Summary
Introduction Vatalanib is an oral receptor tyrosine kinase inhibitor that blocks all known VEGF, PDGF, and c-Kit receptors. This phase I study evaluated the safety, tolerability, and biologic activity of the combination of vatalanib with pemetrexed disodium in patients with advanced solid tumors. Methods Patients were administered escalating twice daily doses of vatalanib in combination with pemetrexed disodium in 21-day cycles. A dose expansion cohort was enrolled to further define the maximum tolerated dose (MTD) and further evaluate efficacy. Results A total of 29 patients were enrolled in the study (dose escalation, 9; dose expansion, 20). Dose-limiting toxicities included grade 4 thrombocytopenia (6.9%) and febrile neutropenia, anorexia, constipation, and dehydration. Other common adverse events were fatigue (75%), nausea (66%), vomiting (48%), oral mucositis (31%) and diarrhea (28%). The majority of these toxicities were Grade 1–2. The MTD was reached at vatalanib 250 mg twice daily continuously combined with pemetrexed disodium 500 mg/m2 day 1. Overall, 2 patients (6.9%) had partial responses, 8 (27.6%) had stable disease for at least 4 cycles, 5 had progressive disease (17.2%) and 5 went off study before disease assessment. Conclusion The combination of vatalanib with pemetrexed disodium was feasible, but not well tolerated. The modest efficacy results are consistent with other results obtained from combinations of chemotherapy and a large number of VEGF tyrosine kinase inhibitors. This combination should not be developed further unless predictive biomarkers can be identified.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Angiogenesis is the process by which new capillary blood vessels are created, thus allowing most solid tumors to grow beyond a limiting size by facilitating delivery of nutrients to the tumor and removal of waste products [1,2,3]. Vascular endothelial growth factor (VEGF) and its receptors play a crucial role in angiogenesis of numerous malignancies. The VEGF family consists of at least five angiogenic factors (VEGF-A, VEGF-B, VEGF-C, VEGF-D), and is the principal growth and survival factor for endothelial cells, rendering it a prime target for anti-neovascularization therapies [4]. VEGF acts by binding to three receptor tyrosine kinases (RTK): VEGFR-1/Flt-1, VEGFR-2/KDR/Flk − 1 and VEGFR-3/Flt-4 located on the host vascular endothelial cells, monocytes, and hematopoietic precursors, resulting in the induction of several proteins, including tissue factor, urokinase, tissue-type plasminogen activator, plasminogen activation inhibitor 1, matrix metalloproteinase, and anti-apoptotic factors facilitating and supporting tumor growth and tumor metastasis formation [5].
Vatalanib, is an oral amino-phthalazine inhibitor of all VEGF RTKs (VEGFR-1, −2, and − 3). It also inhibits platelet-derived growth factor (PDGF) receptor tyrosine kinase, cytokine stem cell factor receptor (c-kit) tyrosine kinase and colony-stimulating factor 1 receptor (CSF − 1R) at concentrations below 10 μM [6, 7]. In phase I/II clinical trials in patients with advanced cancers, vatalanib doses ranging from 50 to 2000 mg once daily and 150 to 1000 mg administered twice daily (BID) have had manageable side effects [8, 9]. The recommended vatalanib dose in phase III studies was 1250 mg once daily. Two phase III trials (CONFIRM 1 and 2) evaluated the efficacy of vatalanib in combination with chemotherapy in advanced colorectal cancer [10, 11]. The improvements in a pre-planned analysis of the progression-free survival (PFS) in the vatalanib arm reported by the investigators were not corroborated upon an independent central review. An exploratory analysis in both studies did show statistically significant PFS benefit among patients with elevated LDH levels (defined as >1.5x ULN) receiving vatalanib. An explanation offered for the negative results was that vatalanib might have been under-dosed since it has a pharmacokinetically short half-life of approximately 6 h. Because of this short half-life [12], one of the explanations for the lack of improvement in survival outcome in the aforementioned trial was the lack of sustained biologic effect with the once-daily dosing scheme. This is in contrast to the established long half-life of the monoclonal antibody bevacizumab which has demonstrated survival benefit in combination with chemotherapy.
Pemetrexed disodium is a multitargeted antifolate inhibitor of 3 enzymes in the folate metabolic pathway essential for cell replication: thymidylate synthase (TS), dihydrofolate reductase (DHFR), and glycinamide ribonucleotide formyl transferase (GARFT) [13]. It also targets other enzymes, including aminoimidazole carboxamide ribonucleotide formyltransferase, a folate dependent enzyme involved in purine synthesis [14]. Pemetrexed disodium is currently approved in combination with cisplatin for first-line treatment of malignant pleural mesothelioma [15] and as a standard treatment in the first-line and maintenance of advanced non-squamous NSCLC, and second-line settings of advanced NSCLC [16,17,18,19,20,21,22,23]. In addition, pemetrexed disodium has an excellent safety profile. Toxicities related to the treatment are mild and tolerable, including neutropenia, leukopenia, anemia, fatigue, nausea, and vomiting [16, 17, 24, 25].
