Summary
Osteosarcoma is the most common primary malignancy of bone and characterized by an appendicular primary tumor with a high rate of metastasis to the lungs. Unfortunately, there is no effective strategy to treat osteosarcoma in current clinical practice. In this study, the anticancer effects and potential mechanisms of nifuroxazide, an oral nitrofuran antibiotic, on two osteosarcoma cell lines were investigated. The results of the antiproliferative activity in vitro showed that nifuroxazide inhibited cell proliferation of UMR106 and MG63 cells in a dose- and time-dependent manner. Interestingly, nifuroxazide showed low toxicity to non-tumor cells (HEK 293 T). In addition, ROS-mitochondrial mediated apoptosis was observed after treatment of nifuroxazide. Moreover, nifuroxazide could significantly inhibit osteosarcoma cells migration and invasion via p-Stat3, MMP-2 and MMP-9 mediated signaling pathway. Taken together, our results suggested that nifuroxazide could be a promising agent for osteosarcoma treatment by inhibiting cell proliferation, inducing cell apoptosis and impairing cell migration and invasion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteosarcoma is the most common type of primary bone malignancy derived from primitive mesenchymal cells [1, 2]. Although osteosarcoma is a relative infrequent cancer, for example with about 900 new cases reported in the United States per year, it accounts for approximately 3.4% of all childhood cancers and even 56% of malignant bone cancers in children [3]. What’s more, osteosarcoma is characterized highly metastatic with lungs as the primary site of metastasis [4,5,6]. For cancer therapy, surgery, radiotherapy and polychemotherapy are dominant treatments. Because of frequent complications and recurrence, surgery of sarcomas of the axial skeleton remains great challenge [7, 8]. It is reported that local treatment of osteosarcomas by radiotherapy is unsatisfactory because that osteosarcoma is long regarded as a radioresistant tumor [9,10,11]. Polychemotherapy is always served as an effective treatment for osteosarcoma, however, more than 90% of patients with osteosarcoma died of pulmonary metastases before the treatment of polychemotherapy [12]. Therefore, there is an urgent need to develop new effective drug which not only kill the primary tumor but also suppress metastasis. Drug discovery is a time-consuming, high-investment, and high-risk process in traditional drug development. Recently, drug repositioning which refers to the identification of new indications from existing drugs and the application of the newly identified drugs to the treatment of diseases other than the drug’s intended disease has become a popular strategy. Different from traditional drug development strategies, the strategy is efficient, economical and riskless [13,14,15]. Nifuroxazide, an oral nitrofuran antibiotic, is a medicine available in a number of countries worldwide. It is used to treat colitis and diarrhea in humans and non-humans. Recently, it was proved to be effective in inhibiting breast, colorectal and melanoma cancer growth and metastasis [16,17,18]. Considering the broad-spectrum of anti-tumor effect, we hypothesized that nifuroxazide might be practicable in the treatment of osteosarcoma. To verify this hypothesis, the anti-tumor effects and potential mechanisms of nifuroxazide were evaluated in osteosarcoma cells in vitro. Our results revealed that nifuroxazide could significantly induce apoptosis in osteosarcoma cells via the reactive oxygen species (ROS)-mitochondria apoptotic pathway. Importantly, nifuroxazide could suppress osteosarcoma cell migration and invasion via p-Stat3, MMP-2 and MMP-9 mediated pathway. These results implied that nifuroxazide could serve as a new agent for the treatment of osteosarcoma.
Materials and methods
Materials
Nifuroxazide was purchased from Xiya Reagent Co. Ltd. (Linyi, China). Dimethyl sulfoxide (DMSO), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), 2′,7′-Dichlorodihydrofluorescein diacetate (DCFH-DA), Rhodamine123 (Rh123) and propidine iodide (PI)were purchased from Sigma Chemical Co. (St Louis, MO, USA). Hoechst 33258 was bought from Beyotime (Beijing, China). The Annexin V-FITC apoptosis detection kit was provided by KeyGen Biotech (Nanjing, China). The antibodies used in this study were Stat3/P-Stat3 Tyr705, inhibitor of metalloproteinase 2 (TIMP2), cleaved caspase-3, Bax (Cell Signaling Technology), MMP2, Bcl-2(Millipore), MMP9 (Abcam), and β-actin (ZSGB-BIO, Beijing, China).
