Summary
Background. We aimed to investigate the efficacy and safety of atezolizumab plus bevacizumab therapy in patients with unresectable hepatocellular carcinoma (u-HCC) based on whether they had previously received systemic therapy, as well as the association of atezolizumab plus bevacizumab with early alpha-fetoprotein (AFP) response in real-world practice. Methods. A total of 52 patients with u-HCC were treated with atezolizumab plus bevacizumab between October 2020 and April 2021. The Response Evaluation Criteria in Solid Tumors (RECIST) and modified RECIST were used to evaluate radiological responses. Results. The patients received atezolizumab plus bevacizumab as 1st-line (n = 23), 2nd-line (n = 16), 3rd-line (n = 6), 4th-line (n = 3), 5th-line (n = 3), or 6th-line (n = 1) therapy. According to RECIST, the objective response rate (ORR) and disease control rate (DCR) in all patients were 15.4% and 57.7%. In the 1st-line patients, ORR and DCR based on RECIST 1.1 were 27.3% and 81.8%. The median time to progression (TTP) assessed by RECIST was significantly longer among patients receiving atezolizumab plus bevacizumab as 1st-line therapy than in patients receiving atezolizumab plus bevacizumab as later-line therapy (P < 0.001). Patients with an AFP response (reduction ≥ 20% from baseline) at 6 weeks had a significantly longer TTP assessed by RECIST than those without an AFP response (P = 0.02). Conclusion. Patients who received atezolizumab plus bevacizumab as 1st-line therapy had better clinical outcome than those who received atezolizumab plus bevacizumab in later lines. The AFP response at 6 weeks could be a predictor of disease progression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the most common primary liver cancer and the fourth most common cause of cancer-related deaths worldwide [1]. HCC is hypervascular, and such angiogenesis is driven by growth factors, such as vascular endothelial growth factor (VEGF). Tyrosine kinase inhibitors targeting VEGF signaling, such as sorafenib [2, 3], regorafenib [4], and lenvatinib [5], have already been approved for the treatment of unresectable HCC (u-HCC) in many countries. Cabozantinib [6] and ramucirumab [7, 8] have been approved as 2nd- or later-line agents for u-HCC in Japan. Ramucirumab was approved for patients with a high alpha-fetoprotein (AFP) level (≥ 400 ng/mL) in July 2019.
VEGF is also associated with cancer immune evasion. Based on this rationale, the combination of programmed cell death-ligand 1 (PD-L1) (atezolizumab) and VEGF (bevacizumab) pathway inhibition is believed to be the most effective therapy for advanced HCC. In September 2020, atezolizumab plus bevacizumab [9] was approved for patients with u-HCC in Japan and became the recommended 1st-line therapy. In Japan, agents approved for 1st- and 2nd-line therapies may be used for later-line treatment in real-world practice. In this study, we aimed to investigate the efficacy and safety of atezolizumab plus bevacizumab as 1st-line and later-line therapies in real-world practice. Moreover, we analyzed the factors associated with time to progression (TTP) and the association of atezolizumab plus bevacizumab therapy with the timing of the AFP response and disease progression.
Materials and methods
Patients
A total of 52 patients with u-HCC received atezolizumab plus bevacizumab therapy at the Musashino Red Cross Hospital between October 2020 and April 2021. Atezolizumab plus bevacizumab therapy was administered after discussions by experts at a tumor board in the institution. HCC diagnosis and evaluation of disease progression were based on guidelines proposed by the Japan Society of Hepatology [10], the American Association for the Study of Liver Disease [11], and the European Association for the Study of the Liver [12]. Written informed consent was obtained from all patients, and the ethics committee of the Musashino Red Cross Hospital approved the study in accordance with the Declaration of Helsinki.