Combination of some VEGF inhibitors with systemic chemotherapy has, in the past few years, demonstrated increased clinical efficacy in comparison with systemic chemotherapy alone in various malignancies including NSCLC, gastrointestinal and gynecological cancers [26,27,28,29,30,31,32,33,34,35,36]. Vatalanib is a well-tolerated, oral tyrosine kinases inhibitor of VEGFR, PDGFR and c-Kit, resulting in a reduction of tumor angiogenesis and tumor growth. As a small molecule inhibitor, vatalanib also has a potential ability to penetrate the blood brain barrier. Based on this and the tolerability of both agents, we evaluated a combination of vatalanib and pemetrexed in advanced solid tumors.
Methods
Study design
This was a phase I study to evaluate the safety, tolerability, and biologic activity of the combination of vatalanib with pemetrexed disodium in patients with advanced solid tumors. Patients were enrolled in a traditional “3 + 3” dose escalation scheme. There were 5 planned dose levels (Table 1), ranging from vatalanib 250 mg twice daily up to 750–1250 mg twice daily. Pemetrexed disodium was administered at a fixed dosage of 500 mg/m2 in all five levels. Three patients were treated at each dose level for 21-day cycles and observed for a minimum of 3 weeks before new patients were treated. Vatalanib was administered continuously orally, while pemetrexed disodium was administered intravenously on day 1, of a 21-day cycle. Patients were assigned their dose level by the Mayo Clinic Cancer Center (MCCC) Randomization Center and continued on treatment until they experienced unacceptable side effects, had disease progression, or withdrew consent. Doses were not escalated in any individual patient.
After the MTD of the combination was identified, 20 additional patients were treated in the expansion cohort to better define the safety and efficacy of these agents.
Dose-limiting toxicity and maximum tolerated dose
The primary objectives were to determine the MTD of the combination. The MTD is defined as the dose level below the lowest dose that induces dose-limiting toxicity (DLT) in at least one-third of patients (at least 2 of a maximum of 6 new patients). The DLT was defined as an adverse event attributed (definitely, probably, or possibly) to the study treatment in the first three weeks of combination therapy and meeting the following criteria: Grade 4 absolute neutrophil count for >5 days or Grade 4 thrombocytopenia of any duration, ≥ Grade 3 non-hematologic toxicity as per NCI Common Terminology Criteria for Adverse Events (CTCAE) v3.0. Grade 3 nausea, vomiting, or diarrhea in spite of maximal supportive treatment(s) was considered dose limiting. Any toxicity causing dose delays of greater than 2 weeks of the intended next dose was also to be considered dose limiting. For grading toxicity for proteinuria when both 24-h urine measurement and random urine sample dipstick results were both available, the 24-h urine protein result was used as the parameter in determining toxicity grade.
Patient selection
Patients older than 18 years with confirmed advanced solid tumors refractory to or failing standard treatment were eligible for the study. Patients were required to have an Eastern Cooperative Oncology Group (ECOG) performance status of 2 or lower and adequate bone marrow, renal, and liver function. In addition, patients should have no contraindications to the intake of folic acid, vitamin B12 or dexamethasone and be able to permanently discontinue aspirin doses of ≥1.3 g/day ≥10 days before through ≥10 days after pemetrexed disodium treatment. Patients with symptomatic, untreated, or uncontrolled CNS metastases or seizure disorder, symptomatic serosal effusion, concurrent severe and/or uncontrolled medical disease, acute or chronic liver disease or severe impairment of gastrointestinal absorption were excluded. Other exclusion criteria included uncontrolled infection; inadequate recovery from previous therapies including surgery, radiation, chemotherapy, biologic therapy, hormonal therapy or immunotherapy, and prior treatment with mitomycin C, nitrosoureas, or bevacizumab within 6 weeks of study entry. Concurrent intake of CYP3A4-inducing agents was not allowed. The study was approved by the Institutional Review Board of Mayo Clinic Cancer Center and all patients provided written informed consent.
Patient treatment
Eligible patients received 500 mg/m2 pemetrexed disodium as a 10-min intravenous infusion on day 1 of a 21-day cycle. They were premedicated with 350 mcg of oral folic acid daily for 7 days before pemetrexed administration, and continuing throughout the study. Vitamin B12 was administered intramuscularly at a dose of 1000 mcg on day 1 of folic acid and repeated every 9 weeks while patient was receiving pemetrexed. Dexamethasone (10 mg) was administrated intravenously on day 1 prior to pemetrexed disodium, and then 4 mg dexamethasone was administrated orally twice daily for 2 days after pemetrexed infusion. In the dose escalation cohort, vatalanib was taken orally continuously on a flat dosing scheme (not weight based). To ensure safety, patients were started on 500 mg twice daily in the first week. Dose escalation/de-escalation by 250 mg BID was done in each subsequent week to reach a final dose level. In the dose expansion cohort, vatalanib was started on day 8 in the first cycle and then given continuously at a dose of 250 mg bid.