Cell lines and cell culture
UMR106, MG63 and HEK 293 T cell lines were obtained from the American Type Culture Collection (Rockville, MD, USA). Cells were cultured in DMEM or RPMI 1640 media containing 10% fetal bovine serum (Hyclone, Logan, UT, USA) and 1% antibiotics (penicillin and streptomycin) in 5% CO2 at 37 °C.
Cell viability assay
The cell viability was evaluated by MTT assay. UMR106, MG63 and HEK 293 T cells were seeded in 96-well plates at a density of 3~5 × 103 cells/well. After 24 h incubation, cells were exposed to different concentrations of nifuroxazide for specific time (24, 48 and 72 h). Then, 20 μL of 5 mg/mL MTT was added to each well, and cells were incubated at 37 °C for another 4 h. Finally, the medium was replaced with 150 μL of DMSO to dissolve the precipitated formazan. Spectrophotometric absorbance at 570 nm was measured by the Spectra MAX M5 microplate spectrophotometer (Molecular Devices, CA, USA). All the results were performed in triplicate, and all data were expressed as the mean ± SD.
Colony formation assay
UMR106 and MG63 cells were plated in the 6-well plates at a density of 200–500 cells/well. After 24 h incubation, the cells were treated with gradient concentrations of nifuroxazide and then incubated for additional 12 days. After washed with cold PBS, the cells were fixed with methanol and stained with 0.5% crystal violet solution for 30 min. The colonies (> 50 cells) were counted under a microscope.
Morphological analysis by Hoechst staining
The cells morphological changes related to apoptosis was stained by Hoechst 33258 to assess the effects of nifuroxazide on apoptosis induction. In brief, UMR106 cells (1 × 105 cells/well) were plated onto 18-mm cover glass in a 6-well plates for 24 h. After treated with gradient concentrations of nifuroxazide for 24 h, the cells were washed with cold PBS and fixed with 4% paraformaldehyde for 15 min and stained with Hoechst 33258 solutions (5 μg/mL). Finally, the morphology of nuclear was observed by fluorescence microscopy (Leica DM4000B, Leica, Wetzlar, Germany).
Apoptotic assays by FCM
The UMR106 cells (2 × 105 cells/well) were seeded in 6-well plates for 24 h. After treatment with gradient concentrations of nifuroxazide for following 24 h, the cells were harvested, washed with cold PBS,and stained with Annexin V-FITC/PI detection kit according to the manufacturer’s instructions. Apoptotic cells induced by nifuroxazide were analyzed by flow cytometry (FCM).
Measurement of mitochondrial membrane potential (ΔΨm) and reactive oxygen species (ROS)
To investigate the change of mitochondrial membrane potential (ΔΨm) as well as ROS, UMR106 cells were treated with different concentrations of nifuroxazide for 24 h. After staining with 5 μg/mL of 2-(6-amino-3-imino-3H-xanthen-9-yl) benzoic acid methyl ester (Rh123) and 10 μM of DCFH-DA at 37 °C in dark for 30 min, the stained cells were washed with cold PBS and analyzed by FCM. Data shown represent the mean value of independent experiment results in triplicate.
Wound-healing assay
Wound-healing migration assay was used to evaluate the ability of cell migration. The UMR106 cells were plated in 6-well plates (5 × 105 cells/well) and incubated for 24 h. When cells grew to about 80% confluence, cell monolayer was scraped by a sterile 10 μL pipette tips. After treatment with different concentrations of nifuroxazide for 24 h, cells were fixed and photographed. Images were taken by microscope (Zeiss, Axiovert 200, Germany). The migrated cell number was quantified by manual counting.
