Summary
Purpose Among alkaloids, abundant secondary metabolites in plants, aporphines constitute a class of compounds with interesting biological activities, including anticancer effects. The present study evaluated the anticancer activities of 14 substances, including four aporphine derivatives acquired through the biomonitoring of (±)-apomorphine hydrochloride total synthesis from 2-phenethylamine and 3,4-dimethoxybenzaldehyde against head and neck squamous cell carcinoma (HNSCC). Methods The cytotoxic effects of compounds against a panel of HNSCC cell lines were determined by PrestoBlue cell viability assay, while the genotoxicity of substances was evaluated by micronucleus test. Cell death was detected by flow cytometry (Annexin V/7AAD) and western blot analysis was used to detect the presence of cleaved Caspase-3 molecules. Results The aporphine and isoquinoline derivatives APO, C1, and A5 significantly reduced HNSCC cell viability and promoted DNA damages in these cells. Further, by activating the Caspase-3 pathway, these substances were able to induce apoptosis. Conclusion Our results revealed that APO, C1, and A5 exhibit cytotoxic effects in HNSCC cells. The mechanisms of action appear to be partly via the generation of DNA damages and apoptosis induction through Caspase-3 pathway activation. This study provides preclinical data that suggest a potential therapeutic role for APO, C1, and A5 against head and neck cancer cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Work on natural compounds has significantly advanced anticancer drug research. Nearly 60% of the approved anticancer drugs have natural origins that include microorganisms, plants, animals, and marine organisms [1]. For example, vinca alkaloids (vinblastine, vincristine, and the semi-synthetic derivative vinorelbine), irinotecan and topotecan (derivatives from a monoterpenoid alkaloid isolated from the Chinese ornamental tree Camptotheca acuminate), podophyllotoxins (the semi-synthetic derivatives etoposide and teniposide), and taxols (paclitaxel and the semi-synthetic derivative docetaxel) all have origins in natural sources and are among the most frequently used anticancer drugs [2,3,4]. Alkaloids are abundant secondary metabolites found in plants and represent one of the most widespread classes of natural products endowed with multiple, varied pharmacological properties [5]. Among alkaloids, aporphines are biosynthetic derivatives of benzylisoquinoline alkaloids and have a skeleton of four rings (A-D), with a nitrogen atom present on ring B [6,7,8]. Aporphine alkaloids compose a class of compounds with interesting biological activities, including anti-HIV activity, antiplatelet aggregation, anticonvulsant, antispasmodic, dopaminergic activities and anticancer effects [9,10,11,12,13,14].
Head and neck cancers are one of the most common cancers worldwide, affecting 740,000 patients and causing 300,000 deaths every year [15, 16]. Head and neck cancers are mostly squamous cell carcinomas (HNSCC) and arise from the mucosa of distinct head and neck topologies including the oral cavity, pharynx, and larynx. Combined efforts in surgery, radiotherapy, and chemotherapy have improved the management of HNSCC patients, yet still, only 50% of patients with locally advanced disease survive more than 5 years [17, 18]. Chemotherapeutic drugs are used in the treatment of HNSCC patients, but their application is limited due to low efficacy, the development of drug resistance, and low tolerance of side effects. Thus, the discovery of new effective compounds to enhance the treatment of patients with HNSCC is urgent.
With this in mind, the aim of the present study is to identify new cytotoxic drugs for the treatment of HNSCC that could help optimize appropriate patient-specific therapeutic interventions, quality of life and outcomes. For this purpose, we first studied in HNSCC cells the cytotoxic effect (IC50 determination) of 14 compounds, including four aporphine prototypes. Secondly, a micronucleus test was used to measure the genotoxic effects induced by these substances. In addition, we characterized the type of cell death induced by some of these compounds in HNSCC cells. The results of this study demonstrated, for the first time, that aporphine and isoquinoline derivatives reduce the viability of HNSCC cells, promote DNA damage and induce apoptosis cell death by regulating Caspase-3 activity.
