Summary
Objective This study aimed to compare the safety and efficacy of the combination therapy of gemcitabine and S-1 (GS) versus gemcitabine and cisplatin (GC) in patients with advanced biliary tract cancer (BTC). Methods In this multicenter retrospective cohort study, a total of 212 patients with advanced BTC receiving GS (n = 125) or GC (n = 87) between July 2006 and August 2015 were analyzed. The primary endpoint was overall survival (OS). The secondary endpoints were progression-free survival (PFS), objective tumor response, and safety. Results Patient characteristics were well balanced between the two groups, except for tumor size (the baseline sum of the largest diameter of the tumor: 6.3 cm in the GS group vs. 8.6 cm in the GC group, p = 0.01). Although the response rate was higher in the GS group than in the GC group (28.8% vs. 10.3%, p = 0.01), the median PFS and OS were comparable between the two groups (PFS of 5.6 vs. 7.6 months, p = 0.74; OS of 12.4 vs. 9.2 months, p = 0.20, respectively). Stomatitis and skin rash were more frequently observed in the GS group, whereas anemia, thrombocytopenia, nausea, and renal toxicity were more commonly observed in the GC group. Conclusion This study demonstrates that GS and GC are similar with regard to their safety and efficacy in patients with advanced BTC. GS could serve as an alternative treatment for advanced BTC as a first-line chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biliary tract cancer (BTC) is a highly lethal malignancy with a 5-year survival rate of <20%; the number of patients with this condition has been increasing in Japan [1]. While only surgery can provide a cure, BTC is often diagnosed as an advanced disease. Moreover, tumor recurrence frequently develops, even after curative surgery. Therefore, palliative chemotherapy plays a crucial role in improving the prognosis of patients with advanced and recurrent BTC.
The current standard of care for patients with advanced BTC is a combination of gemcitabine and cisplatin (GC) chemotherapy; this is based on the results from two randomized controlled trials in which GC resulted in better survival without substantial toxicity compared with treatment with gemcitabine alone [2, 3]. However, cisplatin-containing treatments have several limitations. First, cisplatin is associated with several cumulative toxicities, including dose-dependent nephrotoxicity and neurotoxicity, which may reduce the opportunities for second-line treatment after disease progression as well as long-term treatment. In addition, cisplatin-containing treatments require vigorous hydration or diuresis to minimize the risk and severity of acute nephrotoxicity.
S-1, an oral fluoropyrimidine derivative, has been widely used as one of the key drugs for the treatment of BTC, especially in Asian countries, because of its high anti-tumor activity and its oral administration route [4–6]. In particular, gemcitabine and S-1 (GS) combination therapy has shown promising results in several phase II trials [7–11]. Therefore, considering its long-term safety and convenient route of administration, GS therapy is a candidate for the standard treatment of BTC. However, no study to date has compared the safety and efficacy of GS versus GC in patients with advanced BTC.
Patients and methods
Patients
Between July 2006 and August 2015, consecutive patients receiving GS or GC as a first-line chemotherapy for advanced BTC at the University of Tokyo Hospital and five affiliated hospitals were retrospectively studied. BTC diagnosis was based on pathological or typical radiological findings. At least 6 months of follow-up were required in patients without pathological evidence to confirm that their clinical course was consistent with the malignancy. Clinical outcomes were retrieved from our database and medical records. This study was approved by the local ethics committee of each hospital.
Treatment
GS and GC combination therapies have been described previously [2, 3, 7, 8]. In the GS group, gemcitabine was administered at a dose of 1000 mg/m2 on days 1 and 15, and 80 mg/m2 S-1 was administered orally every 4 weeks on days 1 through 14 [7, 8]. In the GC group, gemcitabine and cisplatin were administered every 3 weeks at doses of 1000 mg/m2 and 25 mg/m2, respectively, on days 1 and 8 [2, 3]. In most patients, the treatment selection was on a chronological basis because S-1 and GC were approved by Japanese medical insurance in 2008 and 2011, respectively. In the remaining patients, treatment was chosen according to the clinical trial protocol [7, 8] or at the discretion of the attending physician.
