Summary
Patients receiving pegfilgrastim (Neulasta®) for the treatment of neutropenia can experience bone pain following the injections required to achieve effective neutrophil levels. The safety, pharmacokinetic (PK), and pharmacodynamic (PD) profiles of ANF-RHO™, a novel pegylated granulocyte colony stimulating factor, were assessed in a randomized, controlled, double-blind Phase 1 clinical study in healthy volunteers. Subjects received a single subcutaneous dose of ANF-RHO over a range of 6 doses (5–50 μg/kg), placebo (saline), or the recommended clinical dose of pegfilgrastim administered at the labeled fixed 6 mg dosage (equivalent to 80–100 μg/kg). The primary outcome measure was safety and tolerability. Secondary outcomes included PK and PD effects on absolute neutrophil count (ANC) and number of CD34+ progenitor cells. Severity of bone pain was also assessed. In healthy volunteers, ANF-RHO was administered at ascending doses up to 50 μg/kg without significant adverse effects; appeared to be better (5 to 30 μg/kg) or equally well (50 μg/kg) tolerated, and had lower mean bone pain scores as compared to pegfilgrastim. ANF-RHO achieved CD34+ and ANC numbers at significantly lower doses, and had a significantly longer circulating half-life than pegfilgrastim. These results suggest that ANF-RHO can be provided less frequently, at a lower dose, and with fewer side effects. ANF-RHO had unique, prolonged PK/PD attributes as compared to marketed pegfilgrastim, suggesting that it may provide an improved clinical benefit in further clinical studies in patients with chemotherapy-induced or chronic idiopathic neutropenia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Febrile neutropenia (FN), defined as an absolute neutrophil count of less than 0.5 × 109/L and a temperature more than 38.5 °C, is a frequent and life-threatening complication in cancer subjects receiving myelosuppressive chemotherapy [1]. Episodes of FN require immediate hospitalization and administration of broad spectrum antibiotics. Mortality rates from FN episodes can average around 9.5% and be as high as 14% or higher depending on the cancer type, number of major comorbidities, and type of systemic infection [2]. Furthermore, FN leads to chemotherapy dose reductions and treatment that are associated with poor treatment outcome, prolonged hospitalization, and pre-disposes the patient to potential life-threatening infection [3].
Endogenous granulocyte colony stimulating factor (G-CSF) is essential for neutrophil survival, release from the bone marrow, differentiation and activation, and assisting in the first line defense of microorganism infection in patients receiving myelosuppressive therapy [4]. Myelosuppression can cause FN and render the patients unable to sustain an initial immune response in the week following chemotherapy administration. The development of recombinant human granulocyte colony stimulating factor (rhG-CSF) has dramatically improved the capacity of clinicians to treat patients with risk of developing severe neutropenia, but the frequent dosing needed and potential for immune reaction with rhG-CSFs were limitations. PEGylated biologics (PEG-rhG-CSF) like the marketed pegfilgrastim, which are administered to patients once-per-cycle of chemotherapy at 24 h following treatment, proved effective at reducing the number of days patients have severe neutropenia (absolute neutrophil count [ANC] less than 0.5 × 109/L), lowering the incidence of FN and the need for intravenous antibiotics, with greater convenience. Because of the well-documented favorable risk/benefit profile of pegfilgrastim, it is now the standard of care in patients receiving myelosuppressive chemotherapy where there is a high incidence of FN. Pegfilgrastim has been previously shown to reduce the neutropenia nadir and duration, but the majority of patients still present with Grade 3 or 4 neutropenia [5]. Additionally, bone pain occurs in a significant number of patients, especially in the first cycle of treatment [6]. A major concern in patients who develop severe bone pain is that they may refuse further administration of pegfilgrastim and hamper the dense chemotherapy schedule [7]. Traditional analgesics, such as non-steroidal anti-inflammatory drugs (NSAIDs) and opioids, can be ineffective in