Boyden chamber migration and invasion assay
Migration and invasion were evaluated with boyden chamber (8 μm pore size) assay as previously described [19, 20]. For migration assay, 100 μL serum-free medium with 1 × 105 UMR106 cells was added in the upper chamber, and 600 μL of medium containing 10% FBS was placed at the bottom. Various concentrations of nifuroxazide were added in both up and bottom chambers. After 24 h incubation, the non-migrated cells in the upper chamber were discarded using a cotton swab. The migrated cells were fixed and stained with 0.5% crystal violet. Invasion assay was performed similarly. Briefly, the surface of upper chamber was coated with the Matrigel (l0 μL/well, BD Biosciences) and the lower chamber was filled with 600 μL of medium containing 10% FBS. 100 μL Serum-free medium with 1 × 105 UMR106 cells was added in upper chamber and cells were exposed to different concentrations of nifuroxazide. After treatment for 24 h, non-migrated cells on the upper side of the filter were removed, and the migrated cells were fixed and stained with 0.5% crystal violet. Images of migrated and invasive cells were taken by a light microscope. Migrated or invasive cells in six randomly selected fields were counted and compared with control group.
Western blot analysis
The change of protein levels which were influenced by nifuroxzide was evaluated by western blot. Briefly, UMR106 cells were treated with different concentrations of nifuroxzide for 24 h, washed with cold PBS and lysed in RIPA buffer (Beyotime, Beijing, China) to obtain total proteins. The protein concentrations were measured with BCA method. The total proteins were separated on SDS-PAGF gel and transferred onto polyvinylidene fluoride (PVDF) membranes (Amersham Bioscience, Piscataway, NJ). Then, the membranes were blocked with 5% milk, target proteins were incubated with primary and secondary antibodies. After that, the target protein bands were detected by the enhanced chemiluminescence kit (Millipore, USA).
Results
Nifuroxazide inhibits osteosarcoma cells proliferation
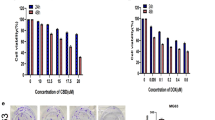
MTT assay was used to test the anticancer activities of nifuroxazide. Briefly, UMR106 and MG63 osteosarcoma cells were treated with gradient concentrations of nifuroxazide from 0 to 80 μM for 24 h, 48 h and 72 h, respectively. As displayed in Fig. 1a, b, nifuroxazide showed dose- and time-dependent cytotoxicity on both UMR106 and MG63 cells. To further evaluate whether nifuroxazide could inhibit the proliferation of osteosarcoma cells, clonogenic assay was conducted after nifuroxazide treatment. As shown in Fig. 1c, clone formations of UMR106 and MG63 cells were significantly diminished in a dose-dependent manner after nifuroxazide treatment. In addition, compared with control group, the size of colonies treated with nifuroxazide were distinctly smaller. Meanwhile, non-tumor cell lines HEK 293 T was treated with nifuroxazide from 0 to 80 μM for 48 h. The results in Fig. 1d demonstrated nifuroxazide exhibited low cytotoxicity for non-tumor cell lines. This result showed that nifuroxazide can selectively kill cancer cells.