Patient evaluation
Patients were monitored continuously throughout the study. A complete history and physical examination was done prior to each cycle, and complete blood count and blood chemistries were done prior to each cycle and weekly during each cycle for the first two cycles. Imaging such as a computed tomography scan or magnetic resonance imaging was done at base-line and every six weeks for the evaluation of efficacy using the Modified Response Evaluation Criteria in Solid Tumors (RECIST version 1.0) criteria [37]. In addition to a baseline scan, confirmatory scans were obtained at least four weeks following initial documentation of objective response. Patients were followed for a maximum of 3 months after completing treatment.
Results
Patient characteristics
A total of 29 patients with advanced solid tumors were enrolled in the study, with 9 patients in the dose escalation portion and 20 in the dose expansion portion. The median age was 60 years (range from 42 to 84 years). Thirty-four percent of patients were male; all had an ECOG performance status of 0–2. Additional patient demographics and pretreatment characteristics are summarized in Table 2.
Dose-limiting toxicity and maximum tolerated dose
All 29 patients were evaluable for DLT assessment. Eight DLT events were observed among two patients from Cohort I at dose level 1, including grade 4 thrombocytopenia, grade 3 febrile neutropenia, anorexia, constipation and dehydration. The dose was therefore de-escalated to dose level − 1, where no DLT’s were observed. The MTD was defined as 250 mg (dose level − 1) of vatalanib orally twice daily continuously combined with 500 mg/m2 of pemetrexed disodium on day 1. Twenty patients were further treated at the MTD in the dose expansion cohort.
Safety and tolerability
All 29 patients were evaluable for safety analysis. The most common side effects (of all grades) of the combination were fatigue (75%), nausea (66%), vomiting (48%), mucositis oral (31%) and diarrhea (28%). The vast majority of these toxicities were Grade 1–2. Three patients experienced grade 4+ adverse events at least possibly related to treatment. As described above, two patients from Cohort I, dose level 1 experienced grade 4 thrombocytopenia. One patient from the MTD cohort experienced grade 4 neutropenia. Two patients from Cohort I, dose level 1 and 11 patients from the MTD cohort, for a total of 13 patients experienced grade 3 or greater toxicity. These included neutropenia, thrombocytopenia, headache, lymphopenia, hemolysis, constipation, febrile neutropenia, increased alanine aminotransferase, gait abnormalities, fatigue, dehydration, anorexia, nausea, vomiting and diarrhea. A summary of the toxicities during cycle 1 and all cycles is provided in Table 3.
Preliminary antitumor activity
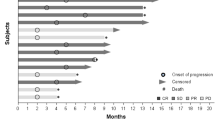
The tumor responses were assessed according to the RECIST (version 1.0) criteria. Overall, 2 patients (6.9%) had partial responses, 17 (58.6%) had stable disease, 5 had progressive disease (17.2%) and 5 went off study before disease assessment (Table 4).
Out of the 17 patients who had stable disease as their best response, three patients had stable disease for 10 or more cycles, one patient had stable disease for 9 cycles, two patients had stable disease for 6 cycles, two patients had stable disease for 4 cycles, and 11 patients had stable disease for 3 or less cycles.
Discussion
The VEGF A ligand inhibitor bevacizumab is an anti-angiogenic agent that has now been approved in combination with standard chemotherapy for patients with metastatic colorectal cancer [26, 27, 29, 30], recurrent/advanced NSCLC [28, 31], advanced cervical cancer [32], and advanced ovarian cancer [33,34,35,36], although its effectiveness is limited and lower than expected, especially in end-stage tumors. It is believed that there is multilevel cross-stimulation among targets along several pathways of signal transduction that lead to malignancy. Thus, a single agent with multiple targets may provide a more complete therapeutic benefit in combination with chemotherapy. Angiogenesis involves signaling via receptor tyrosine kinases that include the VEGFRs and PDGFRs [38, 39]. The antitumor activity of single-agent vatalanib in vitro has been observed as a selective inhibitor of all VEGFRs and a PDGFR/kit inhibitor with anti-proliferation and anti-angiogenic properties [6, 7].
An attempt in the current study was made to combine a standard regimen of pemetrexed disodium with vatalanib. However, there were 8 DLTs at dose level 1. The MTD for vatalanib combined with pemetrexed is 250 mg orally twice daily. In phase I/II clinical trials, vatalanib doses ranging from 50 to 2000 mg once daily and 150 to 1000 mg administered twice daily (BID) have had manageable side effects. The recommended vatalanib dose combined with chemotherapy in phase III was 1250 mg once daily and the recommended single agent dose for administration of vatalanib is 750 mg BID [12, 40, 41]. Because the biologic half-life of vatalanib is between 3 and 6 h [12], the twice-daily schedule of vatalanib may have contributed to a positive impact on OS [8, 12]. This is in contrast to the established long half-life of the monoclonal antibody bevacizumab. One possible reason for the lower MTD in the current study is that there was a relatively less tolerance with heavily pretreated patient population enrolled in the study. In addition, VEGF receptors are present on bone marrow progenitor cells [42] and the possible synergistic hematologic toxicity with pemetrexed disodium may explain the inability to escalate the vatalanib dose in this combination.