severe drug-induced bone pain. With the high frequency of this adverse effect (AE), it is clear that health practitioners need additional treatment options for patients who experience severe pegfilgrastim-induced bone pain. ANF-RHO is a new prophylactic treatment for chemotherapy-induced neutropenia consisting of a novel pegylated version of rhG-CSF protein developed to produce improved pharmacodynamic properties that should be superior to existing products by providing less excessive and more long-lasting levels of circulating neutrophils during the period following chemotherapy administration. A series of pre-clinical in vivo and in vitro pharmacodynamic (PD), pharmacokinetic (PK), including juvenile, genotoxicity and toxicology studies of ANF-RHO in rats and monkeys were conducted in comparison to pegfilgrastim [Misra, Preliminary results from a long-term repeat dose toxicity and toxicokinetic study of ANF-RHO, a novel anti-neutropenic factor; Misra, Toxicology studies and results for determining safety of a novel anti-neutropenic factor–ANF-RHO™; Jubin et al., Development of a novel anti-neutropenic factor clinical candidate for hematopoeisis deficincies, unpublished manuscript]. Results from these preclinical studies have shown that ANF-RHO induced no overt toxicity, no pathology, and no major AEs on hematology, serum chemistry, gross necropsy, or histology evaluations. Limited immunogenicity after up to 13 weeks of repeated administration was seen against both drug products, though significantly more with pegfilgrastim than with ANF-RHO.
Here, we report the results of a Phase I clinical study conducted to determine the safety profile of ANF-RHO and its potential to reduce the occurrence of side effects such as bone pain, as well as its dose-response relationship in healthy adults.
Patients and methods
Patients
Eligible patients had to be healthy volunteers aged ≥18 years and ≤55 years with a Body Mass Index (BMI) between 18 and 30 kg/m2 inclusive; and a weight not <60 kg or >90 kg. Additionally, female volunteers of reproductive age had to have a negative serum pregnancy (β-HCG) test at screening and on Day −1 prior to dosing, be non-lactating and agree to use birth control for 90 days after dosing on Day 1. Exclusion criteria included previous administration of G-CSF, as well as any history or current signs of significant disease, which could interfere with the study or confound the results. Volunteers with positive results for HIV, hepatitis B, or hepatitis C were also excluded. Those with malignancy within the last 5 years were excluded, with the exception of successfully treated basal cell carcinoma and carcinoma in situ uteri.
Study design and objectives
The primary objective of this study was to assess the safety and tolerability of increasing doses of ANF-RHO in healthy volunteers in comparison to placebo and standard of care. This double-blind, randomized, placebo-controlled study enrolled healthy volunteers to receive a single abdominal subcutaneous (sc) administration of either placebo, the recommended clinical dose of pegfilgrastim administered at the labeled fixed 6 mg dosage (equivalent to 80–100 μg/kg), or a single ascending dose of 5, 10, 15, 20 (low concentration vial 2 mg/mL), 20 (high concentration vial 5 mg/mL), 30, and 50 μg/kg of ANF-RHO in seven cohorts. The study schedule consisted of a screening period between Day −28 and Day −1 (admission), an observation period from Day −1 (admission) to Day 7 (approximately 144 h after study drug administration), as well as 4 additional ambulatory visits in the mornings of Days 8, 10, 12 and 14. A follow-up visit was planned for Day 22 (± 2 days).
Secondary objectives included determining the plasma PK profiles of ANF-RHO at ascending single dose in healthy volunteers in comparison to pegfilgrastim, and the effect on ANC and CD34+ cells in increasing doses of ANF-RHO in healthy volunteers in comparison to placebo and the standard of care dose of pegfilgrastim. Additional secondary objectives aimed to assess the immunogenicity potential of ANF-RHO by measuring antibodies and neutralizing antibodies to ANF-RHO following a single sc dose, as well as the occurrence and severity of bone pain in healthy volunteers in comparison to placebo and the standard of care dose of pegfilgrastim.