Treatment protocol
Patients received intravenous atezolizumab (1200 mg) plus bevacizumab (15 mg/kg) every 3 weeks until disease progression or unacceptable toxicity. Treatment interruption was performed based on the manufacturer’s guidelines. Dynamic computed tomography was performed at baseline, 6–8 weeks after atezolizumab plus bevacizumab administration, and every 6–8 weeks thereafter. Treatment response was reported based on the Response Evaluation Criteria in Solid Tumors (RECIST) [13] and the modified RECIST [14]. The AFP level was also evaluated at baseline and every 3 weeks thereafter. Adverse events (AEs) were evaluated according to the Common Terminology Criteria for Adverse Events version 5.0. To assess changes in liver function, the albumin-bilirubin (ALBI) score [15, 16] was analyzed at baseline and at 3, 6, and 9 weeks after treatment. The data cut-off date of clinical response and ALBI score were defined on July 31, 2021.
Statistical analysis
Overall survival (OS) was measured from the date of atezolizumab plus bevacizumab administration to the date of death from any cause. Patients who were lost to follow-up were censored at their last visit or contact. Patients who were still alive on July 31, 2021 were censored. Progression-free survival (PFS) was measured from the date of atezolizumab plus bevacizumab administration to the date of radiological tumor progression or death from any cause. Data are expressed as median (range) or n (%). Fisher's exact test, Mann–Whitney U test, paired t-test, Kaplan–Meier method, and log-rank test were used for statistical analyses. Statistical significance was set at P < 0.05. Statistical analyses were performed using EZR (Saitama Medical Centre, Jichi Medical University, Shimotsuke, Japan), a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria) [17].
Results
Baseline patient characteristics
The baseline characteristics of the 52 patients are presented in Table 1. Atezolizumab and bevacizumab were administered as 1st-line, 2nd-line, 3rd-line, 4th-line, 5th-line, and 6th-line treatments in 23 (44.2%), 16 (30.8%), 6 (11.5%), 3 (5.8%), 3 (5.8%), and 1 (1.9%) patient, respectively. Among the 16 patients who received atezolizumab plus bevacizumab as 2nd-line treatment, 15 were treated with lenvatinib as 1st-line treatment and 1 patient who had been treated with cytotoxic agents as systemic chemotherapy received ramucirumab as the 1st-line molecular targeted agent. Among the 6 patients who received atezolizumab plus bevacizumab as 3rd-line treatment, 3 were previously treated with lenvatinib and sorafenib, 2 received sorafenib and regorafenib, and 1 received axitinib + avelumab (NCT03289533) and lenvatinib. Among the 3 patients who received atezolizumab plus bevacizumab as 4th-line treatment, 2 were treated with sorafenib, regorafenib, and lenvatinib and 1 received lenvatinib, sorafenib, and regorafenib. Among the 3 patients who received atezolizumab plus bevacizumab as 5th-line treatment, 1 was treated with sorafenib, regorafenib, lenvatinib, and ramucirumab, 1 was treated with sorafenib, cabozantinib, lenvatinib, and ramucirumab, and 1 received lenvatinib, sorafenib, ramucirumab, and regorafenib. The 1 patient who received atezolizumab plus bevacizumab as 6th-line treatment was previously treated with axitinib plus avelumab (NCT03289533), cabozantinib, lenvatinib, ramucirumab, and sorafenib.
Therapeutic efficacy
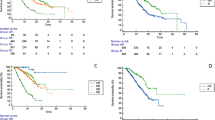
At the end of the data cutoff (July 31, 2021), the median duration of follow-up was 221 days (range, 51–297 days). The median number of courses of atezolizumab plus bevacizumab was 5 (range, 1–15). During the observation period, 18 patients continued atezolizumab plus bevacizumab therapy and 34 patients discontinued atezolizumab plus bevacizumab therapy because of progressive disease (PD) (n = 28) or AEs (n = 6). Six patients died from HCC progression, and one patient died of cerebral hemorrhage. The median OS was not reached, and the median PFS assessed by the RECIST was 4.7 months (Fig. 1). In 23 patients who received atezolizumab plus bevacizumab as 1st-line treatment, the median OS and PFS were not reached, and the PFS at 3 months was 79.8% (Fig. 2).
Radiological evaluation after atezolizumab plus bevacizumab administration was performed in 46 patients. With regard to the best antitumor response according to the RECIST, 1 patient achieved complete response (CR), 7 patients achieved partial response (PR), 22 patients had stable disease (SD), 16 patients had PD, and 6 patients had no evaluable disease (NE). The objective response rate (ORR) and disease control rate (DCR) were 15.4% and 57.7%, respectively. With regard to the best antitumor response according to the modified RECIST, 1 patient achieved CR, 10 patients achieved PR, 21 patients had SD, 14 patients had PD, and 6 patients had NE. The ORR and DCR were 21.2% and 61.5%, respectively.
