Summary
Introduction Germline BRCA mutations may have therapeutic implications as surrogate markers of DNA-damage repair status in pancreatic ductal adenocarcinoma (PDAC). We performed a prospective study to evaluate the efficiency of risk criteria based on personal or family history of breast and ovarian cancer for determining germline BRCA mutations in PDAC patients with Asian ethnicity. Methods Between November 2015 and May 2016, we screened consecutive PDAC patients with locally advanced unresectable or metastatic disease who were referred for systemic chemotherapy. Analyses for germline BRCA mutations were performed if patients had one or more first-degree or second-degree relatives with breast or ovarian cancers or had a personal medical history of these diseases. DNA was extracted from whole blood, and all coding exons and their flanking intron regions of BRCA1 and BRCA2 were sequenced. Results A total of 175 patients were screened for personal and family history and 10 (5.7%) met the inclusion criteria for genetic sequencing. Pathogenic germline BRCA2 mutation [c.7480C>T (p.Arg2494*)] was identified in one male patient, resulting in a frequency of 10% for the risk-stratified patients and 0.6% for the unselected PDAC population. Two patients had germline BRCA2 variants of uncertain significance [c.1744A>C (p.Thr582Pro) and c.68-7T>A]. Conclusion Personal or family history of breast or ovarian cancers is a feasible, cost-effective risk categorization for screening germline BRCA mutations in Asian PDAC patients as 10% of this population had the pathogenic mutation herein. Future validation from a large, prospective cohort is needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a well-known disease with poor prognosis, having a 5-year survival rate of <6% [1]. At the time of diagnosis, 10%–20% patients with PDAC are categorized as potentially curable with surgical resection [2]. However, the overall survival (OS) of patients as reported in large phase 3 trials for adjuvant chemotherapy ranges between 20 and 25 months even after resection and postoperative chemotherapy [3,4,5].
In recent years, the efficacy of systemic chemotherapy for PDAC has been enhanced using combination chemotherapy regimens, such as FOLFIRINOX (fluorouracil [5-FU], leucovorin, irinotecan, and oxaliplatin) and gemcitabine plus nab-paclitaxel [6, 7]. Despite these improvements, overall prognosis for metastatic PDAC is still dismal as median OS is <1 year.
Approximately 5%–10% patients with PDAC are regarded to have hereditary predisposition [8, 9]. Among the inherited cancer susceptibility syndromes, germline mutations in BRCA1 or BRCA2 have been well defined for increased risk of PDAC development compared to the general population (2-fold with BRCA1 and 3.5-fold with BRCA2) [10, 11]. The population frequency of pathogenic BRCA1 and BRCA2 mutations is estimated at 1 in 400 to 1 in 800, with exceptionally high prevalence in the Ashkenazi Jewish population (approximately 2%), but it varies depending on ethnicity [12,13,14].
DNA-damage repair deficiency status is well known for susceptibility to platinum-based chemotherapy or a poly (ADP-ribose) polymerase (PARP) inhibitor [15]. A recent study revealed that genomic instability and BRCA mutational signature status can be identified using whole-genome sequencing and did not necessarily require germline BRCA1 and BRCA2 in pancreatic cancer [16]. However, tests for germline BRCA1 and BRCA2 mutations are still the most widely used surrogate measures of DNA maintenance deficiencies in daily practice. As a previous Israeli retrospective analysis showed that platinum-based chemotherapy was associated with improved survival compared to non-platinum-based chemotherapies in PDAC patients with germline BRCA1 and BRCA2 mutations [17], BRCA mutations may be the valuable biomarker in patients with PDAC.
Previous studies have revealed that the prevalence of germline BRCA1 and BRCA2 mutations in overall PDAC patients ranged between 3% and 21% [18,19,20,21,22]. However, most of these data were from Western populations and limited data is available from Asian populations. Considering that BRCA mutation prevalence is higher in patients with Ashkenazi Jewish ancestry, the frequency of BRCA mutation in the Asian PDAC patient population may be less than previous studied populations. A previous Korean study, which evaluated germline BRCA2 mutation for 60 unselected patients with PDAC, could not find any patient with pathogenic mutation [23].