Study assessments
The primary endpoint in this study was safety of treatment as determined by changes in vital, cardiographic, biochemical, hematological, and urinalytical measures, as well as reported increases in bone pain and other reported AEs. AEs were recorded from admission until completion of the follow-up visit. Any clinically significant observations in results of clinical laboratory, vital signs, 12-lead ECG, cardiac telemetry, physical examination or local tolerability were recorded as AEs. A treatment-emergent AE (TEAE) was defined as any event not present prior to administration of the study drug or any event already present that worsened in either severity or frequency following exposure to the study drug. Secondary PK endpoints included maximum drug concentration (Cmax), time to maximum drug concentration (Tmax), extent of exposure (last measurable exposure) (AUC0-t) and time zero to infinity (AUC0-inf), and terminal half-life (t1/2). The secondary PD endpoint was the change in the CD34+ cell count by treatment group over time. Immunogenicity, defined as the proportion of subjects in each treatment group with antibodies and neutralizing antibodies at Day 14 and Day 22 compared to Baseline, was an additional secondary endpoint. The change in occurrence and severity of bone pain scores by treatment group daily compared to Baseline was investigated as a secondary endpoint as well.
Statistical analysis
This was an ascending single dose trial designed to determine the recommended Phase II dose. Safety analysis was based on all patients who were enrolled into the study and received at least 1 dose of study drug. In view of the explorative character of this study, no formal statistical analysis was performed. Additionally, no prospective calculations of statistical power were made for this preliminary study. All safety and PD parameters are indicated using descriptive statistics. All relevant PK parameters are also indicated by descriptive statistics for number of subjects, mean, standard deviation (SD), minimum, median, maximum, geometric mean, and coefficient of variation (CV%).
Results
Patient disposition and characteristics
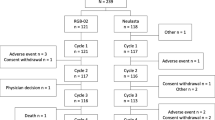
A total of 209 subjects were screened and 76 of these subjects were included and dosed. One subject withdrew consent on Day 8, and 75 subjects completed the study as per protocol. The disposition of the subjects is presented in Table 1. Characteristics of the 76 healthy volunteers enrolled on study are shown in Table 2. The mean patient age ranged between 24 and 34 years among the pegfilgrastim, ANF-RHO, and placebo groups; the mean BMI ranged between 21.5 kg/m2 and 25.2 kg/m2. A total of 35 female and 41 male subjects were enrolled in the study. Among the 76 subjects, 71 were white (93%), 2 were black (3%), 1 was Asian (1%), 1 was mixed Asian and white (1%) and 1 was of mixed race (1%). None of the subjects was of Hispanic/Latino ethnicity.
Safety evaluation
Twenty-two AEs were reported before administration of the study drug. Except for 2 TEAEs of moderate intensity, these were all of mild intensity, and did not preclude randomization of the subjects. There were no deaths, serious AEs (SAEs) or withdrawals due to AEs during this study.
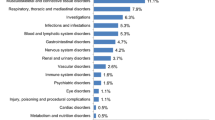
During the study, a total of 354 TEAEs were reported by 71 (93%) of the subjects. The majority of the TEAEs were of mild intensity (308 events [87%]), whereas 46 (13%) TEAEs were of moderate intensity. Most TEAEs of moderate intensity were reported in the 15 μg/kg and 50 μg/kg ANF-RHO dose groups and in the pegfilgrastim group. There were no TEAEs of severe intensity in any of the subjects. All TEAEs were transient and had recovered or were recovering at the time of follow-up. The percentage of subjects reporting at least one TEAE was similar for the ANF-RHO (ranging between 88% and 100%), pegfilgrastim (100%), and placebo group (92%). However, the number of TEAEs was relatively higher in the ANF-RHO and pegfilgrastim subjects compared to the placebo subjects. Within the ANF-RHO dosing groups, the number of TEAEs was higher in the higher dose groups but without a clear relation to dose (51 events in the 15 μg/kg dose group, 36 events in the 20 μg/kg [high] dose group, 38 events in the 30 μg/kg dose group and 58 events in the 50 μg/kg dose group). The number of TEAEs in the pegfilgrastim group was similar to the number of TEAEs reported in the 15 μg/kg ANF-RHO dose and 50 μg/kg ANF-RHO dose group, but higher compared to the other ANF-RHO dose groups (5 μg/kg, 10 μg/kg, 20 μg/kg [low], 30 μg/kg, and 20 μg/kg [high]).