The effects of nifuroxazide on cells proliferation. a, b UMR106 and MG63 cells were incubated with gradient concentrations (0–80 μM) of nifuroxazide for 24, 48 and 72 h. Cell viability was determined by MTT assay. c The effects of nifuroxazide (0–20 μM) on colony formation in UMR106 and MG63 cell lines for 12 days, the statistic results of colony formation assays presented as surviving colonies. d HEK 293 T cells were incubated with gradient concentrations (0–80 μM) of nifuroxazide for 48 h. Data are expressed as means ± SD from three independent experiments (***p < 0.01)
Nifuroxazide induces osteosarcoma cells apoptosis
Inspired by the anti-proliferation efficiency of nifuroxazide, we then investigated whether nifuroxazide could induce UMR106 cells apoptosis. Firstly, Hoechst 33258 staining assay was performed to evaluate the cell apoptosis induced by nifuroxazide. As shown in Fig. 2a, compared with control group, bright-blue fluorescent-condensed nuclei and nuclear fragmentation were observed in nifuroxazide treatment groups. In order to further confirm that apoptosis of UMR106 was induced by nifuroxazide, we evaluated the percent of apoptosis by FCM using the Annexin V-FITC/PI dual labeling method. As displayed in Fig. 2b, nifuroxazide induced apoptosis of UMR106 cells in a dose-dependent manner. After treatment of 20 μM for 24 h, 16.8% of apoptotic UMR106 cells were detected, however, the apoptotic rate in control group was just 1.47%. Therefore, nifuroxazide could significantly induced apoptosis in UMR106 cells. To further confirm that apoptosis induced by nifuroxazide is associated with caspase-3 cascade, we explored Western-blot assay to examine cleaved caspase-3, Bcl-2 and Bax expression levels in UMR106 cell after nifuroxazide treatment. As shown in Fig. 2c, the expression of Bcl-2 significantly decreased and that of Bax and cleaved caspase-3 increased after nifuroxazide treatment, implying that nifuroxazide-induced apoptosis might be associated with mitochondrial apoptotic pathway.
Apoptosis of UMR106 cells induced by nifuroxazide. a The fluorescence microscopic appearance of Hoechst 33258 staining nuclei of UMR106 cells with gradient concentrations of nifuroxazide for 24 h (40x). Data are the representative from three experiments. b UMR106 cells were treated with nifuroxazide at indicated doses for 24 h and the apoptosis rate was treated statistically. Data are expressed as mean ± SD, **p < 0.01, ***p < 0.001 compared to control. c Western blot analyses of UMR106 cells treated with different concentrations of nifuroxazide for 24 h to evaluate protein expression of Bcl-2, Bax, Cleaved caspase-3. β-actin served as a loading control and the protein expression of Bax, Bcl-2, Cleaved caspase-3 was quantified and normalized against β-actin expression
Nifuroxazide causes a mitochondria-mediated apoptosis
To verify the apoptosis induced by nifuroxazide was associated with mitochondrial apoptotic pathway, we evaluated variations in the mitochondrial membrane potential (ΔΨm) using a green fluorochrome Rh123 (2-(6-amino-3-imino-3H-xanthen-9-yl) benzoic acid methyl ester) by FCM. As presented in Fig. 3a, a significant decrease of ΔΨm was observed after treatment of nifuroxazide. In addition, level of ROS which is mainly generated by mitochondrial was also measured by FCM using an indicator of DCFH-DA. Significantly increased levels of ROS were observed after treatment of nifuroxazide in the Fig. 3b. These results suggested that the mitochondria-mediated pathway may be involved in nifuroxazide-induced apoptosis.
The variation in the mitochondrial membrane potential (ΔΨm) and the level of ROS. a UMR106 cells were exposed to gradient concentrations of nifuroxazide (0–20 μM) for 24 h. The mean fluorescence intensity detected in the mitochondrial membrane potential assay was treated statistically. Data are presented as mean ± SD, **p < 0.01, ***p < 0.001 compared to control. b UMR106 cells were treated with nifuroxazide at indicated doses for 24 h followed by analysis of ROS by FCM and the variation of ROS was calculated. Data are expressed as mean ± SD, *p < 0.05, **p < 0.01 compared to control
Nifuroxazide impairs migration and invasion in vitro
The anti-metastasis effect of nifuroxazide was evaluated by cell wound-healing and transwell migration assays. As shown in Fig. 4a, nifuroxazide inhibited migration of UMR106 cells in a concentration-dependent manner. Similar results were observed in transwell migration assay (Fig. 4b). In addition, matrigel invasion assay was performed to investigate anti-metastasis effect of nifuroxazide. As displayed in Fig. 4c, compared with control group, invasion was significantly decreased in UMR106 cells after nifuroxazide treatment. We also investigated whether phosphorylated-Stat3(Tyr705), MMP-2 and MMP-9 which are closely associated with cell migration and invasion are involved in nifuroxazide-associated migration and invasion through western-blot assay. As shown in Fig. 5, expression levels of p-Stat3, MMP-2 and MMP-9 showed significantly decrease after treatment of nifuroxazide. These results revealed that nifuroxazide is effective in inhibiting osteosarcoma cell migration and invasion.