Materials and methods
Substances
2-Phenethylamine (A1), N-phenethylacetamide (A2), 1-methyl-3,4-dihydroisoquinoline (A3), 1-(1-methylene-3,4-dihydroisoquinolin-2(1H)-yl)ethanone (A4), 3,4-dimethoxybenzaldehyde (B1), 3,4-dimethoxyphenol (B2), (3,4-dimethoxy-2-(trimethylsilyl)phenoxy)trimethylsilane (B3), 3,4-dimthoxy-2-(trimethylsilyl)phenol (B4), 3,4-dimethoxy-2-(trimethylsilyl)phenyltrifluoromethanesulfonate (B5), 1-(10,11-Dimethoxy-6a,7-dihydro-4H-dibenzo[de,g]quinolin-6(5H)-yl)ethanone (C1), 10,11-dimethoxy-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]quinolone (C2), and 10,11-dimethoxy-6-methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]quinoline (C3) were obtained through the convergent total synthesis of (±)-apomorphine hydrochloride (APO). This was accomplished by using an approach that employs a sequence of transformations involving a [4 + 2] cycloaddition reaction followed by a hydrogen migration as described by Muraca et al. [19]. 2,2,2-Trifluoro-1-(1-methylene-3,4-dihydroisoquinolin-2(1H)-yl)ethanone (A5) was recently synthesized in our research group by protocols reported in the literature [20, 21] and employed in an alternative route to produce (±)-apomorphine hydrochloride (APO) (Fig. 1). Cisplatin was purchased from Merck (#1134357).
Cell line culture
In order to assess the cytotoxic effects of the chosen substances, a panel of HNSCC cell lines was selected. The human HNSCC cell lines FaDu and SCC25 were purchased from American Type Culture Collection (ATCC), while the NCC-HN120, a patient-derived cell line, was received as a generous gift from Prof. Dr. N. G. Iyer (National Cancer Centre of Singapore, Singapore). All cell lines were maintained in RPMI-1640 or DMEM (ThermoFisher Scientific) supplemented with 10% fetal bovine serum (ThermoFisher Scientific) and 0.01 μg/ml of penicillin-streptomycin (ThermoFisher Scientific). All cell lines were cultured at 37 °C with 5% CO2.
Cell viability assay and determination of IC50 values
The cell viability after drugs treatment was determined by PrestoBlue Cell Viability Reagent (ThermoFisher Scientific), following the manufacturer’s instructions. The HNSCC cells were briefly seeded into 96-well plates at an initial density of 3 × 103 cells per well and incubated at 37 °C for 48 h. Cells were treated with the substances (A1, A2, A3, A4, A5, B1, B2, B3, B4, B5, C1, C2, C3, and APO) and cisplatin for 72 h. Two hours before the end of treatment, 10 μL of PrestoBlue reagent was added to each well. After the total incubation-time, fluorescence (540 nm excitation/ 590 nm emission) was measured using a microplate reader M3 (Molecular Devices). The relative luminescence units from treated wells were normalized against vehicle-treated control cells (DMSO) and expressed as percentage of viable cells. Cisplatin was used as a positive control for cytotoxicity.
The relation between surviving cells and substance concentration was plotted to get the survival curve of each tumor cell line after treatment with each compound. The 50% inhibitory concentration (IC50), required to achieve 50% cell death, was estimated from graphic plots of the dose response curve using Prism 5 (GraphPad software). Cells were briefly treated with different concentrations of each compound for 72 h at concentrations ranging from 0.98 μM to 500 μM for organic substances and 0.78 nM to 100 nM for cisplatin. Drug stocks (100 mM) were prepared in DMSO and all the working dilutions were prepared in culture media. All experiments were performed in triplicate and data was expressed as the mean ± SD of the triplicate assays.
Micronuclei assay
The micronuclei assay was performed as previously reported [22]. The 6 × 104 cells were briefly seeded on slides and incubated for 48 h. Following this, the cells were treated for 72 h with the IC10 of each compound, as recommended by OECD [23]. Next, the cells were fixed with 4% formaldehyde for 15 min. The slides were allowed to air-dry and treated with hydrochloric acid 5 N for 15 min. Then, the slides were incubated with Schiff Reagent solution (#109033, Merck) for 90 min and stained with Fast Green (#F7258, Merck) for 20 min. Slides were coded for “blind” analysis and scored manually using an optical microscope (Nikon Eclipse E-400) by two independent observers. The images were captured and analyzed with Imagelab1 software (version 2.3, Softium Informatica Ltda.). The total number of MN was determined according to the criteria set by da Silva et al. [24], in which circular or ovoid chromatin bodies, smaller than one-third of the main nucleus displaying the same staining and focusing pattern as the main nucleus, were considered as MN. One thousand cells were scored for each treatment to determine the MN frequency. The tests were carried out in triplicate.