Drug-related TEAEs are shown in Table 3. Sixty-eight of the 76 (89%) subjects reported at least one TEAE that was considered possibly or probably related to the study drug. In total, 285 of the 354 TEAEs (81%) were considered possibly or probably to be related to the study drug (ANF-RHO, pegfilgrastim or placebo). The profile of the most frequently reported related TEAEs was similar to the TEAE profile overall. Several TEAEs were treated with concomitant medication, as allowed by protocol. Paracetamol was sufficient for the treatment of back pain or other musculoskeletal pain, except for one subject who was treated with ibuprofen to treat shoulder pain. Concomitant medication was mostly given to subjects in the 50 μg/kg ANF-RHO group and pegfilgrastim group. Overall, treatment with one single sc dose of up to 50 μg/kg ANF Rho was well to moderately well tolerated in subjects. When compared to pegfilgrastim, ANF-RHO appeared to be equally well (50 μg/kg dose group) or better (5 to 30 μg/kg dose groups) tolerated than the reference pegfilgrastim.
Based on the mode of action of pegfilgrastim and as described in the prescribing information for pegfilgrastim [6], the observed AE profile with musculoskeletal and connective tissue disorders was consistent with the safety profile anticipated for both pegfilgrastim and ANF-RHO. However, some minor differences were observed between pegfilgrastim and ANF-RHO groups. The events back pain, chest pain, and headache were reported more frequently in the pegfilgrastim reference group compared to the ANF-RHO group. TEAEs related to bone pain (including pain in arms and shoulders, back, chest, legs, hips and pelvis, skull, and other muscle and skeletal pain) were captured by a pain visual analogue score (VAS) assessment.
There were no clinically significant findings with respect to clinical laboratory, vital signs, ECG, Holter monitoring, physical examination, local tolerability, and bone pain. Although injection site reaction (ISR) and/or VAS scores ≥1 mm were most frequently observed in subjects treated with ANF-RHO, these findings were of short duration (disappeared within 3 to 24 h) and were of mild intensity. Overall, the local tolerability of ANF-RHO as well as pegfilgrastim was considered good. Of the 76 subjects, one subject was found positive for ADA against ANF-RHO post-dose.
Concentration data of PEGylated recombinant human G-CSF (PEG-rhG-CSF) in plasma
Following single sc dosing with ANF-RHO, on average, PEG-rhG-CSF appeared in plasma within 2 to 4 h post-dose, except for the 5 μg/kg dose group for which mean concentrations could be calculated from 36 h post-dose onwards. Maximum mean PEG-rhG-CSF plasma concentrations were observed at 36 h post-dose across all ANF-RHO dose groups. In all ANF-RHO dose groups, an initial smaller peak was observed between 12 and 72 h post-dose. The plasma concentrations increased with dose but with minor differences between the mean plasma concentrations of the 10, 15 and 20 μg/kg (low) dose groups. The rhG-CSF plasma concentrations were higher in the 20 μg/kg (high) dose group compared to the 20 μg/kg (low) dose group [Fig. 1]. In subjects who received ANF-RHO, mean rhG-CSF concentrations could be determined up to 96 h (5 μg/kg), 168 h (20 μg/kg [low]), 216 h (15 μg/kg), 264 h (10 μg/kg) and 312 h (20 μg/kg [high], 30 μg/kg and 50 μg/kg) post-dose. The combined individual plasma time profiles show irregular patterns with multiple peaks in the majority of the subjects. In addition, the rhG-CSF plasma concentrations varied strongly among the subjects within the ANF-RHO dose groups. After dosing with pegfilgrastim, mean rhG-CSF plasma concentrations appeared at 1 h post-dose, which was 1 to 3 h earlier than in the ANF-RHO group. Also, mean rhG-CSF plasma concentrations increased more rapidly compared to dosing with ANF-RHO with a maximum mean hG-CSF concentration at 16 h post-dose. After reaching a peak plasma concentration, the rhG-CSF plasma concentrations decreased more rapidly after treatment with pegfilgrastim compared to ANF-RHO (with the exception of treatment with 5 μg/kg ANF-RHO) with a mean rhG-CSF measurable up to 144 h post-dose.