Inhibition of migration and invasion by nifuroxazide (0–20 μM) treated in UMR106 cells. a UMR106 cells were seeded in 6-well plates. A single scratch was made after the cells grew about 80% confluence. After treatment of nifuroxazide for 24 h, the cells were fixed and photographed (10x). The migrated cells derived from the same area were quantified. b UMR106 cells were plated in the top chamber of transwell with serum-free medium and treated with vehicle or different concentrations of nifuroxazide. After 24 h, migrated cells were fixed, stained, photographed and quantified (20x). c UMR106 cells were treated with different concentrations of nifuroxazide and allowed to invade through matrigel for 24 h. Invaded cell number was counted (20x), *p < 0.05, **p < 0.01, ***p < 0.001 compared with control
The changes of p-Stat3, MMP-2 and MMP-9 expression. a UMR106 cells were treated with different concentrations of nifuroxazide. After 24 h, cells were harvested and lysed. Western blot assay was conducted to detect the expression of p-Stat3, MMP-2 and MMP-9. β-actin served as loading control and protein expression of p-Stat3, MMP-2 and MMP-9 were quantified and normalized against β-actin expression. Data are shown as mean ± SD, *P < 0.05, **P < 0.01, ***P < 0.001 compared to vehicle (0 μM group)
Discussion
Osteosarcoma is the most common type of bone cancer in children and teens. Unfortunately, there is currently no effective therapy to control the recurrence and metastasis of osteosarcoma. Thus, it is urgent to find a potential drug candidate for treatment of osteosarcoma. In this study, nifuroxazide, an oral anti-diarrheal agent identified as an inhibitor of Stat3, was evaluated for its potency against osteosarcoma in vitro. The MTT and colony formation results showed that nifuroxazide significantly inhibited the cell viability and proliferation of osteosarcoma cells in a time- and dose-dependent manner. While nifuroxazide showed lower toxicity in non-tumor cell lines. Taken together, the above results implied that nifuroxazide had a good anti-proliferation effect on osteosarcoma cell.
Apoptosis is a major route to eradicate cancer cells [21, 22] and caspase-3 is activated by upstream effector proteins, leading to apoptosis cascade [23, 24]. Moreover, mitochondria play an important role in the intrinsic apoptosis via changing the mitochondrial transmembrane potential, and the Bcl-2 family proteins which included anti-apoptotic protein Bcl-2 and the pro-apoptotic protein Bax may participate the intrinsic apoptosis pathway [25,26,27,28]. In this study, Hoechst 33258 staining and Annexin V-FITC/PI dual labeling assays both suggested that nifuroxazide could distinctly induce osteosarcoma cells apoptosis. In addition, the activation of cleaved-caspase-3 and Bax as well as down-regulation of Bcl-2 were observed after treatment with nifuroxazide. There was a significant loss of Δ Ψ m and ROS production in osteosarcoma cells after nifuroxazide treatment. Therefore, nifuroxazide treatment significantly induced apoptosis in osteosarcoma cell via ROS-medicated mitochondrial apoptotic pathway.
Osteosarcoma is a cancer that characterized with high metastatic [29, 30]. Moreover, the poor therapeutic efficiency against osteosarcoma could be attributed to high metastatic potential of osteosarcoma cells [31, 32]. Therefore, the anti-metastasis effect of nifuroxazide was evaluated by cell wound-healing and transwell migration assays. Stat3 proved to be a key regulator of cell migration and invasion via controlling the expression levels of MMP-2 and MMP-9 [33, 34], so we also investigated whether phosphorylated-Stat3(Tyr705), MMP-2 and MMP-9 which are closely associated with cell migration and invasion are involved in nifuroxazide-associated migration and invasion through western-blot assay. In our work, the wound-healing assay and transwell-migration and -invasion assays revealed that nifuroxazide exhibited potent anti-migration and anti-invasion effects. Western-blot results showed that nifuroxazide inhibited osteosarcoma cells migration and invasion via the Stat3/MMP2/MMP9 pathway.