The majority of adverse events were fatigue, nausea, vomiting, mucositis and diarrhea. It is noteworthy that angiogenesis inhibitors do not all have similar adverse event profiles. Bevacizumab in combination with pemetrexed disodium in NSCLC demonstrated an increase in hypertension, bleeding and proteinuria compared with pemetrexed disodium alone [43, 44]. This difference in toxicity profiles may be important for patients who have a contraindication to one antiangiogenic agent and potentially allow the use of another drug in this class.
While preliminary anti-tumor activity is seen in the current study in non-small cell lung cancer (NSCLC) in particular, it is unclear if vatalanib added any additional efficacy to the combination. This is because single-agent treatment with pemetrexed results in a response rate of 9.1% and a median survival of 8.3 months in patients with recurrent NSCLC [20]. A number of trials assessing VEGF tyrosine kinase inhibitors in combination with chemotherapy have been done in NSCLC, including cediranib/carboplatin/paclitaxel, sorafenib/carboplatin/paclitaxel, sorafenib/gemcitabine/cisplatin, motesanib/carboplatin/paclitaxel, nintedanib/docetaxel, vandetanib/docetaxel, vandetanib/pemetrexed, and pemetrexed/pazopanib, and none of them demonstrated a superior efficacy to chemotherapy alone [45,46,47,48,49,50,51,52,53,54,55]. In a recent review, Crino et al. described several possible explanations for this observation. First, agents may not effectively combat the redundancy of angiogenic pathways. Owing to the structural similarities of tyrosine kinases most small-molecule TKIs have inhibitory activity over a range of receptors. However, other than nintedanib, no agents with inhibitory potency over all subtypes of VEGFR, PDGFR and FGFR have been tested in a phase III trial. Secondly, the TKIs tested to date may not completely inhibit signaling via their target receptors [56]. It is possible that agents with more favorable pharmacological profiles may be effective at completely abrogating, rather than downregulating, signaling via proangiogenic pathways. Finally, owing to the highly heterogeneous nature of NSCLC, it is probable that certain patients are more likely to respond to antiangiogenic agents than others [57]. In this case, predictive biomarkers for antiangiogenic agents are urgently required to select specific subgroups of patients who are more likely to respond and are a focus of intensive research.
In summary, the combination of vatalanib with pemetrexed disodium was not well tolerated, and had only limited efficacy. Thus, this combination should not be developed further unless predictive biomarkers can be identified.
References
Folkman J (1971) Tumor angiogenesis: therapeutic implications. N Engl J Med 285(21):1182–1186. https://doi.org/10.1056/NEJM197111182852108
Folkman J (1995) Seminars in medicine of the Beth Israel hospital, Boston. Clinical applications of research on angiogenesis. N Engl J Med 333(26):1757–1763. https://doi.org/10.1056/NEJM199512283332608
Hayes AJ, Li LY, Lippman ME (1999) Science, medicine, and the future. Antivascular therapy: a new approach to cancer treatment. BMJ 318(7187):853–856
Keshet E, Ben-Sasson SA (1999) Anticancer drug targets: approaching angiogenesis. J Clin Invest 104(11):1497–1501. https://doi.org/10.1172/JCI8849
Ferrara N, Gerber HP, LeCouter J (2003) The biology of VEGF and its receptors. Nat Med 9(6):669–676. https://doi.org/10.1038/nm0603-669
Wood JM, Bold G, Buchdunger E, Cozens R, Ferrari S, Frei J, Hofmann F, Mestan J, Mett H, O'Reilly T, Persohn E, Rösel J, Schnell C, Stover D, Theuer A, Towbin H, Wenger F, Woods-Cook K, Menrad A, Siemeister G, Schirner M, Thierauch KH, Schneider MR, Drevs J, Martiny-Baron G, Totzke F (2000) PTK787/ZK 222584, a novel and potent inhibitor of vascular endothelial growth factor receptor tyrosine kinases, impairs vascular endothelial growth factor-induced responses and tumor growth after oral administration. Cancer Res 60(8):2178–2189