Annexin V/ 7AAD assay
To demonstrate and quantify the cells undergoing cell death, the Annexin V/ 7AAD binding assay was performed using flow cytometry. The 1 × 105 cells were briefly seeded on each well of a 12-well dish with 1 mL of culture medium. Forty-eight hours after seeding, the substances were added (IC50). After 72 h of incubation, the cells were harvested by centrifugation (250 x g; 5 min), washed with PBS and resuspended in 100 μL of Annexin V (AV) binding buffer (#1006, Biovision). Next, 1 μL of AV-conjugated with FITC and 1 μL of 7-aminoactinomycin D (7AAD) (#640922; Biolegend) were added to the cells and incubated for 60 min at room temperature in the dark. Flow cytometer was performed using standard procedures on an Accuri C6 (BD Biosciences). Cells marked only with 0.5 μL of AV or 0.5 μL of 7AAD were used to calibrate the flow cytometer according to AV and 7AAD labeling. These assays were performed in triplicate. The data were analyzed using FlowJo software.
Caspase-3 activity
The cells were harvested, washed with ice-cold phosphate-buffered saline solution and resuspended in Mammalian Cell Extraction buffer (#K269–500, Biovision) according to manufacturers´ recommendations. After cell lysis, the proteins were resolved on SDS-PAGE and transferred to polyvinylidene difluoride membranes (#WAHP04700, Millipore). The membranes were blocked in Tris-buffered saline solution containing 0.05% of Tween-20 and 5% of bovine serum albumin (#A2153, Merck) for 1 hour at room temperature. Further, the membranes were incubated overnight with the appropriate primary antibodies using the following dilutions: 1:1000 Caspase-3 (#9664, Cell Signaling) and 1:1000 α/β-Tubulin (#2148, Cell Signaling). Goat anti-mouse (#70745, Cell Signaling) conjugated to peroxidase was used as secondary antibody. Proteins were detected using Chemiluminescent HRP Substrate (#WBKLS0550, Merck) and visualized with ImageQuantLass 4000 system (GE Healthcare Life Sciences).
Statistical analyses
Comparisons of the values obtained for cell viability, MN frequency and cell death proportions were performed using one-way ANOVA. Multiple paired comparisons were conducted by means of the Bonferroni’s post-test method to maintain the 5% significance level.
Results
Antitumor effects of aporphine and isoquinoline derivatives in HNSCC cell lines
The in vitro antitumor activities of compounds obtained through convergent total syntheses of (±)-apomorphine hydrochloride (APO) (A1, A2, A3, A4, A5, B1, B2, B3, B4, B5, C1, C2, C3, and APO) were evaluated on three HNSCC cell lines (FaDu, SCC25 and NCC-HN120). Firstly, the HNSCC cells were treated with 1 μM of each compound, but no reduction in the cell viability was observed (data not shown). Next, the cells were treated with 100 μM of each compound. The growth of all HNSCC cell lines tested was significantly reduced by APO, C1, and A5 while the other compounds evaluated (A1, A2, A3, A4, B1, B2, B3, B4, B5, C2, and C3) did not affected the viability of HNSCC cells (Fig. 2). Therefore, APO, C1, and A5 were selected for further assessment in HNSCC cells.