Conclusions
In conclusion, our present study evaluated anti-proliferation and anti-metastasis of nifuroxazide in osteosarcoma cells. Nifuroxazide exhibited time- and dose-dependent anti-proliferation in osteosarcoma cells. In addition, mechanism studies implied that nifuroxazide could significantly induce cell apoptosis via ROS-mitochondrial apoptotic pathway. Moreover, nifuroxazide markedly inhibited osteosarcoma cell migration and invasion via MMP-2 and MMP-9 mediated pathway. Therefore, all these results suggested that nifuroxazide might be developed as a potential therapeutic agent for inhibiting osteosarcoma growth and metastasis.
References
Whelan JS, Davis LE (2018) Osteosarcoma, chondrosarcoma, and chordoma. J Clin Oncol 36:188–193
Wang W, Zhao HF, Yao TF, Gong H (2018) Advanced development of ErbB family-targeted therapies in osteosarcoma treatment. Investig New Drugs. https://doi.org/10.1007/s10637-018-0684-8
Chan RJ, Cooper T, Kratz CP, Weiss B, Loh ML (2009) Juvenile myelomonocytic leukemia: a report from the 2nd international JMML symposium. Leuk Res 33:355–362
Pradelli E, Karimdjee-Soilihi B, Michiels JF, Ricci JE, Millet MA, Vandenbos F et al (2009) Antagonism of chemokine receptor CXCR3 inhibits osteosarcoma metastasis to lungs. Int J Cancer 125:2586–2594
Morrow JJ, Bayles I, Funnell APW, Miller TE, Saiakhova A, Lizardo MM et al (2018) Positively selected enhancer elements endow osteosarcoma cells with metastatic competence. Nat Med 24:178–185
Geller DS, Gorlick R (2010) Osteosarcoma: a review of diagnosis, management, and treatment strategies. Clin Adv Hematol Oncol 8:705–718
Tomita K, Kawahara N, Murakami H, Demura S (2006) Total en bloc spondylectomy for spinal tumors: improvement of the technique and its associated basic background. J Orthop Sci 11:3–12
Deng ZP, Ding Y, Puri A, Wang EH, Gulia A, Durban C et al (2015) The surgical treatment and outcome of nonmetastatic extremity osteosarcoma with pathological fractures. Chin Med J 128:2605–2608
Biazzo A, De Paolis M (2016) Multidisciplinary approach to osteosarcoma. Acta Orthop Belg 82:690–698
Akagunduz OO, Kamer SA, Kececi B, Demirag B, Oniz H, Kantar M et al (2016) The role of radiotherapy in local control of nonextremity Ewing sarcomas. Tumori 102:162–167
Wang CC, Jing JH, Cheng L (2018) Emerging roles of non-coding RNAs in the pathogenesis, diagnosis and prognosis of osteosarcoma. Investig New Drugs 36:1116–1132
Ritter J, Bielack SS (2010) Osteosarcoma. Ann Oncol 21(Suppl 7):vii320–vii325
Xue HQ, Li J, Xie HZ, Wang YD (2018) Review of drug repositioning approaches and resources. Int J Biol Sci 14:1232–1244
Würth R, Thellung S, Bajetto A, Mazzanti M, Florio T, Barbieri F (2016) Drug-repositioning opportunities for cancer therapy: novel molecular targets for known compounds. Drug Discov Today 21:190–199
Shim JS, Liu JO (2014) Recent advances in drug repositioning for the discovery of new anticancer drugs. Int J Biol Sci 10:654–663
Zhu Y, Ye T, Yu X, Lei Q, Yang F, Xia Y et al (2016) Nifuroxazide exerts potent anti-tumor and anti-metastasis activity in melanoma. Sci Rep 6:20253
Ye TH, Yang FF, Zhu YX, Li YL, Lei Q, Song XJ et al (2017) Inhibition of Stat3 signaling pathway by nifuroxazide improves antitumor immunity and impairs colorectal carcinoma metastasis. Cell Death Dis 8:e2534