Treatment after atezolizumab plus bevacizumab
During the observation period, 30 of 34 patients (88.2%) who discontinued atezolizumab plus bevacizumab received further anticancer therapy (Table 2). The other 4 patients (11.8%) received palliative care. The transition rates to post-treatment among patients receiving atezolizumab plus bevacizumab as 1st-line therapy (n = 9) and those receiving atezolizumab plus bevacizumab as later-line therapy (n = 25) were 100% and 84%, respectively.
Changes in liver function during atezolizumab plus bevacizumab therapy
The changes in the ALBI score in 52 patients who received atezolizumab plus bevacizumab are shown in Fig. 3. The median ALBI scores at baseline, 3 weeks, 6 weeks, and 9 weeks were − 2.26 (range, − 3.28 to − 1.37), − 2.19 (range, − 3.13 to − 1.17), − 2.25 (range, − 3.07 to − 1.13), and − 2.02 (range, − 3.2 to − 1.26), respectively. The median ALBI score was significantly worsened at 3 weeks compared to baseline (P = 0.04). There were no significant differences in the median ALBI scores at baseline and 6 weeks (P = 0.24), or at baseline and 9 weeks (P = 0.26). Among patients receiving atezolizumab plus bevacizumab as 1st-line treatment (n = 23), the median ALBI scores at baseline, 3 weeks, 6 weeks, and 9 weeks were − 2.28 (range, − 3.28 to − 1.37), − 2.30 (range, − 3.13 to − 1.17), − 2.31 (range, − 3.07 to − 1.13), and − 2.39 (range, − 3.2 to − 1.26), respectively. In patients receiving atezolizumab plus bevacizumab as 2nd- or later-line therapy (n = 29), the median ALBI scores at baseline, 3 weeks, 6 weeks, and 9 weeks were − 2.25 (range, − 2.99 to − 1.48), − 2.14 (range, − 3.04 to − 1.26), − 2.14 (range, − 2.82 to − 1.20), and − 1.96 (range, − 3.04 to − 1.26), respectively. There were no significant differences in the median ALBI scores at baseline and at 3 weeks, at baseline and at 6 weeks or at baseline and at 9 weeks in either group (Fig. 4).
Adverse events during atezolizumab plus bevacizumab therapy
AEs observed during atezolizumab plus bevacizumab therapy are shown in Table 3. Any grade AE was observed in 36 of the 52 patients. The rates of total AEs in patients receiving atezolizumab plus bevacizumab as 1st-line therapy (n = 23) and those receiving atezolizumab plus bevacizumab as 2nd- or later-line therapy (n = 29) were 60.9% and 75.9%, respectively (P = 0.37). When comparing AEs with an incidence of 10% or more, the rates of AEs were 56.5% in patients receiving atezolizumab plus bevacizumab as 1st-line therapy and 75.9% in patients receiving atezolizumab plus bevacizumab as 2nd- or later-line therapy (P = 0.23). No infusion reaction was reported in any patient. During the observation period, six patients discontinued atezolizumab plus bevacizumab therapy owing to AEs, as follows: two patients with transaminase increase, one patient with interstitial pneumonia, one patient with meningitis and transaminase increase, one patient with renal dysfunction, and one patient with gastrointestinal bleeding. Four of the six patients were treated with corticosteroids. The rates of discontinuation due to AEs among patients treated with atezolizumab plus bevacizumab as 1st-line and 2nd- or later-line therapy were 8.7% and 13.8%, respectively (P = 0.68). No AEs other than those reported in the IMbrave150 trial were observed.