Treatment was temporarily suspended upon the development of grade 3/4 hematological or grade 2 or higher non-hematological adverse events, graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 3.0 or 4.0. After recovery to grade 2 or lower in hematological toxicities or grade 1 or lower in non-hematological toxicities, treatment was restarted at reduced doses. Dose re-escalation was not applied in this setting. Treatment continued until the disease progressed, until unacceptable toxicity was reached, or until the patient refused treatment.
Response and toxicity assessment
The primary endpoint was overall survival (OS). The secondary endpoints included progression-free survival (PFS), the objective tumor response, and safety. OS was defined as the time from treatment initiation to the final follow-up or death from any cause. PFS was defined as the time from treatment initiation to disease progression or death from any cause. The follow-up time was defined as the time from treatment discontinuation to the final follow-up or death from any cause. The objective tumour response was assessed via computed tomography (CT) using Response Evaluation Criteria in Solid Tumors (RECIST) version 1.0 or 1.1 [12, 13]. The evaluation was repeated at least after every two or three courses or more frequently in patients with clinically suspected progression. The baseline sum of the largest diameter (BSLD) was defined as a sum of the longest diameter for all target lesions identified at baseline, which were according to RECIST version 1.1 [13]. Adverse events were evaluated and graded according to CTCAE version 3.0 or 4.0. Carcinoembryonic antigen (CEA) and carbohydrate antigen 19–9 (CA19–9) were measured on day 1 of each cycle.
Statistical analysis
Fisher’s exact test was used to compare patient characteristics and tumor responses between the two groups. The Mann–Whitney U test was used to compare quantitative variables where appropriate. OS and PFS were calculated using the Kaplan–Meier method and compared using the log-rank test. Hazard ratios (HRs) with 95% confidence intervals (CIs) for OS and PFS were estimated using a Cox proportional hazards model. Exploratory analyses were performed to identify subgroups that may benefit from each treatment. A P value <0.05 was considered to indicate statistical significance. JMP 11.0 (SAS Institute Inc., Cary, NC, USA) was used for all statistical analyses. The final analysis was based on the follow-up information, which was received until October 2016.
Results
Patient characteristics
From July 2006 to August 2015, a total of 375 patients were diagnosed with advanced or recurrent BTC, of whom 212 patients were included in this analysis (125 in the GS group and 87 in the GC group). The remaining 163 patients were excluded based on the following criteria: patients receiving gemcitabine monotherapy (n = 68), S-1 monotherapy (n = 11), GS plus leucovorin combination therapy (n = 20), radiation therapy (n = 29), or best supportive care alone (n = 35) (Fig. 1). Among these 212 patients, 42 received GS in clinical trials [7, 8]. Patient characteristics are summarized in Table 1. There were no significant differences between the two groups except for the BSLD (6.3 cm in the GS group and 8.6 cm in the GC group, p = 0.01). At the time of analysis, four patients in the GS group were still alive with a median follow-up time of 5.1 (range, 0–34.4) months, and two patients were still receiving GS therapy. Two patients in the GC group were still alive and receiving GC therapy with a median follow-up time of 4.6 (range, 0–33.9) months.
Overall survival, progression-free survival, and tumor response
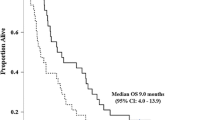
A median of 4 (range, 1–68) cycles per patient in the GS group and a median of 5 (range, 1–20) cycles per patient in the GC group were delivered. The median OS was 12.4 (95% CI, 9.0–15.8) months in the GS group and 9.2 (95% CI, 7.6–13.7) months in the GC group. The HR of GS to GC for OS was 0.81 (95% CI, 0.59–1.12; p = 0.20) (Fig. 2a). The median PFS was 5.6 months (95% CI, 7.6–13.7) in the GS group and 6.4 months (95% CI, 7.6–13.7) in the GC group. The HR of GS to GC for PFS was 1.06 (95% CI, 0.73–1.52; p = 0.74) (Fig. 2b).
