulnerable to predation by relatively small Nile perch. The greater body depth of detritivores did not seem to determine predation by Nile perch in the studied size range, since their mouth gape was always large enough to allow them to ingest detritivorous haplochromines. Thus, Nile perch mouth gape is not serious restriction to feed on detritivores. There was an increase in body depth with increasing Nile perch size, but it should be stressed that the increase was extremely small, and only just significant. This may be an artifact caused by the small size of the sample. Nile perch <13 cm TL could find it more difficult to ingest detritivores than zooplanktivores owing to their deeper bodies. However, as only a relatively small fraction of Nile perch <13 cm included haplochromines in their diet (Kishe-Machumu et al. 2012), the effect of differential predation by these small Nile perch may not be large. Moreover, if there is any relationship between body depth and prey selectivity, then predation pressure on the deeper bodied detritivorous haplochromines would be expected to be lower than on the zooplanktivores.
In this study, the maximum length of prey was 38 % of the Nile perch total length (a Nile perch of 14.8 cm TL). This is between the values of 25–30 % reported for Lake Victoria (Hamblyn 1966; Gee 1969; Ogutu-Ohwayo 1985) and the maximum of 50 % in Lake Chad by Hopson (1972), but since haplochromines do not grow as large as Nile perch, their relative size decreases in the stomachs of larger fish (Fig. 3b). If Nile perch in Lake Victoria could eat haplochromines with a length up to 50 % of their own total length, the Nile perch in the size range of 6.8 cm TL (the smallest that had eaten a haplochromine; Kishe-Machumu et al. 2012) and 15.4 cm TL (the longest haplochromine was 7.7 cm TL; this study) would not be able to swallow all haplochromines. For a prey to predator ratio of 30 %, a Nile perch should be even 25.6 cm to swallow a haplochromine of 7.7 cm (Fig. 3b). However, as the mean lengths of detritivores and zooplanktivores did not differ, it is unlikely that prey length will cause a differential predation effect by Nile perch smaller than those in the present study.
Body shape of zooplanktivores might be better adapted to burst swimming because of a relatively low ratio between head surface area and caudal peduncle area. This would facilitate predator escape as is described by van Rijssel and Witte 2013 (and references therein). In both resurgent groups, detritivorous and zooplanktivorous haplochromines of Lake Victoria, it has been found that the ratio between head surface area and caudal peduncle area decreased compared to that before the Nile perch boom (van Rijssel and Witte 2013). Nevertheless, detritivores had and still have a higher head surface/caudal peduncle area ratio than zooplanktivores, which theoretically might make them more vulnerable to Nile perch predation. But, in spite of this, detritivores were under-represented in the Nile perch diet. It is unlikely therefore that difference in size or body shape between the two trophic groups has had a major impact on their vulnerability to predation by Nile perch.
Habitat differences
It was suggested that detritivores which live closer to the bottom than zooplanktivores had a larger habitat overlap with Nile perch and may therefore have been more susceptible to Nile perch predation than zooplanktivores (Witte and Goudswaard 1985; Goudswaard et al. 2004; Witte et al. 2007, 2012). In the past, detritivores were mainly caught in the lowest three meters of the water column (Goldschmidt et al. 1993; Goudswaard et al. 2004; Witte et al. 2012), but in 2006 and 2008, a major part of the detritivorous population migrated to the surface at night along with zooplanktivores (Kishe-Machumu 2012). It seems unlikely that differences in habitat between the two trophic groups can explain why in 2006 the contribution of detritivores to the Nile perch diet was lower than expected from their frequency in the environment.
The similarity in size and distribution of the resurgent zooplanktivores and detritivores seems to provide little proof for selective feeding on one of the two groups. Thus, the hypothesis that the slow recovery of detritivores, which were more numerous than zooplanktivores in the pre-Nile perch era, was a result of Nile perch predation has to be refuted as the data suggest the Nile perch selectively preyed more upon zooplanktivores.
The resurgent zooplanktivores revealed ecological and morphological adaptations to the new environmental conditions (Witte et al. 2008; van der Meer et al. 2012; van Rijssel and Witte 2013) and this could be the cause of their successful recovering. However, a detailed morphological study is of great importance for both detritivores and zooplanktivores. In case there are more morphological constraints in detritivores than in zooplanktivores, the potentials to adapt to the new environmental conditions could be smaller in detritivores than in zooplanktivores, and this could explain their slow recovery.