Given the high cost and low positive rate, genetic tests for germline BRCA1 and BRCA2 mutations are not currently recommended for unselected PDAC patients in the daily practice setting. In contrast that several criteria have been suggested for BRCA mutations in patients with breast or ovarian cancer [24], but no such criteria exist for patients with PDAC. Therefore, we performed a prospective study to evaluate the efficiency of risk criteria based on personal and family history of breast and ovarian cancer in PDAC patients of Asian ethnicity.
Patients and methods
Patients
Between November 2015 and May 2016, we screened consecutive patients with locally advanced unresectable or metastatic PDAC who were referred for systemic chemotherapy to the Department of Oncology, Asan Medical Center, Seoul, Korea. Analyses for germline BRCA1 and BRCA2 mutations were performed if patients had one or more first-degree or second-degree relatives with breast or ovarian cancers or had a personal medical history of breast or ovarian cancer.
Participants provided informed consent, a cancer family history and personal medical history, and allowed access to current and previous cancer treatment records. This study was approved by the Institutional Review Board of the Asan Medical Center and conducted in accordance with the Declaration of Helsinki and the Guidelines for Good Clinical Practice.
BRCA mutation analysis
DNA was extracted from EDTA-anticoagulated whole blood using the QIAamp DSP DNA Mini Kit according to the manufacturer’s instructions (Qiagen, Hilden, Germany). The concentration of extracted DNA was measured using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Carlsbad, CA, USA). All coding exons and their flanking intron regions of BRCA1 and BRCA2 were amplified by polymerase chain reaction using primer pairs designed by Primer3 software (Whitehead Institute for Biomedical Research, Cambridge, MA). Relevant regions were sequenced using a BigDye Terminator Cycle Sequencing Ready Reaction Kit (Applied Biosystems) and an ABI 3730 Genetic Analyzer (Applied Biosystems Foster City, CA).
We classified the variants in accordance with the American College of Medical Genetics and Genomics guidelines. The mutation status was assessed using the Human Genome Mutation Database (http://www.hgmd.cf.ac.uk/ac/index.php), ClinVar (http://www.ncbi.nlm.nih.gov/clinvar/), or UMD (http://www.umd.be/BRCA1/, http://www.umd.be/BRCA2/). Functional effects of variants of unknown significance (VUSs) were predicted by sorting intolerant from tolerant, polymorphism phenotyping-2 (PolyPhen), LRT, FATHMM, MutationTaster, MutationAssesor, MaxEnt, and Genomic Evolutionary Rate Profiling score. The allele frequency (AF) of the VUSs were estimated on the basis of the 1000 Genome Project (1000GP, http://browser.1000genomes.org/index.html), the Exome Sequencing Project (ESP6500, http://evs.gs.washington.edu/EVS/), and the Exome Aggregation Consortium (ExAC, http://exac.broadinstitute.org/). Sequences were visualized for analysis using Sequencher 4.10.1 (Gene Codes Corporation, Ann Arbor, MI). The “A” of the ATG translation initiation codon is described as position number 1 in BRCA1 (NM_007294.3) and BRCA2 (NM_000059.3).
Response evaluation and treatment
Baseline radiological tumor evaluations were performed at diagnosis and the response was evaluated every 6 or 8 weeks of treatment by the same imaging techniques used at baseline. Additional imaging was performed if disease progression was suspected Tumor response was determined according to the Response Evaluation Criteria in Solid Tumor version 1.1. In this study, treatment was not prespecified and was administered at the discretion of the attending physicians.
Statistical analysis
Progression-free survival (PFS) was defined as the duration from the first day of chemotherapy to disease progression or death from any cause, whichever occurred first. OS was calculated from the date of first chemotherapy to the date of death because of any cause. Data were censored if the disease had not progressed on last evaluation or patients were still alive at the time of analysis (December 31, 2016). PFS and OS were estimated using the Kaplan–Meier method. All statistical analyses, including descriptive statistics, were performed using IBM SPSS Statistics for Windows, Version 21.0 (Armonk, NY: IBM Corp).