Kaplan-Meier estimates for overall survival and progression-free survival. Kaplan–Meier estimates of overall survival (Fig. 2a) and progression-free survival (Fig. 2b) in patients with advanced biliary tract cancer receiving gemcitabine and S-1 (black line) versus gemcitabine and cisplatin (dot-line)
The response rate (RR) was significantly higher in the GS group than in the GC group (29% vs. 14%, p = 0.01). However, the disease control rate (DCR) was similar between the two groups (70% vs. 77%, respectively, p = 0.27). A complete response (CR) was achieved in 4 patients, all of whom were in the GS group. Among these 4 patients who achieved CR, 2 discontinued GS after 35 and 68 cycles of treatment and were still alive without tumor progression, with a median follow-up time of 49 months. The remaining 2 patients were still receiving GS therapy at the time of the last follow-up. Conversion surgery was performed in 3 patients, including 2 (2%) in the GS group and 1 (1%) in the GC group.
Adverse events and reasons for treatment discontinuation
Table 2 summarizes the incidences of major adverse events during this study. No treatment-related death occurred during the treatment period in either group. In regard to hematological adverse events, grade 3/4 anemia and thrombocytopenia occurred less frequently in the GS group than in the GC group (anemia 18% vs. 31% and thrombocytopenia 6% vs. 15%, respectively). In regard to non-hematological adverse events, all grades of stomatitis, diarrhea, pigmentation, and skin rash were significantly more frequent in the GS group, whereas nausea, anorexia, renal dysfunction, and peripheral neuropathy were more common in the GC group.
The major reasons for treatment discontinuation were disease progression (80% and 60% of patients in the GS and GC groups, respectively, p < 0.01) and adverse events (10% and 21% of patients in the GS and GC groups, respectively, p = 0.04). The most common adverse events that led to the cessation of treatment were skin rash (n = 5) in the GS group and renal dysfunction (n = 7) in the GC group. Details are shown in Table 3.
Second-line chemotherapy
A second-line chemotherapy was similarly introduced after discontinuation of the GS or GC regimen (63% in the GS group and 59% in the GC group, p = 0.50). In the GS group, second-line chemotherapy included GC (n = 56), gemcitabine monotherapy (n = 12), S-1 monotherapy (n = 1), and other regimens (n = 5). In the GC group, GS (n = 17), gemcitabine monotherapy (n = 8), and S-1 monotherapy (n = 25) were given as second-line chemotherapies. The chance of treatment cross-over, meaning GC after GS or GS after GC, was significantly higher in the GS group than in the GC group (45% vs. 20%, respectively, p < 0.01).
Subgroup analyses
A forest plot of OS by each subgroup is shown in Fig. 3. The median OS of the GS group was similar to that of the GC group in most subgroups. For example, among patients with gallbladder cancer, the median OS was similar between the two groups (9.5 months in the GS vs. 7.6 months in the GC group, p = 0.33). However, in patients with recurrent disease, the median OS was significantly longer in the GS group than in the GC group (16.6 vs. 8.7 months, respectively, p < 0.01).
The median PFS in patients with gallbladder cancer was similar between the two groups [4.6 (GS group) vs. 4.5 months (GC group), p = 0.92]. Conversely, in patients with recurrent disease, the median PFS was longer in the GS group than in the GC group (10.7 vs. 4.1 months, respectively, p = 0.07).
Discussion
Our multicenter retrospective study demonstrates similar OS in patients receiving GS and GC for advanced BTC. Although several studies have reported GS as a promising regimen in patients with advanced BTC, no randomized study has directly compared GS to GC, the current standard treatment. Given the comparable efficacy of GS and GC in our study cohort, GS can serve as an alternative treatment for advanced BTC as a first-line chemotherapy and may be selected according to specific adverse events.