References
Acere TO (1988) Recent trends in the fisheries of Lake Victoria (Uganda, Northern part). FAO Tech. Fish. Pap. No. 338, Rome, pp 75–85
Balirwa JS, Chapman CA, Chapman LJ, Cowx G, Geheb K, Kaufman L, Lowe-McConnell RH, Seehausen O, Wanink JH, Welcomme RL, Witte F (2003) Biodiversity and fishery sustainability in the Lake Victoria basin: an unexpected marriage? Bioscience 53:703–715
Barel CDN, van Oijen MJP, Witte F, Witte-Maas ELM (1977) An introduction to the taxonomy and morphology of the haplochromine Cichlidae from Lake Victoria. Neth J Zool 27:333–389
Barel CDN, Dorit R, Greenwood PH, Fryer G, Hughes N, Jackson PBN, Kanawabe H, Lowe-McConnell RH, Nagoshi M, Ribbink AJ, Trewavas E, Witte F, Yamaoka K (1985) Destruction of fisheries in Africa’s lakes. Nature 315:19–20
de Zeeuw MP, Mietes M, Niemantsverdriet P, ter Huurne S, Witte F (2010) Seven new species of detritivorous and phytoplanktivorous haplochromines from Lake Victoria. Zool Med 84:201–250
Gee JM (1969) A comparison of certain aspects of the biology of Lates niloticus (Linnaeus) in some East African lakes. Rev Zool Bot Afr 80:244–262
Getabu A, Tumwebaze R, MacLennan DN (2003) Spantial distribution and temporal change in the fish populations of Lake Victoria. Aquat Living Resour 16:159–165
Goldschmidt T, Witte F, Wanink J (1993) Cascading effects of the introduced Nile perch on the detritivorous/phytoplanktivorous species in the sublittoral areas of Lake Victoria. Conserv Biol 7:686–700
Goudswaard PC, Wanink JH, Witte F, Katunzi EFB (2004) Diel vertical migration of major fish species in Lake Victoria, East Africa. Hydrobiologia 513:141–152
Goudswaard KPC, Witte F, Katunzi EFB (2008) The invasion of an introduced predator, Nile perch (Lates niloticus, L.) in Lake Victoria (East Africa): chronology and causes. Environ Biol Fish 81:127–139
Hamblyn EL (1966) The food and feeding habits of Nile perch: Lates niloticus (Linn’e) (Pisces: Centropomidae). Rev Zool Bot Afr 74:1–28
Hecky RE (1993) The eutrophication of Lake Victoria. Ver Int Ver Limnol 25:39–48
Hecky RE, Bugenyi FWB, Ochumba P, Talling JF, Mugidde R, Gophen M, Kaufman L (1994) Deoxygenation of the deep water of Lake Victoria, East Africa. Limnol Oceanogr 39:1476–1481
Hecky RE, Mugidde R, Ramlal PS, Talbot MR, Kling GW (2010) Multiple stressors cause rapid ecosystem change in Lake Victoria. Freshw Biol 55(suppl 1):19–42
Hopson AJ (1972) A study of the Nile perch (Lates niloticus L., Pisces: Centropomidae) in Lake Chad. Overseas Research Publications, London
Hughes NF (1986) Changes in the feeding biology of the Nile perch, Lates niloticus (L) (Pisces, Centropomidae), in Lake-Victoria, East-Africa since its introduction in 1960, and its impact on the native fish community of the Nyanza-Gulf. J Fish Biol 29:541–548
Kayanda R, Taabu AM, Tumwebaze R, Muhoozi L, Jembe T, Mlaponi E, Nzungi P (2009) Status of the major commercial fish stocks and proposed species-specific management plans for Lake Victoria. Afr J Trop Hydrobiol Fish 12:60–66
Kishe-Machumu, MA (2012) Inter-guild differences and possible causes of the recovery of cichlid species in Lake Victoria, Tanzania. Thesis, University of Leiden
Kishe-Machumu MA, Witte F, Wanink JH (2008) Dietary shift in benthivorous cichlids after the ecological changes in Lake Victoria. Anim Biol 58:401–417
Kishe-Machumu MA, Witte F, Wanink JH, Katunzi EFB (2012) The diet of Nile perch, Lates niloticus (L.) after resurgence of haplochromine cichlids in the Mwanza Gulf of Lake Victoria. Hydrobiologia 682:111–119
Kudhongania AW, Cordone AJ (1974) Batho-spatial distribution patterns and biomass estimates of the major demersal fishes in Lake Victoria. Afr J Trop Hydrob Fish 3:15–31
Ligtvoet W, Mkumbo OC (1990) Synopsis of ecological and fishery research on Nile perch (Lates Niloticus) in Lake Victoria. FAO Fish. Tech. pap. No. 430, Rome, pp 35–74
Matsuishi T, Muhoozi LI, Mkumbo OC, Budeba YO, Njiru M, Asila A, Othina A, Cowx IG (2006) Are the exploitation pressures on the Nile perch fisheries resources of lake Victoria a case for concern? Fish Manag Ecol 13:53–71
Mkumbo OC (2002) Assessment and management of Nile perch (Lates niloticus L.) stocks in the Tanzanian waters of Lake Victoria. Thesis, University of Hull
Mkumbo OC, Nsinda P, Ezekiel CN, Cowx IG, Aeron M (2007) Towards sustainable exploitation of Nile perch consequential to regulated fisheries in Lake Victoria. Aquat Ecosyst Health Manag 10:398–406