Results
Patient characteristics
During the study period, a total of 175 patients received systemic chemotherapy for locally advanced unresectable or metastatic PDAC. Among them, 10 (5.7%) patients met the prespecified criteria for analysis of germline BRCA1 and BRCA2 mutations. Of these 10, six (60%) patients had family history of breast or ovarian cancers and four (40%) patients had previous medical history of breast cancer. All patients agreed to undergo the genetic testing for germline BRCA1 and BRCA 2.
Patient characteristics and treatment outcomes are listed in Table 1. The median age was 60 years (range 49–72 years) and five patients (50%) were male. All patients received chemotherapy for locally advanced unresectable (n = 3, 30%) and metastatic or recurrent disease (n = 7, 70%). FOLFIRINOX was most commonly used (n = 6, 60%), followed by gemcitabine-based regimens (n = 3, 30%) and FOLFOX (n = 1, 10%).
Germline BRCA mutations
Among 10 patients undergoing germline variant evaluation, pathogenic variants and VUSs were found in one (10%) and two (20%) patients, respectively (Table 1). These three variants have been previously reported in cancer patients. The pathogenic variant detected in our study was BRCA2 c.7480C > T (p.Arg2494*) and VUSs were BRCA2 c.1744A > C (p.Thr582Pro) and c.68-7 T > A, a single-nucleotide variant. The frequency data, including ExAC, ESP6500, and 1000GP, showed that the AF of these two VUSs was <0.1% in the general population. According to in silico analyses, they were predicted to have benign or neutral effects. Recent updates indicate that these two variants have benign or uncertain effects according to ClinVar.
Clinical outcomes
In overall patients, median PFS and OS were 4.2 months (95% confidence interval [CI], 0.8–7.7) and 9.3 months (95% CI, 6.0–12.5), respectively (Table 1). Partial response was observed in 2 patients (20%) and progressive disease was the best response in 2 patients (20%). The patient with pathogenic BRCA2 mutation showed partial response and median PFS of 4.6 months with modified FOLFIRINOX and median OS was 9.3 months. This patient had exposure to cisplatin after failure of FOLFIRINOX. Two patients with VUSs of BRCA2 showed median PFS of 2.2 with modified FOLFIRINOX and 5.5 months with nab-paclitaxel plus gemcitabine.
Clinical courses of patients with BRCA-mutant PDAC
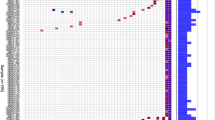
The patient (No.3, Table 1) with pathogenic germline BRCA2 mutation was a 63-year-old male with pancreatic head adenocarcinoma and liver metastases. His younger sister was diagnosed with breast cancer. He had a nonsense mutation (p.Arg2494*) in BRCA2. He received modified FOLFIRINOX as first-line chemotherapy. Although a partial response was achieved after four cycles, the response duration was only 10 weeks (Fig. 1). As second-line chemotherapy, a combination of gemcitabine and cisplatin was administered. Despite a slight decrease in pancreas and liver tumor sizes after 6 weeks of gemcitabine plus cisplatin, the tumors progressed after 3 months of treatment. Although the patient received subsequent third-line S-1, an oral fluoropyrimidine, disease progressed very rapidly, and he died 9 months after being diagnosed with PDAC.
Two patients, found to have BRCA2 VUSs, were both women previously diagnosed and treated for breast cancer. The patient harboring a BRCA2 c.1744A > C (p.Thr582Pro) mutation (No.4) received modified FOLFIRINOX, but PFS was only 2.2 months. The patient with BRCA2 c.68-7 T > A mutation (No.9) was treated with nab-paclitaxel plus gemcitabine. For this patient, partial response was achieved and the disease remained stable at the time of analysis (7.1 months after the start of chemotherapy).