Our study revealed a similar efficacy of GS and GC in patients with advanced BTC. A recent meta-analysis reported that RR, DCR, and median PFS were correlated with the median OS in patients with advanced BTC receiving chemotherapy [14]. In the present study, however, the median OS was similar between the two groups despite the higher RR in the GS group (RR of 29% vs. 14%, respectively, p = 0.01). These outcomes are consistent with those from several previous clinical trials [2, 3, 8–11]. The median OS of the GS group was more than 3 months longer than that of the GC group, although the difference was not statistically significant. This could be explained by the imbalance of the baseline characteristics and the impact of second-line treatment. We previously reported that BSLD, which represents tumor burden, was associated with OS in advanced BTC patients receiving chemotherapy [15]. Therefore, a smaller BSLD in the GS group may result in a longer OS [16, 17]. In addition, second-line treatment could influence the OS. Combination chemotherapy was often intolerable after GC failure because more than 20% of patients discontinued GC due to adverse events. Although the overall induction rate of second-line chemotherapy was similar between the two groups (63% in the GS group vs. 59% in the GC group, p = 0.50), the cross-over rate (i.e., GC after GS or GS after GC) was higher in the GS group than in the GC group (45% vs. 20%, respectively, p < 0.01), which may prolong the post-progression survival in patients receiving GS.
Because the efficacy was comparable between GS and GC, the safety profile could be informative for treatment selection. Although both GS and GC were generally well tolerated in this study, the major adverse events differed. The major adverse events that led to discontinued treatment were skin rash in the GS group and renal dysfunction in the GC group. Cisplatin-induced nephrotoxicity is dose-dependent and may limit the chance of subsequent treatment as well as long-term GC treatment. Therefore, it is important to identify risk factors for cisplatin-induced nephrotoxicity to avoid subjecting high-risk patients to cisplatin. One study reported that cardiac comorbidities and the use of non-steroidal anti-inflammatory drugs (NSAIDs) were risk factors for cisplatin-induced nephrotoxicity in patients with thoracic malignancy [18]. This can also be applied in patients with BTC, where GS may serve as an alternative treatment option.
Our subgroup analyses showed that the median OS in patients with recurrent disease was longer in the GS group than in the GC group (16.6 vs. 8.7 months, respectively, p < 0.01). We previously reported that treatment outcomes, including efficacy as well as toxicity and dose intensity, were significantly different between initially unresectable and recurrent BTC [19]. This difference may be caused by low tumor burden due to short-interval surveillance after surgery and by some changes in drug metabolism after surgery. However, favorable outcomes of GS in recurrent BTC should be interpreted with caution since our analysis was limited by its retrospective design and the small number of patients with recurrent disease. Only prospective randomized trials stratified for these subgroups can define the benefits of GS in this subgroup.
This study has several limitations. First, this was a retrospective study with different patient characteristics between the GS and GC groups, including the baseline sum of the largest diameter of the tumor and a smaller BSLD in the GS group, which may have overestimated the efficacy of GS. Second, this study had a relatively long-term registration period and the treatment selection was mostly on a chronological basis. The multidisciplinary approach is mandatory in patients with BTC, and the clinical management of patients with BTC is improving in general. Thus, the outcomes of GC may have been overestimated in our analysis. Despite these inherent biases, this study included a large number of patients from five referral hospitals; therefore, the results from this study reflect what is observed in our daily practice and can be easily generalized. A relatively high rate of patients with biliary drainage, which is often necessary for the management of BTC, compared to two previous RCTs suggests the generalisability of our results.
In conclusion, this study demonstrates that GS and GC are similarly safe and effective in patients with advanced BTC. GS can serve as an alternative treatment for advanced BTC as a first-line chemotherapy. A large prospective randomized controlled trials comparing GS with GC are now underway [20].