Clinical outcomes in the 1st-line group and the 2nd- or later-line group
We compared TTP between the patients treated with atezolizumab plus bevacizumab as 1st-line (n = 22) and 2nd or later-line (n = 24). The baseline characteristics are shown in Table 4. The proportions of patients receiving a full dose of atezolizumab plus bevacizumab in the 1st-line group and the 2nd- or later-line group were 72.7% and 58.3%, respectively (P = 0.23). Among 22 patients who received atezolizumab plus bevacizumab as 1st-line treatment, 1 patient achieved CR, 5 patients achieved PR, 12 patients had SD, and 4 patients had PD according to the RECIST. The ORR was 27.3% and the DCR was 81.8% according to the RECIST. According to the modified RECIST, 1 patient achieved CR, 6 achieved PR, 11 had SD, and 4 had PD. The ORR was 31.8% and the DCR was 81.8% using the modified RECIST. Among the 24 patients who received atezolizumab plus bevacizumab as 2nd- or later-line treatment, 2 achieved PR, 10 had SD, and 12 had PD according to the RECIST. The ORR was 8.3% and the DCR was 50.0% using the RECIST. According to the modified RECIST, 4 patients achieved PR, 10 had SD, and 10 had PD. The ORR was 16.7% and the DCR was 58.3% using the modified RECIST. The median TTP assessed by the RECIST was significantly longer in the 1st-line group than in the 2nd- or later-line group (not reached vs. 3.8 months, P < 0.001) (Fig. 5).
We next analyzed the factors associated with TTP in patients receiving atezolizumab plus bevacizumab (n = 46). In the multivariate analysis, receiving atezolizumab plus bevacizumab as 2nd- or later-line therapy was the only significant factor (hazard ratio 3.16, 95% confidence interval 1.03–9.74, P = 0.04) (Table 5).
1st-line group: received atezolizumab plus bevacizumab as 1st-line therapy.
2nd- or later-line group: received atezolizumab plus bevacizumab as 2nd-, 3rd-, 4th-, 5th-, or 6th-line therapy.
First-line group: received atezolizumab plus bevacizumab as 1st-line therapy.
Second- or later-line group: received atezolizumab plus bevacizumab as 2nd-, 3rd-, 4th-, 5th-, or 6th-line therapy.
The association between the AFP response and therapeutic efficacy in patients receiving atezolizumab plus bevacizumab therapy.
We investigated the AFP response as a predictor of DCR according to RECIST in patients receiving atezolizumab plus bevacizumab therapy. The AFP response was defined as a reduction in AFP from the baseline of ≥ 20%. Thirty-two patients continued atezolizumab plus bevacizumab therapy for more than 6 weeks and were included in the analysis. At 3 weeks, 12 patients showed an AFP response. The AFP response at 3 weeks had a positive predictive value (PPV) of 91.7% and a negative predictive value (NPV) of 40.0%. At 6 weeks, 12 patients showed an AFP response. The AFP response at 6 weeks had a PPV of 91.7% and an NPV of 40.0%. The TTPs of patients with and without an AFP response at 3 weeks and 6 weeks are presented in Fig. 6. There were no significant differences in the median TTP between patients with and without an AFP response at 3 weeks (3.8 months vs. 4.8 months, P = 0.09). By contrast, there was a significant difference in the median TTP between patients with and without an AFP response at 6 weeks (2.6 months vs. 4.7 months, P = 0.02). The results were similar when patients with a baseline AFP < 20 ng/mL were excluded. Although there were no significant differences in the median TTP between patients with and without an AFP response at 3 weeks (3.8 months vs. 4.1 months, P = 0.08), there was a significant difference in the median TTP between patients with and without an AFP response at 6 weeks (2.5 months vs. 4.7 months, P = 0.001) (Fig. 7).
We also analyzed the association between the AFP response at 6 weeks and therapeutic efficacy stratified by patients with baseline AFP < 20 ng/mL, 20–400 ng/mL, and > 400 ng/mL (Table 6). Among 10 patients with a baseline AFP < 20 ng/mL, 3 had an AFP response at 6 weeks. According to the RECIST, 2 patients achieved PR and 1 had SD. Among 10 patients with a baseline AFP 20–400 ng/mL, 4 had an AFP response at 6 weeks. One patient achieved PR, 2 had SD, and 1 had PD. Among 12 patients with a baseline AFP > 400 ng/mL, 5 had an AFP response at 6 weeks. One patient achieved CR, 1 achieved PR, and 3 patients had SD. There were no significant differences in the median TTP among patients with baseline AFPs < 20 ng/mL, 20–400 ng/mL, and > 400 ng/mL (5.2 months, 4.0 months, and 4.8 months, respectively; P = 0.19) in patients with an AFP response at 6 weeks (Fig. 8).