Discussion
Our study prospectively evaluated the frequency of germline BRCA1 and BRCA2 mutations in advanced PDAC patients with Asian ethnicity screened by personal and family cancer history. We screened 175 patients for personal and family cancer history and found 10 patients who met the prespecified criteria—a personal history of breast or ovarian cancers or one first-degree or second-degree relative with breast or ovarian cancers—and underwent genetic testing for BRCA1 and BRCA2 mutations. There was one patient with pathogenic mutation of BRCA2, indicating a frequency of 10% in the risk-stratified patient group and 0.6% in the unselected patient population. Our findings suggest that determining germline BRCA mutation risk category using personal and family history of breast or ovarian cancer might be feasible in the Asian population.
Although previous studies reported a BRCA mutation prevalence in patients with PDAC of up to 21%, most recent large cohort studies have reported approximately a 3% BRCA mutation prevalence in PDAC patients overall [22, 25, 26]. The discrepancy in BRCA mutation prevalence among the studies may be because of the proportion of PDAC patients with Ashkenazi Jewish ancestry, as this population has a high reported prevalence of BRCA mutations (10%–14%) [22, 27]. In these studies, mostly using a Western patient population, the BRCA mutation prevalence ranged from 4 to 7% in PDAC patients with non-Ashkenazi Jewish ancestry (Table 2).
Until recently, only a few studies have evaluated the prevalence of BRCA mutations in PDAC patients with Asian ethnicity. In a previous Korean study testing BRCA2 mutation only, no pathogenic mutation was found in 60 unselected patients with PDAC [23]. A recently published Japanese study reported the prevalence of germline mutations in familial PDAC patients with at least one first-degree relative with PDAC [28]. In this study, targeted deep sequencing of peripheral blood was performed for 21 genes known to be associated with hereditary predispositions to pancreatic, breast, and ovarian cancers, including BRCA1 and BRCA2. BRCA2 mutations were detected in three (5.6%) of 54 patients with familial pancreatic cancer. The results of these Asian studies, including ours, indicate that germline BRCA mutations may be less frequent in Asian PDAC patients. However, because there was no large cohort study evaluating all unselected patients with PDAC, the exact prevalence of BRCA1 and BRCA2 mutations in Asian PDAC patients remains unclear.
Because BRCA1 and BRCA2 mutations may have therapeutic implications for patients with PDAC, relevant risk criteria for genetic testing are needed considering their low prevalence in PDAC patients, except those with Ashkenazi Jewish ancestry. Our study suggests that personal and family history of breast and ovarian cancer might be a good indicator for genetic screening for BRCA1 and BRCA2 mutations in PDAC patients with non-Ashkenazi Jewish ancestry, at least in terms of cost effectiveness. Our results are consistent with those in previous studies. A study conducted in the United States showed that prevalence of BRCA1 and BRCA2 mutation prevalence was 9% (4 of 44) in PDAC patients with non-Ashkenazi Jewish ancestry and ≥1 first-degree or second-degree relatives with breast, ovarian, or pancreatic cancer [27]. A large Canadian cohort study showed that BRCA mutation positive rates were 13.3% (2 of 15) in patients with a personal history of breast cancer, 10% (5 of 50%) in patients with ≥1 first-degree relatives with breast cancer, and 22.2% (2 of 9) in patients with ≥1 first-degree relatives with ovarian cancer, although there was only marginal significance (p = 0.06) for increased BRCA mutation frequency compared to those without family history of breast or ovarian cancers [22]. A Japanese study also showed that BRCA positive rates were 15.4% (2 of 13) in patients with a personal history of breast or ovarian cancers [28]. Although familial history of pancreatic cancer has also been regarded as potential risk category for genetic testing of BRCA1 and BRCA2 mutations, frequency of BRCA mutations were 5.4% (2 of 35 patients) in a Canadian study and 5.6% (3 of 54) in a Japanese study, which are much lower positive rates than categorization by family history of breast or ovarian cancers. [22, 28].