Abbreviations
- BTC:
-
Biliary tract cancer
- GS:
-
Gemcitabine and S-1 combination therapy
- GC:
-
Gemcitabine and cisplatin combination therapy
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
References
Miyakawa S, Ishihara S, Horiguchi A et al (2009) Biliary tract cancer treatment: 5,584 results from the biliary tract cancer statistics registry from 1998 to 2004 in Japan. J Hepato-Biliary-Pancreat Surg 16:1–7
Valle J, Wasan H, Palmer DH et al (2010) Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 362:1273–1281
Okusaka T, Nakachi K, Fukutomi A et al (2010) Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer 103:469–474
Furuse J, Okusaka T, Boku N et al (2008) S-1 monotherapy as first-line treatment in patients with advanced biliary tract cancer: a multicenter phase II study. Cancer Chemother Pharmacol 62:849–855
Sasaki T, Isayama H, Yashima Y et al (2009) S-1 monotherapy in patients with advanced biliary tract cancer. Oncology 77:71–74
Park I, Lee JL, Ryu MH et al (2009) Efficacy and safety of S-1 monotherapy in patients with advanced biliary tract adenocarcinoma: retrospective analysis of 162 patients. Oncology 76:126–132
Sasaki T, Isayama H, Nakai Y et al (2013) A randomized phase II study of gemcitabine and S-1 combination therapy versus gemcitabine monotherapy for advanced biliary tract cancer. Cancer Chemother Pharmacol 71:973–979
Sasaki T, Isayama H, Nakai Y et al (2010) Multicenter, phase II study of gemcitabine and S-1 combination chemotherapy in patients with advanced biliary tract cancer. Cancer Chemother Pharmacol 65:1101–1107
Morizane C, Okusaka T, Mizusawa J et al (2013) Randomized phase II study of gemcitabine plus S-1 versus S-1 in advanced biliary tract cancer: a Japan clinical oncology group trial (JCOG 0805). Cancer Sci 104:1211–1216
Kanai M, Yoshimura K, Tsumura T et al (2011) A multi-institution phase II study of gemcitabine/S-1 combination chemotherapy for patients with advanced biliary tract cancer. Cancer Chemother Pharmacol 67:1429–1434
Kim HS, Kim HY, Zang DY et al (2015) Phase II study of gemcitabine and S-1 combination chemotherapy in patients with metastatic biliary tract cancer. Cancer Chemother Pharmacol 75:711–718
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Moriwaki T, Yamamoto Y, Gosho M et al (2016) Correlations of survival with progression-free survival, response rate, and disease control rate in advanced biliary tract cancer: a meta-analysis of randomised trials of first-line chemotherapy. Br J Cancer 114:881–888
Sasaki T, Isayama H, Nakai Y et al (2011) Prognostic factors in patients with advanced biliary tract cancer receiving chemotherapy. Cancer Chemother Pharmacol 67:847–853
Lamarca A, Hubner RA, David Ryder W, Valle JW (2014) Second-line chemotherapy in advanced biliary cancer: a systematic review. Ann Oncol 25:2328–2338
Brieau B, Dahan L, De Rycke Y et al (2015) Second-line chemotherapy for advanced biliary tract cancer after failure of the gemcitabine-platinum combination: a large multicenter study by the association des gastro-Enterologues Oncologues. Cancer 121:3290–3297
Sato K, Watanabe S, Ohtsubo A et al (2016) Nephrotoxicity of cisplatin combination chemotherapy in thoracic malignancy patients with CKD risk factors. BMC Cancer 16:222
Sasaki T, Isayama H, Nakai Y et al (2014) Treatment outcomes of chemotherapy between unresectable and recurrent biliary tract cancer. World J Gastroenterol 20:18452–18457
Mizusawa J, Morizane C, Okusaka T et al (2016) Randomized phase III study of gemcitabine plus S-1 versus gemcitabine plus cisplatin in advanced biliary tract cancer: Japan clinical oncology group study (JCOG1113, FUGA-BT). Jpn J Clin Oncol 46:385–388
Acknowledgements
We thank all participating patients and their families, as well as the investigators Drs. Ryunosuke Hakuta, Gyotane Umefune, Tomotaka Saito, Takeo Watanabe, and Kaoru Takagi, and the research coordinators Miyuki Tsuchida and Chiho Takeda (Department of Gastroenterology, the University of Tokyo Hospital).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest related to this article.
Funding
None.
Ethical approval
This study was performed in accordance with the Declaration of Helsinki and was approved by each institutional review board.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Takahara, N., Isayama, H., Nakai, Y. et al. Gemcitabine and S-1 versus gemcitabine and cisplatin treatment in patients with advanced biliary tract cancer: a multicenter retrospective study. Invest New Drugs 35, 269–276 (2017). https://doi.org/10.1007/s10637-017-0430-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-017-0430-7