ANF-RHO administration resulted in a dose-dependent increase in hG-CSF plasma concentrations. The analysis of pegfilgrastim in plasma samples was performed by Prolong using a validated Enzyme Linked Immunosorbent Assay (ELISA). All study samples were analyzed with analytical runs that complied with the acceptance ranges for the quality control samples
Pharmacokinetic parameters of rhG-CSF in plasma
Summary statistics of pharmacokinetic parameters for rhG-CSF in plasma are shown in Table 4. After treatment with single sc doses of ANF-RHO, median tmax of rhG-CSF was independent of dose and occurred at 36 h across all ANF-RHO dose levels tested. After treatment with the reference pegfilgrastim, tmax occurred earlier compared to the ANF-RHO group with a median tmax of 16 h. Single sc administration of ANF-RHO resulted in a more or less dose-dependent but not dose-linear increase in Cmax, AUC0-t and AUC0-inf for rhG-CSF. With respect to the difference in formulation strength (20 μg/kg [low] versus 20 μg/kg [high]), Cmax and AUC0-t and AUC0-inf were respectively 1.5-fold, 1.5-fold and 1.2-fold higher for the 20 μg/kg (high) compared to the 20 μg/kg (low) ANF-RHO group. After treatment with 50 μg/kg ANF-RHO (highest ANF-RHO dose level tested), the mean Cmax of hG-CSF was approximately 1.8-fold lower, whereas the mean AUC0-t and AUC0-inf of hG-CSF were approximately 1.5-fold higher compared to treatment with 6 mg pegfilgrastim. The higher AUC for the 50 μg/kg ANF-RHO was mainly due to the longer half-life observed for ANF-RHO compared to pegfilgrastim. The mean half-life of hG-CSF was independent of the ANF-RHO dose and was longer (mean t1/2 ranging between 38.5 to 51.0 h) after treatment with ANF-RHO compared to treatment with pegfilgrastim (28.0 h). The mean half-life of the 5 μg/kg dose group could not be calculated as there was a limited number of time-points at which hG-CSF concentrations were observed.
Absolute neutrophil counts (ANC)
After single sc dosing with ANF-RHO the geometric mean ANC started to increase compared to placebo and baseline levels within 4 to 6 h post-dose across all dose groups in a dose-related but not dose linear manner. The increase was rapid over the first 12 h post-dose and increased more gradually thereafter. All ANF-RHO dose groups tested showed a minor decrease in ANC around Day 3. In addition, the 10 μg/kg dose group showed a minor decrease in ANC between 1 and 2 h post-dose before the ANC started to increase. The ANC further increased in a more gradual manner from Day 3 onwards. A maximum mean ANC was reached between Day 6 and Day 10 across the dose levels tested, with maximum geometric mean ANC values ranging between 8.6 × 109/L (5 μg/kg) and 45 × 109/L (50 μg/kg). The ANC values started to gradually decrease thereafter and had returned to baseline on Day 14 at the 5 to 15 μg/kg ANF-RHO dose levels, and were decreasing but still above baseline levels at the higher dose levels (20 μg to 50 μg/kg). With respect to the difference in formulation strength (20 μg/kg [low] versus 20 μg/kg [high]), the ANC was approximately 1.8-fold higher for the 20 μg/kg (high) compared to the 20 μg/kg (low) ANF-RHO group [Figs. 2 and 3]. The mean ANC in the pegfilgrastim group showed a minor decrease in ANC between 0.5 and 3 h post-dose before starting to increase. Thereafter, the ANC increased rapidly. A plateau phase was observed between Day 2 