Despite the potential feasibility of using personal or family history of breast or ovarian cancer to determine genetic testing of BRCA1 and BRCA2 mutations, there are some issues to be globally accepted. The most important one is that this risk category can determine only a subset of patients with BRCA mutations, as only 30%–40% BRCA mutation carriers met various definitions for family breast/ovarian cancer history in a previous Canadian study [22]. This indicates that our study may miss the subgroup of patients with BRCA-mutated PDAC. Nevertheless, because there were no other predictive factors for BRCA mutations in PDAC patients, except Ashkenazi Jewish ancestry [22, 27], personal or family history of breast or ovarian cancer may be a reasonable and cost-effective option for finding BRCA mutation in PDAC patients.
The prospective design of this study was beneficial for determining the risk category for detection of BRCA mutation carriers in PDAC patients with Asian ethnicity. However, the number of patients who met the prespecified criteria for genetic testing was too small, as only 10 of 175 patients were included. Because personal and family cancer history were based on the patients’ self-reports, our study may underestimate the number of patients qualifying for our risk category. Some patients, particularly those with old age, may have had difficulty recalling family cancer history. Moreover, the number of patients with pathogenic BRCA mutation was too small to investigate the clinical phenotype of BRCA-mutated PDAC, such as the association between clinical characteristics and BRCA mutations, prognostic implication of BRCA mutation in PDAC, and efficacy of platinum-based treatment in BRCA-mutated PDAC.
In conclusion, personal or family history of breast or ovarian cancers is a feasible and reasonable risk categorization for germline BRCA1 and BRCA2 mutations in Asian PDAC patients. Despite of our results, as recent whole-genome study suggested that BRCA mutation signatures, potential biomarkers for platinum or PARP inhibitors, in PDAC are not limited in patients with germline BRCA mutation carriers [16], further studies are needed to define the subgroup of PDAC patients with this phenotype.
References
Siegel R, Ma J, Zou Z, Jemal A (2014) Cancer statistics, 2014. CA Cancer J Clin 64(1):9–29
Heestand GM, Murphy JD, Lowy AM (2015) Approach to patients with pancreatic cancer without detectable metastases. J Clin Oncol 33(16):1770–1778
Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H et al (2004) A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 350(12):1200–1210
Neoptolemos JP, Stocken DD, Bassi C, Ghaneh P, Cunningham D, Goldstein D et al (2010) Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA 304(10):1073–1081
Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K et al (2013) Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA 310(14):1473–1481
Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y et al (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364(19):1817–1825
Shridhar R, Almhanna K, Hoffe SE, Fulp W, Weber J, Chuong MD et al (2013) Increased survival associated with surgery and radiation therapy in metastatic gastric cancer: a surveillance, epidemiology, and end results database analysis. Cancer 119(9):1636–1642
Brand RE, Lynch HT (2000) Hereditary pancreatic adenocarcinoma. A clinical perspective. Med Clin North Am 84(3):665–675
Carnevale J, Ashworth A (2015) Assessing the significance of BRCA1 and BRCA2 mutations in pancreatic cancer. J Clin Oncol 33(28):3080–3081
Breast Cancer Linkage C (1999) Cancer risks in BRCA2 mutation carriers. J Natl Cancer Inst 91(15):1310–1316
Thompson D, Easton DF (2002) Breast cancer linkage C. Cancer incidence in BRCA1 mutation carriers. J Natl Cancer Inst 94(18):1358–1365
Levy-Lahad E, Catane R, Eisenberg S, Kaufman B, Hornreich G, Lishinsky E et al (1997) Founder BRCA1 and BRCA2 mutations in Ashkenazi Jews in Israel: frequency and differential penetrance in ovarian cancer and in breast-ovarian cancer families. Am J Hum Genet 60(5):1059–1067