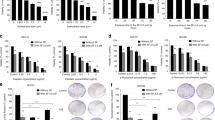
Cell viability level of FaDu (a), SCC25 (b), and NCC-HN120 (c) after treatment with the selected substances tested in this study. The cells were treated for 72 h with 100 μM of each compound, as well as 10 nM of cisplatin. Cell viability was determined using PrestoBlue reagent. The proportion of viable cells observed in the treatment with DMSO alone (drug vehicle) was considered 100% and the relative luminescence units from treated cells was normalized according to vehicle-treated control cells. This assay was conducted in triplicate, error bars represent mean ± SD. Asterisks denote significance as determined by one-way ANOVA multiple comparisons followed by Bonferroni test (post-test); *p < 0.0001 and **p < 0.05
Given the evidence that APO, C1, and A5 have cytotoxic activity against HNSCC cells, a 72-h treatment with increasing concentrations of these compounds resulted in dose-dependent reduction of cell viability in all cell lines tested (Fig. 3a). The IC50 value of each cell line was determined and ranged from 11.93 ± 0.15 to 25.95 ± 0.23 μM for APO; 14.83 ± 0.25 to 49.3 ± 0.6 μM for C1, 24.37 ± 0.5 to 67.23 ± 0.75 μM for A5; and 2.8 ± 0.2 to 13.81 ± 0.2 nM for cisplatin (Table 1). The highest anticancer effects were recorded by APO on SCC25 (IC50 = 11.9 μM) and C1 on NCC-HN120 (IC50 = 14.8 μM). The IC50 values of these three compounds at low concentrations (ranging from 11.93 ± 0.15 to 67.23 ± 0.75 μM) suggest that these substances are possible antitumor agents for HNSCC therapy (Fig. 3b).
Assessment of IC50 values of APO, C1, and A5. a The HNSCC cell lines (FaDu, SCC25, and NCC-HN120) were treated for 72 h with increasing concentrations of APO, C1, and A5. Cell viability was determined using PrestoBlue reagent. b The lowest dose (μM) of APO, C1, and A5 required for 50% inhibition on the viability of HNSCC cells (IC50) is indicated. This assay was conducted in triplicate
Aporphine and isoquinoline derivatives increase micronuclei frequency
In order to analyze the geno/cytotoxic effect of APO, C1, and A5, we evaluated micronuclei (MN) frequency in FaDu and SCC25 cell lines after 72 h of treatment with the IC10 of each compound. As observed in Fig. 4a, this analysis showed that MN frequencies in FaDu and SCC25 lines prior to treatment with the aporphine and isoquinoline derivatives were 13.6% and 9.6%, respectively. After treatment with these compounds, the MN frequency observed in FaDu cells was 18.92% for A5, 30.16% for C1, and 27.60% for APO. Higher micronuclei frequencies were also observed in SCC25 (32.9% for APO, 25.2% for C1, and 29.8% for A5). The significant increase in micronuclei frequency after treatment with the three substances tested suggest DNA damage was the potential mechanism for the geno/cytotoxic activity of these compounds in HNSCC cells.
APO, C1, and A5 promote DNA damage and induce apoptosis cell death. a MN frequency per 1000 HNSCC cells (FaDu and SCC25) treated with the IC10 of APO, C1, and A5. All three aporphine and isoquinoline derivatives significantly increased the micronuclei frequency in the assessed cell lines. *p < 0.0001. b Treatment with IC50 of APO, C1, and A5 induced apoptosis as shown by the proportion of AV staining cells. Data are shown as mean ± SD and asterisks denote significance as determined by one-way ANOVA multiple comparisons followed by Bonferroni test (post-test); *p < 0.0001. c Western blot analysis of cleaved Caspase-3. APO, C1, and A5 were able to induce Caspase-3 activation in HNSCC cell lines. Cisplatin (Cis) was used as the positive control of Caspase-3 cleavage. αβTUBULIN blot served as loading control
Aporphine and isoquinoline derivatives are able to induce apoptosis in HNSCC cells
To detect whether aporphine and isoquinoline derivatives induced apoptosis in HNSCC cells, an Annexin V-7AAD dual staining assay was conducted. The FaDu and SCC25 cell lines were treated with APO, C1, and A5 half maximal inhibition values (IC50). After a 72-h treatment, the proportions of FaDu apoptotic cells (early- and late-stage apoptotic cells) in APO, C1, and A5 treated groups were 39.23%, 39.83%, and 36.96%, respectively, while the ratio of apoptotic cells was 8.77% in the control group. It is evident that the apoptotic cell populations were significantly higher in the treated groups than in the untreated control group. Similar ratios of apoptosis were observed when SCC25 cells were treated with APO (42.03%), C1 (36.5%), and A5 (31.73%) in comparison to the control group (11.18%) (Fig. 4b).
To further clarify the underlying mechanism by which these aporphine and isoquinoline derivatives induced apoptosis in HNSCC cells, we examined the presence of cleaved Caspase-3 in FaDu and SCC25 cell lines treated with these compounds. As shown in Fig. 4c, we were able to detect cleaved Caspase-3 after treatment with the three compounds. Cisplatin was used as the positive control. These results indicated that these drugs can induce apoptosis by regulating Caspase-3 in HNSCC cells.