and Day 5, with a transient decrease in ANC on Day 3, as also observed for the ANF-RHO groups. A maximum mean ANC of 21.5 × 109/L was reached on Day 4, which was 2 to 6 days earlier compared to the ANF-RHO levels of ≥10 μg/kg. The ANC values were back to baseline on approximately Day 10. The maximum mean ANC observed for the pegfilgrastim group (21.5 × 109/L) was closest to the 20 μg/kg (high) ANF-RHO dose group (27.1 × 109/L) and was approximately 2-fold lower compared to 50 μg/kg ANF-RHO dose group (43.0 × 109/L). In addition, the increase in ANC was more sustained in ANF-RHO groups across all dose levels compared to the pegfilgrastim reference group [Figs. 2 and 3]. Individual ANC and CD34+ counts-time profiles showed irregular patterns (large day-to-day variation) in the cohorts for which the low strength ANF-RHO dose formulation was used (5 μg/kg, 10 μg/kg, 15 μg/kg and 20 μg/kg [low]) and in the pegfilgrastim reference group. Clearly more regular individual ANC and CD34+ counts-time profiles were observed in the cohorts for which the high strength ANF-RHO dose formulation was used (20 μg/kg [high], 30 μg/kg and 50 μg/kg ANF-RHO dose groups). In addition, the individual profiles in the 20 μg/kg (high), 30 μg/kg and 50 μg/kg ANF-RHO showed less inter-individual variation among the subjects.
Administration of ANF-RHO produces a dose-related increase in absolute neutrophil counts. Neutrophil count was determined via white blood cell differentials. The pre-dose ANC counts were used as baseline. In all ANF-RHO dose groups, a gradual increase in ANC was noted around Day 3 after a single sc dosing. The maximum mean ANC was reached between Day 6 and Day 10 across dose levels
Geometric mean absolute neutrophil counts-time profiles. Neutrophil count was determined via white blood cell differentials. The pre-dose ANC counts were used as baseline. Following with ANF-RHO, mean ANC levels increased from baseline, and to a greater degree than in the placebo group, within 4 to 6 h post-dose across all dose groups
CD34+ cell counts
After single sc dosing with ANF-RHO, the mean number of CD34+ cells increased rapidly starting from Day 3 onwards compared to placebo and baseline levels. A maximum geometric mean number of CD34+ cells was reached on Day 7 across all ANF-RHO dose groups. The maximum geometric mean CD34+ cell counts ranged between 10.74 (5 μg/kg) and 71.4 (50 μg/kg) cells/μL. The number of CD34+ cells increased in a dose-related manner. However, the number of CD34+ cells was similar for the 10 and 15 μg/kg dose groups and were in both 10 and 15 μg/kg groups higher compared to the 20 μg/kg (low) group. With respect to the difference in formulation strength (20 μg/kg [low] versus 20 μg/kg [high]), the number of CD34+ cells was approximately 2-fold higher for the 20 μg/kg (high) compared to the 20 μg/kg (low) ANF-RHO group. After reaching a maximum on Day 7, the number of CD34+ cells decreased rapidly and were close to baseline level between Day 12 and Day 14 (Fig. 4). After single sc dosing with pegfilgrastim, the number of CD34+ cells increased more rapidly compared to the ANF-RHO dose groups. A maximum geometric mean number of CD34+ cells (66.98 cells/μL) was reached on Day 5, which was 2 days earlier compared to the ANF-RHO groups. The maximum number of CD34+ cells was similar for the 6 mg pegfilgrastim group and the 30 μg/kg ANF-RHO dose group [Fig. 4].