Discussion
Atezolizumab plus bevacizumab is recommended as a 1st-line therapy for advanced HCC in many guidelines, including the ASCO [18] and ESMO guidelines [19]. Some recent studies have reported the efficacy and safety of atezolizumab plus bevacizumab in Japanese patients with u-HCC in real-world practice [20,21,22]. To the best of our knowledge, this is the first report to reveal the difference in clinical outcome between patients receiving atezolizumab plus bevacizumab as 1st-line and 2nd- or later-line therapies and the association between the timing of the AFP response and therapeutic efficacy.
We included patients who received atezolizumab plus bevacizumab as 2nd- or later-line therapy in this study. The IMbrave150 trial [23] included only patients who had not previously received systemic therapy, and the ORR and DCR were 27.3% and 73.6%, respectively, according to the RECIST. Recent studies [20,21,22] showed there were no significant differences in radiological responses to atezolizumab plus bevacizumab between 1st-line and 2nd- or later-line patients. In our study, the median TTP was significantly shorter in the 2nd- or later-line group than in the first-line group. In patients previously treated with other agents, dose modification and interruption of bevacizumab were more frequent than in patients receiving atezolizumab plus bevacizumab as 1st-line therapy. However, there were no significant differences in the rate of AEs between the two groups.
Previous reports reported deterioration in liver function during the early period among patients treated with sorafenib [24] and lenvatinib [25]. Sangro et al. [26] reported that patients receiving nivolumab were more likely to have preserved liver function than patients receiving sorafenib. Kudo et al. [27] reported that ramucirumab did not worsen the ALBI score during the early treatment period. Changes in liver function during atezolizumab plus bevacizumab therapy were not examined in the IMbrave150 trial. Recent studies [20, 21] showed the liver function was slightly worsened after 2–3 weeks and recovered at 6 weeks during atezolizumab plus bevacizumab therapy. In our study, the ALBI score at 3 weeks transiently worsened compared to baseline but improved at 6 weeks and 9 weeks. The temporary worsening of liver function may be related to the therapeutic response, which leads to appetite loss and fatigue during the early treatment period. Liver function and these AEs improved spontaneously at 6 and 9 weeks. Atezolizumab plus bevacizumab therapy can be maintained while preserving liver function in many patients.
In this study, we focused on the AFP response at 3 and 6 weeks after the administration of the combination therapy. There were no significant differences in the median TTP between patients with and without an AFP response at 3 weeks. By contrast, there was a significant difference in the median TTP between patients with and without an AFP response at 6 weeks. Previous studies reported early AFP response is useful to predict response to sorafenib [28, 29], lenvatinib [30] and checkpoint inhibitor therapy [31]. However, in patients receiving atezolizumab plus bevacizumab therapy, the antitumor response is delayed in some patients, and early treatment decisions based on the AFP response should be avoided.
In Japan, five regimens (sorafenib, regorafenib, lenvatinib, ramucirumab if AFP ≥ 400 ng/mL, and cabozantinib) can be administered after atezolizumab plus bevacizumab therapy based on the national health insurance guidelines. Because of the small cohort and short observation period, we could not examine the established clinical outcomes of post-combination therapies in real-world practice. Among the patients who received lenvatinib after combination therapy, the ORR and DCR were 50% and 75%, respectively. Aoki et al. [32] revealed that lenvatinib demonstrated considerable antitumor effects with acceptable safety in patients with progressive and u-HCC when administered immediately after PD-1/PD-L1 blockade failure (ORR 55.6%, DCR 86.1%). Further multicenter studies on treatment after atezolizumab plus bevacizumab therapy are necessary to develop an effective treatment strategy for patients with u-HCC.
There are certain limitations to our study. The study was a retrospective single-center study with a small number of patients and a short observation period, and tumor response was not evaluated in all patients.