Ogutu-Ohwayo R (1985) The effects of predation by Nile perch introduced in Lake Kyoga in relation to the fisheries of Lake Kyoga and Victoria. FAO Fish. Tech. pap. No. 335, Rome, pp 18–41
Ogutu-Ohwayo R (1990) The decline of the native fishes of Lakes Victoria and Kyoga (East Africa) and the impact of introduced species, especially the Nile perch, Lates niloticus and the Nile tilapia, Oreochromis niloticus. Environ Biol Fish 27:81–96
Pitcher TJ, Bundy A (1995) Assessment of the Nile perch fishery in Lake Victoria. In: Pitcher TJ, Hart PJB (eds) The impact of species changes in African Lakes. Chapman and Hall, London, pp 163–180
Pringle RM (2005) The origins of the Nile perch in Lake Victoria. Bioscience 55:780–787
Seehausen O, van Alphen JJM, Witte F (1997a) Cichlid fish diversity threatened by eutrophication that curbs sexual selection. Science 277:1808–1811
Seehausen O, Witte F, Katunzi EFB, Smits J, Bouton N (1997b) Patterns of the remnant cichlid fauna in southern Lake Victoria. Conserv Biol 11:890–904
van der Meer HJ, van Rijssel JC, Wagenaar LC, Witte F (2012) Photopic adaptations to a changing environment in two Lake Victoria Cichlids. Biol J Linn Soc 106:328–341
van Rijssel JC, Witte F (2013) Adaptive responses in resurgent Lake Victoria cichlids over the past 30 years. Evol Ecol 27:253–267
Witte F, Goudswaard PC (1985) Aspects of the haplochromine fishery in southern Lake Victoria. FAO, Tech. pap. No. 335, Rome, pp 81–88
Witte F, van Oijen MJP (1990) Taxonomy, ecology and fishery of haplochromine trophic groups. Zool Ver Leiden 262:1–47
Witte F, Goldschmidt T, Goudswaard PC, Ligtvoet W, van Oijen MJP, Wanink JH (1992a) Species extinction and concomitant ecological changes in Lake Victoria. Neth J Zool 42:214–232
Witte F, Goldschmidt T, Wanink J, van Oijen M, Goudswaard PC, Witte-Maas ELM, Bouton N (1992b) The destruction of an endemic species flock: quantitative data on the decline of the haplochromine cichlids of Lake Victoria. Environ Biol Fish 34:1–28
Witte F, Msuku BS, Wanink JH, Seehausen O, Katunzi EFB, Goudswaard PC, Goldschmidt T (2000) Recovery of cichlid species in Lake Victoria: an examination of factors leading to differential extinction. Rev Fish Biol Fish 10:233–241
Witte F, Wanink JH, Kishe-Machumu MA, Mkumbo OC, Goudswaard PC, Seehausen O (2007) Differential decline and recovery of haplochromine trophic groups in the Mwanza Gulf of Lake Victoria. Aquat Ecosyst Health Manag 10:416–433
Witte F, Welten M, Heemskerk M, van der Stap I, Ham L, Rutjes H, Wanink J (2008) Major morphological changes in a Lake Victoria cichlid fish within two decades. Biol J Linn Soc 94:41–52
Witte F, Silsbe GM, Hecky RE, Goudswaard PC, Guildford SJ, Kishe-Machumu MA, Wanink JH (2012) Did the loss of phytoplanktivorous fish contribute to algal blooms in the Mwanza Gulf of Lake Victoria? Hydrobiologia 679:283–29
Acknowledgments
We thank the Division of Fisheries under the Ministry of Livestock and Fisheries Development (MLFD) of the United Republic of Tanzania (URT) for approval and grant permission to conduct this research. We express our gratitude to Tanzania Fisheries Research Institute (TAFIRI) for providing office space and research facilities. Special thanks to Martien van Oijen of Naturalis, Leiden for so well caring for samples sent from Tanzania. Appreciation is expressed to Brian Marshall for his comments on earlier drafts of this paper. Thanks to Jacco van Rijssel for shaping the figures and reviewing the manuscript. We thank the captain and engineer of the Research Vessel (R.V.) Lake Victoria Explorer; R. Mamelela and H. Kalima, and their entire crew for their assistance during data collection. We also thank the crew members of the small trawler; Mhoja, Mohammed and Rajabu for their invaluable field assistance. This study was financially supported by The Netherlands Foundation for the Advancement of Tropical Research (WOTRO; grant WOTRO84-587), the Netherlands Organization for International Cooperation in Higher Education (NUFFIC) and the International Foundation for Sciences (IFS).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kishe-Machumu, M.A., Voogd, T., Wanink, J.H. et al. Can differential resurgence of haplochromine trophic groups in Lake Victoria be explained by selective Nile perch, Lates niloticus (L.) predation?. Environ Biol Fish 98, 1255–1263 (2015). https://doi.org/10.1007/s10641-014-0356-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-014-0356-2