Drevs J, Müller-Driver R, Wittig C, Fuxius S, Esser N, Hugenschmidt H, Konerding MA, Allegrini PR, Wood J, Hennig J, Unger C, Marmé D (2002) PTK787/ZK 222584, a specific vascular endothelial growth factor-receptor tyrosine kinase inhibitor, affects the anatomy of the tumor vascular bed and the functional vascular properties as detected by dynamic enhanced magnetic resonance imaging. Cancer Res 62(14):4015–4022
Roboz GJ, Giles FJ, List AF, Cortes JE, Carlin R, Kowalski M, Bilic S, Masson E, Rosamilia M, Schuster MW, Laurent D, Feldman EJ (2006) Phase 1 study of PTK787/ZK 222584, a small molecule tyrosine kinase receptor inhibitor, for the treatment of acute myeloid leukemia and myelodysplastic syndrome. Leukemia 20(6):952–957. https://doi.org/10.1038/sj.leu.2404213
Langenberg MH, Witteveen PO, Lankheet NA, Roodhart JM, Rosing H, van den Heuvel IJ, Beijnen JH, Voest EE (2010) Phase 1 study of combination treatment with PTK 787/ZK 222584 and cetuximab for patients with advanced solid tumors: safety, pharmacokinetics, pharmacodynamics analysis. Neoplasia 12(2):206–213
Hecht JR, Trarbach T, Hainsworth JD, Major P, Jäger E, Wolff RA, Lloyd-Salvant K, Bodoky G, Pendergrass K, Berg W, Chen BL, Jalava T, Meinhardt G, Laurent D, Lebwohl D, Kerr D (2011) Randomized, placebo-controlled, phase III study of first-line oxaliplatin-based chemotherapy plus PTK787/ZK 222584, an oral vascular endothelial growth factor receptor inhibitor, in patients with metastatic colorectal adenocarcinoma. J Clin Oncol 29(15):1997–2003. https://doi.org/10.1200/JCO.2010.29.4496
Van Cutsem E, Bajetta E, Valle J, Köhne CH, Hecht JR, Moore M, Germond C, Berg W, Chen BL, Jalava T, Lebwohl D, Meinhardt G, Laurent D, Lin E (2011) Randomized, placebo-controlled, phase III study of oxaliplatin, fluorouracil, and leucovorin with or without PTK787/ZK 222584 in patients with previously treated metastatic colorectal adenocarcinoma. J Clin Oncol 29(15):2004–2010. https://doi.org/10.1200/JCO.2010.29.5436
Thomas AL, Morgan B, Horsfield MA, Higginson A, Kay A, Lee L, Masson E, Puccio-Pick M, Laurent D, Steward WP (2005) Phase I study of the safety, tolerability, pharmacokinetics, and pharmacodynamics of PTK787/ZK 222584 administered twice daily in patients with advanced cancer. J Clin Oncol 23(18):4162–4171. https://doi.org/10.1200/JCO.2005.09.034
Hanauske AR, Chen V, Paoletti P, Niyikiza C (2001) Pemetrexed disodium: a novel antifolate clinically active against multiple solid tumors. Oncologist 6(4):363–373
Adjei AA (2004) Pemetrexed (ALIMTA), a novel multitargeted antineoplastic agent. Clin Cancer Res 10(12 Pt 2):4276s–4280s. https://doi.org/10.1158/1078-0432.CCR-040010
Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, Gatzemeier U, Boyer M, Emri S, Manegold C, Niyikiza C, Paoletti P (2003) Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 21(14):2636–2644. https://doi.org/10.1200/JCO.2003.11.136
Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U, Digumarti R, Zukin M, Lee JS, Mellemgaard A, Park K, Patil S, Rolski J, Goksel T, de Marinis F, Simms L, Sugarman KP, Gandara D (2008) Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 26(21):3543–3551. https://doi.org/10.1200/JCO.2007.15.0375
Ciuleanu T, Brodowicz T, Zielinski C, Kim JH, Krzakowski M, Laack E, Wu YL, Bover I, Begbie S, Tzekova V, Cucevic B, Pereira JR, Yang SH, Madhavan J, Sugarman KP, Peterson P, John WJ, Krejcy K, Belani CP (2009) Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet 374(9699):1432–1440. https://doi.org/10.1016/S0140-6736(09)61497-5
Paz-Ares L, de Marinis F, Dediu M, Thomas M, Pujol JL, Bidoli P, Molinier O, Sahoo TP, Laack E, Reck M, Corral J, Melemed S, John W, Chouaki N, Zimmermann AH, Visseren-Grul C, Gridelli C (2012) Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol 13(3):247–255. https://doi.org/10.1016/S1470-2045(12)70063-3
Paz-Ares LG, de Marinis F, Dediu M, Thomas M, Pujol JL, Bidoli P, Molinier O, Sahoo TP, Laack E, Reck M, Corral J, Melemed S, John W, Chouaki N, Zimmermann AH, Visseren-Grul C, Gridelli C (2013) PARAMOUNT: final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol 31(23):2895–2902. https://doi.org/10.1200/JCO.2012.47.1102
Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J, Gatzemeier U, Tsao TC, Pless M, Muller T, Lim HL, Desch C, Szondy K, Gervais R, Shaharyar MC, Paul S, Paoletti P, Einhorn L, Bunn PA (2004) Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 22(9):1589–1597. https://doi.org/10.1200/JCO.2004.08.163