To our knowledge, our report is the first to reveal the difference in clinical outcome between 1st-line and 2nd- or later-line atezolizumab plus bevacizumab therapy and the association between the timing of the AFP response and therapeutic efficacy. Our data can provide useful information to develop useful therapeutic strategies for patients with u-HCC and effectively administer combination therapy.
Conclusion
Atezolizumab plus bevacizumab therapy was found to have similar radiological responses to the phase III IMbrave150 trial regimen in u-HCC patients when administered as 1st-line therapy. Patients receiving atezolizumab plus bevacizumab therapy as 1st-line therapy tended to have longer TTP than those receiving atezolizumab plus bevacizumab as 2nd- or later-line therapy. The AFP response at 6 weeks could be a predictive indicator of disease progression.
Data availability
The data presented in this study are available upon request from the corresponding author.
Code availability
Not applicable.
References
Bray F, Ferlay J, Soerjomataram I et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424
Llovet JM, Ricci S, Mazzaferro V et al (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359:378–390
Cheng AL, Kang YK, Chen Z et al (2009) Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 10:25–34
Bruix J, Qin S, Merle P et al (2017) Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 389:56–66
Kudo M, Finn RS, Qin S et al (2018) Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 391:1163–1173
Abou-Alfa GK, Meyer T, Cheng AL et al (2018) Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med 379:54–63
Park JO, Ryoo BY, Yen CJ et al (2016) Second-line ramucirumab therapy for advanced hepatocellular carcinoma (REACH): An East Asian and non-East Asian subgroup analysis. Oncotarget 7:75482–75491
Zhu AX, Kang YK, Yen CJ et al (2019) Ramucirumab in advanced hepatocellular carcinoma and elevated alpha-fetoprotein following sorafenib (REACH-2): A randomised, double-blind, placebocontrolled phase 3 trial. Lancet Oncol 20:282–296
Finn RS, Ducreux M, Qin S et al (2018) IMbrave150: A randomized phase III study of 1L atezolizumab plus bevacizumab vs sorafenib in locally advanced or metastatic hepatocellular carcinoma. J Clin Oncol 36:TPS4141–TPS4141
Kokudo N, Takemura N, Hasegawa K et al (2019) Clinical practice guidelines for hepatocellular carcinoma: The Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol Res 49:1109–1113
Marrero JA, Kulik LM, Sirlin C et al (2018) Diagnosis, staging and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 68:723–750
European Association for the Study of the Liver (2018) EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol 69:182–236
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Lencioni R, Llovet JM (2010) Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 30:52–60
Johnson PJ, Berhane S, Kagebayashi C et al (2014) Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol 33:550–558
Hiraoka A, Kumada T, Tsuji K et al (2019) Validation of modified ALBI Grade for more detailed assessment of hepatic function in hepatocellular carcinoma patients: A multicenter analysis. Liver Cancer 8:121–129
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48:452–445
Gordan JD, Kennedy EB, Abou-Alfa GK et al (2020) Systemic Therapy for Advanced Hepatocellular Carcinoma: ASCO Guideline. J Clin Oncol 38:4317–4345
Vogel A, Martinelli E (2021) Updated treatment recommendations for hepatocellular carcinoma (HCC) from the ESMO Clinical Practice Guidelines. Ann Oncol 32:801–805
Kuzuya T, Kawabe N, Hashimoto S et al (2021) Initial experience of atezolizumab plus bevacizumab for advanced hepatocellular carcinoma in clinical practice. Cancer Diagnosis & Prognosis 1:19–22
Hiraoka A, Kumada T, Tada T et al (2021) Atezolizumab plus bevacizumab treatment for unresectable hepatocellular carcinoma: Early clinical experience. Cancer Rep (Hoboken) e1464