Petrucelli N, Daly MB, Feldman GL (2010) Hereditary breast and ovarian cancer due to mutations in BRCA1 and BRCA2. Genet Med 12(5):245–259
Maxwell KN, Domchek SM, Nathanson KL, Robson ME (2016) Population frequency of germline BRCA1/2 mutations. J Clin Oncol 34(34):4183–4185
Kaufman B, Shapira-Frommer R, Schmutzler RK, Audeh MW, Friedlander M, Balmana J et al (2015) Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol 33(3):244–250
Waddell N, Pajic M, Patch AM, Chang DK, Kassahn KS, Bailey P et al (2015) Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 518(7540):495–501
Golan T, Kanji ZS, Epelbaum R, Devaud N, Dagan E, Holter S et al (2014) Overall survival and clinical characteristics of pancreatic cancer in BRCA mutation carriers. Br J Cancer 111(6):1132–1138
Lucas AL, Frado LE, Hwang C, Kumar S, Khanna LG, Levinson EJ et al (2014) BRCA1 and BRCA2 germline mutations are frequently demonstrated in both high-risk pancreatic cancer screening and pancreatic cancer cohorts. Cancer 120(13):1960–1967
Hahn SA, Greenhalf B, Ellis I, Sina-Frey M, Rieder H, Korte B et al (2003) BRCA2 germline mutations in familial pancreatic carcinoma. J Natl Cancer Inst 95(3):214–221
Couch FJ, Johnson MR, Rabe KG, Brune K, de Andrade M, Goggins M et al (2007) The prevalence of BRCA2 mutations in familial pancreatic cancer. Cancer Epidemiol Biomark Prev 16(2):342–346
Caro M, Verlaan MG, Julian O, Finkers R, Wolters AM, Hutton SF et al (2015) Assessing the genetic variation of ty-1 and ty-3 alleles conferring resistance to tomato yellow leaf curl virus in a broad tomato germplasm. Mol Breed 35(6):132
Holter S, Borgida A, Dodd A, Grant R, Semotiuk K, Hedley D et al (2015) Germline BRCA mutations in a large clinic-based cohort of patients with pancreatic adenocarcinoma. J Clin Oncol 33(28):3124–3129
Cho JH, Bang S, Park SW, Chung JB, Song SY (2008) BRCA2 mutations as a universal risk factor for pancreatic cancer has a limited role in Korean ethnic group. Pancreas 36(4):337–340
Daly MB, Pilarski R, Axilbund JE, Berry M, Buys SS, Crawford B et al (2016) Genetic/familial high-risk assessment: breast and ovarian, version 2.2015. J Natl Compr Cancer Netw 14(2):153–162
Gloria M. Petersen KGC, Robert R. McWilliams, Neil Majithia, Brian Allen, John Kidd, Nanda Singh, Anne-Renee Hartman, Ann L. Oberg (2016). Mayo Clinic, Rochester, MN; Myriad Genetics, Inc., Salt Lake City, UT. Genetic heterogeneity and survival among pancreatic adenocarcinoma (PDAC) patients with positive family history. In: 2016 ASCO Annual Meeting: J Clin Oncol
Golan T, Sela T, Margalit O, Amit U, Halpern N, Aderka D, et al. Short and long-term survival in metastatic pancreatic adenocarcinoma, 1993–2013. J Clin Oncol 2017;35(4_suppl):232–232
Salo-Mullen EE, O'Reilly EM, Kelsen DP, Ashraf AM, Lowery MA, Yu KH et al (2015) Identification of germline genetic mutations in patients with pancreatic cancer. Cancer 121(24):4382–4388
Takai E, Yachida S, Shimizu K, Furuse J, Kubo E, Ohmoto A et al (2016) Germline mutations in Japanese familial pancreatic cancer patients. Oncotarget 7(45):74227–74235
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This study was supported by a grant (2015–0753) from the Asan Institute for Life Sciences, Asan Medical Center, Seoul, Korea.
Conflict of interest
The authors indicate no potential conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
This study was approved by the Institutional Review Board of the Asan Medical Center and informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Lee, K., Yoo, C., Kim, Kp. et al. Germline BRCA mutations in Asian patients with pancreatic adenocarcinoma: a prospective study evaluating risk category for genetic testing. Invest New Drugs 36, 163–169 (2018). https://doi.org/10.1007/s10637-017-0497-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-017-0497-1