Yang F, Hu M, Lei Q, Xia Y, Zhu Y, Song X et al (2015) Nifuroxazide induces apoptosis and impairs pulmonary metastasis in breast cancer model. Cell Death Dis 6:e1701
Zhang T, Li J, Dong Y, Zhai D, Lai L, Dai F et al (2012) Cucurbitacin E inhibits breast tumor metastasis by suppressing cell migration and invasion. Breast Cancer Res Treat 135:445–458
Ye T, Xiong Y, Yan Y, Xia Y, Song X, Liu L et al (2014) The anthelmintic drug niclosamide induces apoptosis, impairs metastasis and reduces immunosuppressive cells in breast cancer model. PLoS One 9:e85887
Thandapani P, Aifantis I (2017) Apoptosis, up the ante. Cancer Cell 32:402–403
Hahn WC (2004) Cancer: surviving on the edge. Cancer Cell 6:215–222
Slee EA, Adrain C, Martin SJ (2001) Executioner caspase-3, −6, and −7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J Biol Chem 276:7320–7326
Benhar M, Forrester MT, Hess DT, Stamler JS (2008) Regulated protein denitrosylation by cytosolic and mitochondrial thioredoxins. Science 320:1050–1054
Wang C, Youle RJ (2009) The role of mitochondria in apoptosis. Annu Rev Genet 43:95–118
Singh PK, Roukounakis A, Frank DO, Kirschnek S, Das KK, Neumann S et al (2017) Dynein light chain 1 induces assembly of large Bim complexes on mitochondria that stabilize Mcl-1 and regulate apoptosis. Genes Dev 31:1754–1769
Pyakurel A, Savoia C, Hess D, Scorrano L (2015) Extracellular regulated kinase phosphorylates mitofusin 1 to control mitochondrial morphology and apoptosis. Mol Cell 58:244–254
Bhola PD, Letai A (2016) Mitochondria-judges and executioners of cell death sentences. Mol Cell 61:695–704
Mirabello L, Koster R, Moriarity BS, Spector LG, Meltzer PS, Gary J et al (2015) A genome-wide scan identifies variants in NFIB associated with metastasis in patients with osteosarcoma. Cancer Discov 5:920–931
Ellegast J, Barth TF, Schulte M, Bielack SS, Schmid M, Mayer-Steinacker R (2011) Metastasis of osteosarcoma after 16 years. J Clin Oncol 29:e62–e66
Lu J, Song G, Tang Q, Zou C, Han F, Zhao Z et al (2015) IRX1 hypomethylation promotes osteosarcoma metastasis via induction of CXCL14/NF-kappaB signaling. J Clin Invest 125:1839–1856
Hattori H, Yamamoto K (2012) Lymph node metastasis of osteosarcoma. J Clin Oncol 30:e345–e349
Liotta LA, Tryggvason K, Garbisa S, Hart I, Foltz CM, Shafie S (1980) Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature 284:67–68
Eissa S, Ali-Labib R, Swellam M, Bassiony M, Tash F, El-Zayat TM (2007) Noninvasive diagnosis of bladder cancer by detection of matrix metalloproteinases (MMP-2 and MMP-9) and their inhibitor (TIMP-2) in urine. Eur Urol 52:1388–1396
Funding
This work was supported by the Support Program for Science and Technology of Sichuan Province (2017SZ0106).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors confirm that they have no conflict of interest with the content of this article.
Ethical approval
This article does not contain studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Luo, Y., Zeng, A., Fang, A. et al. Nifuroxazide induces apoptosis, inhibits cell migration and invasion in osteosarcoma. Invest New Drugs 37, 1006–1013 (2019). https://doi.org/10.1007/s10637-019-00724-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-019-00724-4