Scagliotti G, Hanna N, Fossella F, Sugarman K, Blatter J, Peterson P, Simms L, Shepherd FA (2009) The differential efficacy of pemetrexed according to NSCLC histology: a review of two phase III studies. Oncologist 14(3):253–263. https://doi.org/10.1634/theoncologist.2008-0232
Stinchcombe TE, Borghaei H, Barker SS, Treat JA, Obasaju C (2016) Pemetrexed with platinum combination as a backbone for targeted therapy in non-small-cell lung Cancer. Clin Lung Cancer 17(1):1–9. https://doi.org/10.1016/j.cllc.2015.07.002
Masters GA, Temin S, Azzoli CG, Giaccone G, Baker S, Brahmer JR, Ellis PM, Gajra A, Rackear N, Schiller JH, Smith TJ, Strawn JR, Trent D, Johnson DH, Practice ASCOC (2015) Systemic therapy for stage IV non-small-cell lung Cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 33(30):3488–3515. https://doi.org/10.1200/JCO.2015.62.1342
Paz-Ares LG, Altug S, Vaury AT, Jaime JC, Russo F, Visseren-Grul C (2010) Treatment rationale and study design for a phase III, double-blind, placebo-controlled study of maintenance pemetrexed plus best supportive care versus best supportive care immediately following induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small cell lung cancer. BMC Cancer 10:85. https://doi.org/10.1186/1471-2407-10-85
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP (1982) Toxicity and response criteria of the eastern cooperative oncology group. Am J Clin Oncol 5(6):649–655
Giantonio BJ, Catalano PJ, Meropol NJ, O'Dwyer PJ, Mitchell EP, Alberts SR, Schwartz MA, Benson AB, E3200 ECOGS (2007) Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the eastern cooperative oncology group study E3200. J Clin Oncol 25(12):1539–1544. https://doi.org/10.1200/JCO.2006.09.6305
Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F, Couture F, Sirzén F, Cassidy J (2008) Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 26(12):2013–2019. https://doi.org/10.1200/JCO.2007.14.9930
Reck M, von Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, Leighl N, Mezger J, Archer V, Moore N, Manegold C (2009) Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol 27(8):1227–1234. https://doi.org/10.1200/JCO.2007.14.5466
Tebbutt NC, Wilson K, Gebski VJ, Cummins MM, Zannino D, van Hazel GA, Robinson B, Broad A, Ganju V, Ackland SP, Forgeson G, Cunningham D, Saunders MP, Stockler MR, Chua Y, Zalcberg JR, Simes RJ, Price TJ (2010) Capecitabine, bevacizumab, and mitomycin in first-line treatment of metastatic colorectal cancer: results of the Australasian gastrointestinal trials group randomized phase III MAX study. J Clin Oncol 28(19):3191–3198. https://doi.org/10.1200/JCO.2009.27.7723
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350(23):2335–2342. https://doi.org/10.1056/NEJMoa032691
Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH (2006) Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 355(24):2542–2550. https://doi.org/10.1056/NEJMoa061884
Tewari KS, Sill MW, Long HJ, Penson RT, Huang H, Ramondetta LM, Landrum LM, Oaknin A, Reid TJ, Leitao MM, Michael HE, Monk BJ (2014) Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med 370(8):734–743. https://doi.org/10.1056/NEJMoa1309748
Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, Mannel RS, Homesley HD, Fowler J, Greer BE, Boente M, Birrer MJ, Liang SX, Group GO (2011) Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med 365(26):2473–2483. https://doi.org/10.1056/NEJMoa1104390
Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, Carey MS, Beale P, Cervantes A, Kurzeder C, du Bois A, Sehouli J, Kimmig R, Stähle A, Collinson F, Essapen S, Gourley C, Lortholary A, Selle F, Mirza MR, Leminen A, Plante M, Stark D, Qian W, Parmar MK, Oza AM, Investigators I (2011) A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med 365(26):2484–2496. https://doi.org/10.1056/NEJMoa1103799
Stark D, Nankivell M, Pujade-Lauraine E, Kristensen G, Elit L, Stockler M, Hilpert F, Cervantes A, Brown J, Lanceley A, Velikova G, Sabate E, Pfisterer J, Carey MS, Beale P, Qian W, Swart AM, Oza A, Perren T (2013) Standard chemotherapy with or without bevacizumab in advanced ovarian cancer: quality-of-life outcomes from the international collaboration on ovarian neoplasms (ICON7) phase 3 randomised trial. Lancet Oncol 14(3):236–243. https://doi.org/10.1016/S1470-2045(12)70567-3