Discussion
Although improvements in multimodal treatment protocols (surgery, radiotherapy and chemotherapy) have significantly advanced the management and prognosis of patients with HNSCC, the five-year survival rate remains unchanged at 50%. One major reason is the development of therapeutic resistance as an inevitable endpoint of anticancer approaches [17, 18]. As such, the discovery of new potential anticancer agents is an urgent and unmet clinical need.
Aporphine alkaloids isolated from plants or obtained by semi-synthesis and total synthesis display interesting cytotoxic activities against tumor cell lines in vitro. Hints of anticancer activities in vivo have been reported in several cases [14]. Although several reports have demonstrated that aporphine alkaloids exhibit an anti-proliferative effect in some cancer cells, there is not enough information to show that these compounds act against the growth of HNSCC [12,13,14]. To our knowledge, this is the first study presenting aporphine prototypes (APO and C1) that are able to promote a dose-dependent decrease in HNSCC cell viability.
APO ((±)-apomorphine hydrochloride), C1 (1-(10,11-dimethoxy-6a,7-dihydro-4H-dibenzo[de,g]quinolin-6(5H)-yl)ethanone), and A5 (2,2,2-trifluoro-1-(1-methylene-3,4-dihydroisoquinolin-2(1H)-yl)ethanone) were obtained by sequences of transformations through convergent total syntheses of (±)-apomorphine hydrochloride [19,20,21]. Apomorphine, commonly obtained via treatment of morphine with acid [25], was originally used as an emetic drug. However, in recent decades, apomorphine therapy has shown to be effective in the treatment of Parkinson’s disease [26, 27]. C1 is a key intermediate in the synthesis of (±)-apomorphine hydrochloride [19] and A5 is an isoquinoline compound that was recently synthesized in our research group by protocols reported in the literature [20, 21] and employed as an alternative route to produce (±)-apomorphine hydrochloride.
Until now, the anticancer mechanisms of aporphine alkaloids have not been clearly defined. However, the induction of apoptosis in cancer cells; the prevention of cell proliferation; DNA topoisomerase inhibition; the reduction of the drug-resistant cellular side populations or cancer stem cells; and tyrosine kinase inhibition of epidermal growth factor receptor (EGFR) seem to play important roles in the aporphine anticancer activity [14]. Kondo and collaborators [28] were the first to report the anticancer activities of apomorphine in vitro and in vivo. According to their results, apomorphine showed a potent inhibitory activity against leukemia cell lines, while mice inoculated with these cells treated with APO demonstrated prolonged survival time. Lin and his team [29] showed that the aporphines thalifaberidine, thalifaberine and thalifasine exhibited cytotoxic effects on various human cancer cell lines with IC50 values ranging from 1 to 25 μM. Similarly, upon testing the cytotoxic effects of the same aporphines, Chen and collaborators [30, 31] observed that (S)-ovigerine and (S)-N-methylovigerine presented high cytotoxic activities (IC50 values <4 μM) against the four cell lines evaluated, while noraporphine shernovine exhibited IC50 values ranging from 0.7 to 45 μM. In our study, the cytotoxic effects of 14 substances, including four aporphine derivatives, were tested against three HNSCC cell lines in vitro. Among the compounds, three (APO, C1, and A5) showed general cytotoxic activities (IC50 values ranging from 11 to 67 μM) against all cell lines evaluated. Given that these compounds exert moderate cytotoxic effects on three distinct HNSCC cell lines, it is reasonable to speculate that they may be useful in halting head and neck cancer cell growth. It is important to note that, this is the first study to observe cytotoxic activity induced by C1 and A5 intermediates of total syntheses of (±)-apomorphine hydrochloride.