Geometric mean CD34+ cell counts-time profiles. The pre-dose CD34+ counts were used as baseline. After a single sc dosing with ANF-RHO, the mean number of CD34+ cells increased rapidly starting on Day 3 as compared to baseline levels and to levels observed for placebo-treated subjects. The maximum mean number of CD34+ cells was seen on Day 7 across all ANF-RHO dose groups. For those receiving a single sc dosing of pegfilgrastim, the maximum number of CD34+ cells was seen two days earlier, although the maximum number of CD34+ cells was similar for the pegfilgrastim group and the 30 μg/kg ANF-RHO dose group
Discussion
No safety concerns were raised during this study. All TEAEs were transient and had recovered or were recovering at the time of last follow-up. The percentage of subjects reporting TEAEs was similar for the ANF-RHO, pegfilgrastim, and placebo groups. However, the number of TEAEs was higher in the higher ANF-RHO dose groups and pegfilgrastim reference group compared to the lower ANF-RHO dose groups and placebo without a clear relationship to dose. The highest number of TEAEs was reported in the 50 μg/kg ANF-RHO (highest dose tested) and pegfilgrastim reference group. The most frequently reported TEAEs by system organ class were Musculoskeletal and Connective Tissue Disorders followed by General Disorders and Nervous System Disorders. The TEAE profile was similar for both ANF-RHO and pegfilgrastim groups, except for the Nervous System Disorders (mainly headache) which was clearly more frequently reported in subjects who received pegfilgrastim. The TEAEs in this study were expected based on clinical data of pegfilgrastim already registered for clinical use and based on the mode of action of pegfilgrastim.
The local tolerability of ANF-RHO was good and was similar to pegfilgrastim. No relationship between dose and ISR and/or VAS score was observed. However, most findings with respect to IRS and VAS score were observed in the ANF-RHO receiving the higher dose volumes. Also subjects receiving 20 μg/kg (low) showed slightly more TEAEs related to ISR compared to the 20 μg/kg (high) ANF-RHO groups. Overall, these might suggest a relation between the injection volume and the local tolerability. Whilst most ISR and VAS score of ≥1 mm occurred in subjects who received ANF-RHO, these observations were of short duration (disappeared within 3 to 24 h) and were of mild intensity.
Apart from 2 placebo subjects, bone pain was only reported in subjects who received ANF-RHO or pegfilgrastim. In the ANF-RHO and pegfilgrastim groups, bone pain was most frequently reported in the back, legs, hips and pelvis. In addition, chest pain was reported in subjects who received pegfilgrastim whereas none of the ANF-RHO subjects reported chest pain. The highest VAS scores for bone pain were observed in the 50 μg/kg and pegfilgrastim groups around Day 3. Bone pain was more sustained in the ANF-RHO group compared to the pegfilgrastim reference group.
After sc single dosing with ANF-RHO (all dose levels) and pegfilgrastim, median tmax of hG-CSF occurred at 36 h and 16 h, respectively. The half-life of hG-CSF was independent of dose and longer after treatment with ANF-RHO compared to treatment with pegfilgrastim. After dosing with ANF-RHO, Cmax, AUC0-t, and AUC0-inf values for hG-CSF increased with increasing dose but without a dose-linear relationship. Although the Cmax of hG-CSF was higher in the pegfilgrastim group than in the 50 μg/kg ANF-RHO dose group, the AUC0-t and AUC0-inf values of hG-CSF were approximately 1.5-fold higher after treatment with 50 μg/kg ANF-RHO than after treatment with pegfilgrastim. The higher exposure to hG-CSF after treatment with ANF-RHO was consistent with the longer half-life of hG-CSF after treatment with ANF-RHO compared to pegfilgrastim.
With respect to the difference in formulation strength (20 μg/kg [low] versus 20 μg/kg [high]), after treatment with the high strength formulation (20 μg/kg [high]) the PK profile showed a higher Cmax and higher AUC values compared to the low strength formulation (20 μg/kg [low]). The potential loss of protein during preparation of the injection solution as well as difference in absorption at the injection site might contribute to this difference in PK profile.