Della Pepa C, Tonini G, Pisano C, Di Napoli M, Cecere SC, Tambaro R, Facchini G, Pignata S (2015) Ovarian cancer standard of care: are there real alternatives? Chin J Cancer 34(1):17–27. https://doi.org/10.5732/cjc.014.10274
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92(3):205–216
Board R, Jayson GC (2005) Platelet-derived growth factor receptor (PDGFR): a target for anticancer therapeutics. Drug Resist Updat 8(1–2):75–83. https://doi.org/10.1016/j.drup.2005.03.004
Hicklin DJ, Ellis LM (2005) Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol 23(5):1011–1027. https://doi.org/10.1200/JCO.2005.06.081
Chiorean EG, Malireddy S, Younger AE, Jones DR, Waddell MJ, Sloop MI, Yu M, Hall SD, Schneider B, Sweeney CJ (2010) A phase I dose escalation and pharmacokinetic study of vatalanib (PTK787/ZK 222584) in combination with paclitaxel in patients with advanced solid tumors. Cancer Chemother Pharmacol 66(3):441–448. https://doi.org/10.1007/s00280-009-1179-2
Sharma S, Freeman B, Turner J, Symanowski J, Manno P, Berg W, Vogelzang N (2009) A phase I trial of PTK787/ZK222584 in combination with pemetrexed and cisplatin in patients with advanced solid tumors. Investig New Drugs 27(1):63–65. https://doi.org/10.1007/s10637-008-9157-9
Hattori K, Heissig B, Wu Y, Dias S, Tejada R, Ferris B, Hicklin DJ, Zhu Z, Bohlen P, Witte L, Hendrikx J, Hackett NR, Crystal RG, Moore MA, Werb Z, Lyden D, Rafii S (2002) Placental growth factor reconstitutes hematopoiesis by recruiting VEGFR1(+) stem cells from bone-marrow microenvironment. Nat Med 8(8):841–849. https://doi.org/10.1038/nm740
Spigel DR, Hainsworth JD, Joseph MJ, Shipley DL, Hagan MK, Thompson DS, Burris HA, Greco FA (2018) Randomized phase 2 trial of pemetrexed, pemetrexed/bevacizumab, and pemetrexed/carboplatin/bevacizumab in patients with stage IIIB/IV non-small cell lung cancer and an eastern cooperative oncology group performance status of 2. Cancer 124(9):1982–1991. https://doi.org/10.1002/cncr.30986
Karayama M, Inui N, Fujisawa T, Enomoto N, Nakamura Y, Kuroishi S, Yokomura K, Koshimizu N, Sato M, Toyoshima M, Shirai T, Masuda M, Yamada T, Imokawa S, Suda T (2016) Maintenance therapy with pemetrexed and bevacizumab versus pemetrexed monotherapy after induction therapy with carboplatin, pemetrexed, and bevacizumab in patients with advanced non-squamous non small cell lung cancer. Eur J Cancer 58:30–37. https://doi.org/10.1016/j.ejca.2016.01.013
Reck M, Kaiser R, Mellemgaard A, Douillard JY, Orlov S, Krzakowski M, von Pawel J, Gottfried M, Bondarenko I, Liao M, Gann CN, Barrueco J, Gaschler-Markefski B, Novello S, Group L-LS (2014) Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol 15(2):143–155. https://doi.org/10.1016/S1470-2045(13)70586-2
Hanna NH, Kaiser R, Sullivan RN, Aren OR, Ahn MJ, Tiangco B, Voccia I, Pawel JV, Kovcin V, Agulnik J, Gaschler-Markefski B, Barrueco J, Sikken P, Schloss C, Kim JH, group L-LS (2016) Nintedanib plus pemetrexed versus placebo plus pemetrexed in patients with relapsed or refractory, advanced non-small cell lung cancer (LUME-lung 2): a randomized, double-blind, phase III trial. Lung Cancer 102:65–73. https://doi.org/10.1016/j.lungcan.2016.10.011
Herbst RS, Sun Y, Eberhardt WE, Germonpré P, Saijo N, Zhou C, Wang J, Li L, Kabbinavar F, Ichinose Y, Qin S, Zhang L, Biesma B, Heymach JV, Langmuir P, Kennedy SJ, Tada H, Johnson BE (2010) Vandetanib plus docetaxel versus docetaxel as second-line treatment for patients with advanced non-small-cell lung cancer (ZODIAC): a double-blind, randomised, phase 3 trial. Lancet Oncol 11(7):619–626. https://doi.org/10.1016/S1470-2045(10)70132-7
de Boer RH, Arrieta Ó, Yang CH, Gottfried M, Chan V, Raats J, de Marinis F, Abratt RP, Wolf J, Blackhall FH, Langmuir P, Milenkova T, Read J, Vansteenkiste JF (2011) Vandetanib plus pemetrexed for the second-line treatment of advanced non-small-cell lung cancer: a randomized, double-blind phase III trial. J Clin Oncol 29(8):1067–1074. https://doi.org/10.1200/JCO.2010.29.5717