Iwamoto H, Shimose S, Noda Y et al (2021) Initial experience of atezolizumab plus bevacizumab for unresectable hepatocellular carcinoma in real-world clinical practice. Cancers 13:2786
Finn RS, Qin S, Ikeda M et al (2020) Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 382:1894–1905
Terashima T, Yamashita T, Sunagozawa H et al (2018) Analysis of the liver functional reserve of patients with advanced hepatocellular carcinoma undergoing sorafenib treatment: prospects for regorafenib therapy. Hepatol Res 48:956–966
Hiraoka A, Kumada T, Atsukawa M et al (2019) Early relative change in hepatic function with Lenvatinib for Unresectable hepatocellular carcinoma. Oncology 97:334–340
Sangro B, Park J, Finn R et al (2020) CheckMate 459: long-term (minimum follow-up 33.6 months) servival outcomes with nivolmab versus sorafenib as first-line treatment in patients with advanced hepatocellular carcinoma. Ann Oncol 31:241–242
Kudo M, Galle PR, Brandi G et al (2021) Effect of ramucirumab on ALBI grade in patients with advanced HCC: results from REACH and REACH-2. JHEP Rep 3:100215
Kuzuya T, Asahina Y, Tsuchiya K et al (2011) Early decrease in alpha-fetoprotein, but not des-gamma-carboxy prothrombin, predicts sorafenib efficacy in patients with advanced hepatocellular carcinoma. Oncology 81:251–258
Kuzuya T, Ishigami M, Ishizu Y et al (2016) Fever within 2 weeks of Sorafenib therapy predicts favorable treatment efficacy in patients with advanced hepatocellular carcinoma. Oncology 91:261–266
Kodama K, Kawaoka T, Namba M et al (2019) Correlation between early tumor marker response and imaging response in patients with advanced hepatocellular carcinoma treated with lenvatinib. Oncology 97:75–81
Lee PC, Chao Y, Chen MH, Lan KH et al (2020) Predictors of response and survival in immune checkpoint inhibitor-treated unresectable hepatocellular carcinoma. Cancers 12:182
Aoki T, Kudo M, Ueshima K et al (2020) Exploratory analysis of lenvatinib therapy in patients with unresectable hepatocellular carcinoma who have failed prior PD−1/PD-L1 checkpoint blockade. Cancers 12:3048
Acknowledgements
We thank the patients and medical staff who contributed to this study.
Funding
This study was supported by a grant-in-aid from the Japanese Ministry of Health, Labour and Welfare and from the Japan Agency for Medical Research and Development (JP20fk0210067h0001).
Author information
Authors and Affiliations
Contributions
Study design: Yuka Hayakawa and Kaoru Tsuchiya Data collection: Yuka Hayakawa, Kaoru Tsuchiya, Masayuki Kurosaki, Yutaka Yasui, Shun Kaneko, Yuki Tanaka, Shun Ishido, Kento Inada, Sakura Kirino, Koji Yamashita, Tsubasa Nobusawa, Hiroaki Matsumoto, Tatsuya Kakegawa, Mayu Higuchi, Kenta Takaura, Shohei Tanaka, Chiaki Maeyashiki, Nobuharu Tamaki, Hiroyuki Nakanishi, Jun Itakura, Yuka Takahashi and Namiki Izumi Data analysis: Yuka Hayakawa and Kaoru Tsuchiya Data interpretation: Yuka Hayakawa, Kaoru Tsuchiya, Masayuki Kurosaki, Shun Kaneko, Yutaka Yasui, Yasuhiro Asahina, Ryuichi Okamoto and Namiki Izumi Manuscript writing: Yuka Hayakawa and Kaoru Tsuchiya Manuscript revision: Masayuki Kurosaki, Shun Kaneko, Yutaka Yasui, Yasuhiro Asahina, Ryuichi Okamoto and Namiki Izumi All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of Musashino Red Cross Hospital (No. 28077).
Informed consent
Informed consent was obtained from all subjects involved in the study.
Conflict of interest
Kaoru Tsuchiya, Masayuki Kurosaki, and Namiki Izumi received advisory board fees and honoraria from the speakers’ bureau from Bayer, Eisai, Eli Lilly Japan, and Roche. The Japanese Ministry of Health, Labour and Welfare had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hayakawa, Y., Tsuchiya, K., Kurosaki, M. et al. Early experience of atezolizumab plus bevacizumab therapy in Japanese patients with unresectable hepatocellular carcinoma in real-world practice. Invest New Drugs 40, 392–402 (2022). https://doi.org/10.1007/s10637-021-01185-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-021-01185-4