As with other drug molecules, the pharmacological activities of aporphine alkaloids are closely related to with their structures. Previous studies revealed that these compounds can present themselves in a relatively planar conformation that could easily intercalate into the DNA double helix, promoting DNA sequence alterations, stimulating DNA strand breaks, or inhibiting DNA topoisomerase II (TOP2) activity [32]. In light of these considerations, we also observed a significant increase in the level of DNA damage after treatment with APO, C1, and A5 by using a micronuclei frequency genotoxicity assay. Such assay offer a relatively simple scoring methodology to assess the degree of genomic damage based on the presence of fragments of broken chromosomes that remain in the main cell during cell division and are not integrated into the daughter nucleus [33]. Of note, this assay was conducted with IC10 concentrations because the lower dosage was sufficient to promote DNA damage while still allowing the cells to escape cell-cycle arrest and enter into mitosis even carrying damaged DNA (broken chromosomes). The genotoxic properties observed in those compounds are consistent with other studies, suggesting that aporphine alkaloids have genotoxic effects [34,35,36].
The accumulation of DNA damages is considered a key determinant of tumor cell death. The mechanisms of action of chemotherapy drugs that induce cancer cell death by targeting DNA typically involve induction of apoptosis [37, 38], and it is well known that the ability to induce apoptosis is a critical factor in the effective treatment of cancer [39]. Previous studies showed the inhibitory effect of some aporphine alkaloids on cancer cell growth via induction of apoptosis [40,41,42]. Our results provide strong evidence that APO, C1, and A5 induce apoptosis in HNSCC cells via activation of the proteolytic effector Caspase-3. Activated Caspase-3 can cleave multiple structural and regulatory proteins that ultimately cause the morphological and biochemical changes seen in apoptotic cells [43]. The ability of APO, C1, and A5 to induce cell death via apoptosis makes these compounds promising candidates for the treatment of HNSCC.
It is worth mentioning that this study has some limitations. First, our findings, as well as those of most previous studies, evaluated anticancer activities of aporphine compounds in vitro by cell culture-based assays. Therefore, in vivo studies on the efficacy of these substances need to be conducted. Second, the cytotoxic effect of APO, C1, and A5 must be evaluated in normal cells because some aporphine alkaloids not only kill tumor cells, they also damage normal cells and tissues. Third, some aporphine compounds with anticancer effects present poor absorption and low bioavailability, thus, these aspects should be better evaluated before accepting APO, C1, and A5 as anticancer agents. If necessary, obtaining higher activity through chemical structural modifications, increased bioavailability, and fewer side effects should be considered. Fourth, although some compounds examined here represent potentially interesting anticancer agents, a more complete examination of the mechanism of action of those compounds should be performed.
In conclusion, the present study illustrates the anticancer effect of 14 compounds, including four aporphine derivatives obtained as intermediates of convergent total syntheses of (±)-apomorphine hydrochloride, on a panel of HNSCC cell lines. We demonstrated the ability of APO, C1, and A5 to exert cytotoxic effects (IC50 < 70 μM) against HNSCC cells. Furthermore, these compounds were also able to promote DNA damage and induce apoptosis via activation of Caspase-3. However, it is necessary to confirm the effects of APO, C1, and A5 on other HNSCC cell lines and further in vivo studies are required. Moreover, additional studies that better characterize the molecular mechanism of action of these compounds are needed.
References
Cragg GM, Grothaus PG, Newman DJ (2009) Impact of natural products on developing new anti-cancer agents. Chem Rev 109:3012–3043