Lee JS, Hirsh V, Park K, Qin S, Blajman CR, Perng RP, Chen YM, Emerson L, Langmuir P, Manegold C (2012) Vandetanib versus placebo in patients with advanced non-small-cell lung cancer after prior therapy with an epidermal growth factor receptor tyrosine kinase inhibitor: a randomized, double-blind phase III trial (ZEPHYR). J Clin Oncol 30(10):1114–1121. https://doi.org/10.1200/JCO.2011.36.1709
Laurie SA, Solomon BJ, Seymour L, Ellis PM, Goss GD, Shepherd FA, Boyer MJ, Arnold AM, Clingan P, Laberge F, Fenton D, Hirsh V, Zukin M, Stockler MR, Lee CW, Chen EX, Montenegro A, Ding K, Bradbury PA (2014) Randomised, double-blind trial of carboplatin and paclitaxel with daily oral cediranib or placebo in patients with advanced non-small cell lung cancer: NCIC clinical trials group study BR29. Eur J Cancer 50(4):706–712. https://doi.org/10.1016/j.ejca.2013.11.032
Goss GD, Arnold A, Shepherd FA, Dediu M, Ciuleanu TE, Fenton D, Zukin M, Walde D, Laberge F, Vincent MD, Ellis PM, Laurie SA, Ding K, Frymire E, Gauthier I, Leighl NB, Ho C, Noble J, Lee CW, Seymour L (2010) Randomized, double-blind trial of carboplatin and paclitaxel with either daily oral cediranib or placebo in advanced non-small-cell lung cancer: NCIC clinical trials group BR24 study. J Clin Oncol 28(1):49–55. https://doi.org/10.1200/JCO.2009.22.9427
Scagliotti G, Novello S, von Pawel J, Reck M, Pereira JR, Thomas M, Abrão Miziara JE, Balint B, De Marinis F, Keller A, Arén O, Csollak M, Albert I, Barrios CH, Grossi F, Krzakowski M, Cupit L, Cihon F, Dimatteo S, Hanna N (2010) Phase III study of carboplatin and paclitaxel alone or with sorafenib in advanced non-small-cell lung cancer. J Clin Oncol 28(11):1835–1842. https://doi.org/10.1200/JCO.2009.26.1321
Paz-Ares LG, Biesma B, Heigener D, von Pawel J, Eisen T, Bennouna J, Zhang L, Liao M, Sun Y, Gans S, Syrigos K, Le Marie E, Gottfried M, Vansteenkiste J, Alberola V, Strauss UP, Montegriffo E, Ong TJ, Santoro A, Group Nns-clcREUSNIS (2012) Phase III, randomized, double-blind, placebo-controlled trial of gemcitabine/cisplatin alone or with sorafenib for the first-line treatment of advanced, nonsquamous non-small-cell lung cancer. J Clin Oncol 30(25):3084–3092. https://doi.org/10.1200/JCO.2011.39.7646
Scagliotti GV, Vynnychenko I, Park K, Ichinose Y, Kubota K, Blackhall F, Pirker R, Galiulin R, Ciuleanu TE, Sydorenko O, Dediu M, Papai-Szekely Z, Banaclocha NM, McCoy S, Yao B, Hei YJ, Galimi F, Spigel DR (2012) International, randomized, placebo-controlled, double-blind phase III study of motesanib plus carboplatin/paclitaxel in patients with advanced nonsquamous non-small-cell lung cancer: MONET1. J Clin Oncol 30(23):2829–2836. https://doi.org/10.1200/JCO.2011.41.4987
Scagliotti GV, Felip E, Besse B, von Pawel J, Mellemgaard A, Reck M, Bosquee L, Chouaid C, Lianes-Barragán P, Paul EM, Ruiz-Soto R, Sigal E, Ottesen LH, Lechevalier T (2013) An open-label, multicenter, randomized, phase II study of pazopanib in combination with pemetrexed in first-line treatment of patients with advanced-stage non-small-cell lung cancer. J Thorac Oncol 8(12):1529–1537. https://doi.org/10.1097/JTO.0000000000000005
Aggarwal C, Somaiah N, Simon G (2012) Antiangiogenic agents in the management of non-small cell lung cancer: where do we stand now and where are we headed? Cancer Biol Ther 13(5):247–263. https://doi.org/10.4161/cbt.19594
Gasparini G, Longo R, Fanelli M, Teicher BA (2005) Combination of antiangiogenic therapy with other anticancer therapies: results, challenges, and open questions. J Clin Oncol 23(6):1295–1311. https://doi.org/10.1200/JCO.2005.10.022
Acknowledgements
This study was funded by the National Cancer Institute P30CA015083 grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
All authors have no conflict of interest to report.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Wang, F., Molina, J., Satele, D. et al. A phase I study of the vascular endothelial growth factor inhibitor Vatalanib in combination with Pemetrexed disodium in patients with advanced solid tumors. Invest New Drugs 37, 658–665 (2019). https://doi.org/10.1007/s10637-018-0690-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-018-0690-x