After sc dosing with ANF-RHO, the ANC values appeared to increase with increasing dose without a dose-linear relationship. Of note, the ANC showed a minor decrease in ANC at approximately 1 to 2 h after treatment with pegfilgrastim and 10 μg/kg ANF-RHO, which was not observed in any of the other ANF-RHO dose groups. A decrease in ANC was also observed after treatment with pegfilgrastim. This decrease in ANC after treatment with pegfilgrastim has been described in literature [6]. The number of ANC increased more gradually compared to treatment with the reference pegfilgrastim. In addition, the increase of ANC was more sustained in the ANF-RHO groups compared to the pegfilgrastim reference group. The maximum geometric mean number of ANC was 2-fold higher in the 50 μg/kg ANF-RHO dose group (highest ANF-RHO dose level tested) compared to the 6 mg pegfilgrastim dose group.
Following ANF-RHO sc dosing, the number of CD34+ cells increased with increasing dose without a dose-linear relationship. The increase in the number of CD34+ cells was more gradual after treatment with ANF-RHO compared to treatment with pegfilgrastim. The maximum CD34+ count was reached 2 days later after treatment with ANF-RHO compared to treatment with pegfilgrastim.
The inter-subject as well as the intra-subject PD variability was less in dose groups treated with the high strength formulation (5 mg/mL; 20 μg/kg [high], 30 μg/kg and 50 μg/kg) compared to the low strength formulation dose levels (2 mg/mL; 5 μg/kg, 10 μg/kg, 15 μg/kg and 20 μg/kg [low]).
From the ANF-RHO dose levels tested, the 20 μg/kg (high) ANF-RHO dose group showed a PD effect (ANC and CD34+) that was most comparable to a dose of 6 mg of the reference pegfilgrastim, whereas it appeared that the 20 μg/kg (high) ANF-RHO dose level was better tolerated in healthy volunteers.
In conclusion, the results from this Phase I study suggest that ANF-RHO can be provided less frequently at a lower dose and with fewer side effects than the reference pegfilgrastim. Phase II trials of ANF are planned to begin shortly in patients with chemotherapy-induced neutropenia and chronic idiopathic neutropenia.
References
Bennett CL, Djulbegovic B, Norris LB, Armitage JO (2013) Colony-stimulating factors for febrile neutropenia during cancer therapy. N Engl J Med 368(12):1131–1139
Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH (2006) Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer 106(10):2258–2266
Aapro MS, Bohlius J, Cameron DA et al (2011) 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer 47(1):8–32
Kaushansky K (2006) Lineage-specific hematopoietic growth factors. N Engl J Med 354(19):2034–2045
Brugger W, Bacon P, Lawrinson S, Romieu G (2009) Neutrophil recovery in elderly breast cancer patients receiving adjuvant anthracycline-containing chemotherapy with pegfilgrastim support. Crit Rev Oncol Hematol 72(3):265–269
Neulasta® Full Prescribing Information (2016). Amgen, Inc., Thousand Oaks
Kirshner JJ, Heckler CE, Janelsins MC, Dakhil SR, Hopkins JO, Coles C, Morrow GR (2012) Prevention of Pegfilgrastim-induced bone pain: a phase III double-blind placebo-controlled randomized clinical trial of the University of Rochester Cancer Center Clinical Community Oncology Program Research Base. J Clin Oncol. doi:10.1200/JCO.2011.37.8364
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All financial disclosures and conflict of interest statements have been satisfied.
Funding
All authors are employees of Prolong Pharmaceuticals.
Ethical approval
This study was performed in accordance with the ethical principles that originate in the Declaration of Helsinki, comply with the Dutch ‘Wet Medisch-Wetenschappelijk Onderzoek met Mensen’ (WMO) (Medical Research Involving Human Subjects Act), and are consistent with the International Conference on Harmonization/Good Clinical Practice and applicable regulatory requirements. The protocol was institutional review board-approved. The data were analyzed by PRA Health Sciences, and all authors had access to the primary data.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Misra, H., Berryman, J., Jubin, R. et al. A Phase I study to determine safety, pharmacokinetics, and pharmacodynamics of ANF-RHO™, a novel PEGylated granulocyte colony-stimulating factor, in healthy volunteers. Invest New Drugs 36, 75–84 (2018). https://doi.org/10.1007/s10637-017-0490-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-017-0490-8