Hostettmann K, Potterat O, Wolfender JL (1998) The potential of higher plants as a source of new drugs. Chimia 52:10–17
Cragg GM, Newman DJ (1999) Discovery and development of antineoplastic agents from natural sources. Cancer Investig 17:153–163
Simmonds MS (2003) Novel drugs from botanical sources. Drug Discov Today 8:721–722
Stévigny C, Bailly C, Quetin-Leclercq J (2005) Cytotoxic and antitumor potentialities of aporphinoid alkaloids. Curr Med Chem Anticancer Agents 5:173–182
Guinaudeau H, Lebœuf M, Cavé A (1994) Aporphinoid Alkaloids, V. J Nat Prod 57:1033–1135
Protais P, Arbaoui J, Bakkali EH, Bermejo A, Cortes D (1995) Effects of various isoquinoline alkaloids on in vitro 3H-dopamine uptake by rat striatal synaptosomes. J Nat Prod 58:1475–1484
Yan R, Wang W, Guo J, Liu H, Zhang J, Yang B (2013) Studies on the alkaloids of the bark of Magnolia officinalis: isolation and on-line analysis by HPLC-ESI-MS(n). Molecules 18:7739–7750
Kashiwada Y, Aoshima A, Ikeshiro Y, Chen YP, Furukawa H, Itoigawa M, Fujioka T, Mihashi K, Cosentino LM, Morris-Natschke SL, Lee KH (2005) Anti-HIV benzylisoquinoline alkaloids and flavonoids from the leaves of Nelumbo nucifera, and structure-activity correlations with related alkaloids. Bioorg Med Chem 13:443–448
Zhang A, Zhang Y, Branfman AR, Baldessarini RJ, Neumeyer JLJ (2007) Advances in development of dopaminergic aporphinoids. Med Chem 50:171–181
Singh IP, Bodiwala HS (2010) Recent advances in anti-HIV natural products. Nat Prod Rep 27:1781–1800
Ponnala S, Chaudhary S, González-Sarrias A, Seeram NP, Harding WW (2011) Cytotoxicity of aporphines in human colon cancer cell lines HCT-116 and Caco-2: an SAR study. Bioorg Med Chem Lett 21:4462–4464
Suresh HM, Shivakumar B, Shivakumar SI (2012) Cytotoxicity of Aporphine alkaloids from the roots of Annona Reticulata on human cancer cell lines. Int J Plant Res 2:57–60
Liu Y, Liu J, Di D, Li M, Fen Y (2013) Structural and mechanistic bases of the anticancer activity of natural Aporphinoid alkaloids. Curr Top Med Chem 13:2116–2126
Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J et al (2012) Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 49:1374–1403
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61:69–90
Chin D, Boyle GM, Williams RM, Ferguson K, Pandeya N, Pedley J, Campbell CM, Theile DR, Parsons PG, Coman WB (2005) Novel markers for poor prognosis in head and neck cancer. Int J Cancer 113:789–797
Iyer NG, Tan DS, Tan VK et al (2015) Randomized trial comparing surgery and adjuvant radiotherapy versus concurrent chemoradiotherapy in patients with advanced, nonmetastatic squamous cell carcinoma of the head and neck: 10-year update and subset analysis. Cancer 121:1599–1607
Muraca A, Perecim G, Rodrigues A, Raminelli C (2017) Convergent Total synthesis of (±)-Apomorphine via Benzyne chemistry: insights into the mechanisms involved in the key step. Synthesis 49:3546–3557
Rossini AF, Muraca AC, Casagrande GA, Raminelli C (2015) Total syntheses of Aporphine alkaloids via Benzyne chemistry: an approach to the formation of Aporphine cores. J Organomet Chem 80:10033–10040
Perecim GP, Rodrigues A, Raminelli C (2015) A convenient formation of aporphine core via benzyne chemistry: conformational analysis and synthesis of (R)-aporphine. Tetrahedron Lett 56:6848–6851
Sarto F, Finotto S, Giacomelli L, Mazzotti D, Tomanin R, Levis AG (1987) The micronucleus assay in exfoliated cells of the human buccal. Mutagenesis 2:11–17
Morales-Ramírez P, Vallarino-Kelly T, Cruz-Vallejo VL (2017) The OECD's micronucleus test guideline for single exposure to an agent and the genotox-kinetic alternative. Mutagenesis 32:411–415
da Silva J, de Freitas TR, Heuser V et al (2000) Effects of chronic exposure to coal in wild rodents (Ctenomys torquatus) evaluated by multiple methods and tissues. Mutat Res 10:39–51
Zurich FM (1913) Apomorphine. The formation of Apomorphine on heating and preserving morphine solutions. Z Physiol Chem 84:363–378
Schwab RS, Amador LV, Lettvin JY (1951) Apomorphine in Parkinson's disease. Trans Am Neurol Assoc 56:251–253
Antonini A, Jenner P (2018) Apomorphine infusion in advanced Parkinson disease. Nat Rev Neurol 14:693–694
Kondo Y, Imai Y, Hojo H, Endo T, Nozoe S (1990) Suppression of tumor cell growth and mitogen response by aporphine alkaloids, dicentrine, glaucine, corydine, and apomorphine. J Pharmacobio-Dyn 13:426–431
Lin LZ, Hu SF, Zaw K, Angerhofer CK, Chai H, Pezzuto JM, Cordell GA, Lin J, Zheng DM (1994) Thalifaberidine, a cytotoxic aporphine-benzylisoquinline alkaloid from Thalictrum faberi. J Nat Prod 57:1430–1436
Chen JJ, Ishikawa T, Duh CY, Tsai IL, Chen IS (1996) New dimeric aporphine alkaloids and cytotoxic constituents of Hernandia nymphaeifolia. Planta Med 62:528–533
Chen IS, Chen JJ, Duh CY, Tsai IL, Chang CT (1997) New aporphine alkaloids and cytotoxic constituents of Hernandia nymphaeifolia. Planta Med 63:154–157
Woo SH, Sun NJ, Cassady JM, Snapka RM (1999) Topoisomerase II inhibition by aporphine alkaloids. Biochem Pharmacol 57:1141–1145
Stopper H, Schmitt E, Gregor C, Mueller SO, Fischer WH (2003) Increased cell proliferation is associated with genomic instability: elevated micronuclei frequencies in estradiol-treated human ovarian cancer cells. Mutagen 18:243–247
dos Santos EBR, Daval J, Koziel V, Netter P, Minn A (2001) Toxic effects of apomorphine on rat cultured neurons and glial C6 cells, and protection with antioxidants. Biochem Pharmacol 61:73–85
Gören AC, Zhou BN, Kingston DG (2003) Cytotoxic and DNA damaging activity of some aporphine alkaloids from Stephania dinklagei. Planta Med 69:867–868
Picada JN, Roesler R, Henriques JA (2005) Genotoxic, neurotoxic and neuroprotective activities of apomorphine and its oxidized derivative 8-oxo-apomorphine. Braz J Med Biol Res 38:477–486
Hwang PM, Bunz F, Yu J, Rago C, Chan TA, Murphy MP, Kelso GF, Smith RAJ, Kinzler KW, Vogelstein B (2001) Ferredoxin reductase affects p53-dependent, 5-fluorouracil-induced apoptosis in colorectal cancer cells. Nat Med 7:1111–1117
Kim KK, Kawar NM, Singh RK, Lange TS, Brard L, Moore RG (2011) Tetrathiomolybdate induces doxorubicin sensitivity in resistant tumor cell lines. Gynecol Oncol 122:183–189
Deigner HP, Kinscherf R (1999) Modulating apoptosis: current applications and prospects for future drug development. Curr Med Chem 6:399–414
Chen Q, Peng WL, XU AL (2002) Apoptosis of a human non-small cell lung cancer (NSCLC) cell line, PLA-801, induced by acutiaporberine, a novel bisalkaloid derived from Thalictrum acutifolium (Hand.-Mazz) boivin. Biochem Pharmacol 63:1389–1396
Montririttigri K, Moongkarndi P, Joongsomboonkusol S, Chitkul B, Pattanapanyasat K (2008) Apoptotic activity of aporphine from Stephania venosa on human ovarian cancer cells. Mahidol University J Pharm Sci 35:52–56
Sun HF, Hou HL, Lu P, Zhang L, Zhao F, Ge C, Wang T, Yao M, Li J (2012) Isocorydine inhibits cell proliferation in hepatocellular carcinoma cell lines by inducing G2/M cell cycle arrest and apoptosis. PLoS One 7:e36808
Slee EA, Adrain C, Martin SJ (2001) Executioner caspase-3, −6, and −7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J Biol Chem 276:7320–7326
Funding
This study was supported by grants from the Fundação de Amparo à Pesquisa do Estado de São Paulo – FAPESP (2015/09182–0 and 2017/21990–0). D.M.R.-Jr received a scholarship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, 99999.007922/2014–00) and the São Paulo Research Foundation (FAPESP, 2015/21420–3). G.P.P. also received a scholarship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, 1655331). Both N.M.A.P. and A.L.V. had scholarships from the Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq (123641/2017–9 and 300936/2015–0, respectively).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval
This study was approved by the institutional ethics committee (CEP-UNIFESP: 7428290317).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rodrigues-Junior, D.M., de Almeida Pontes, N.M., de Albuquerque, G.E. et al. Assessment of the cytotoxic effects of aporphine prototypes on head and neck cancer cells. Invest New Drugs 38, 70–78 (2020). https://doi.org/10.1007/s10637-019-00784-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-019-00784-6