Summary
Inhibitors of kinesin spindle protein Eg5 are characterized by pronounced antitumor activity. Our group has recently synthesized and screened a library of 1,3,4-thiadiazoline analogues with the pharmacophoric structure of K858, an Eg5 inhibitor. We herein report the effects of K858 on four different breast cancer cell lines: MCF7 (luminal A), BT474 (luminal B), SKBR3 (HER2 like) and MDA-MB231 (basal like). We demonstrated that K858 displayed anti-proliferative activity on every analyzed breast cancer cell line by inducing apoptosis. However, at the same time, we showed that K858 up-regulated survivin, an anti-apoptotic molecule. We then performed a negative regulation of survivin expression, with the utilization of wortmannin, an AKT inhibitor, and obtained a significant increase of K858-dependent apoptosis. These data demonstrate that K858 is a potent inhibitor of replication and induces apoptosis in breast tumor cells, independently from the tumor phenotype. This anti-proliferative response of tumor cells to K858 can be limited by the contemporaneous over-expression of survivin; consequently, the reduction of survivin levels, obtained with AKT inhibitors, can sensitize tumor cells to K858-induced apoptosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mitotic kinesins are important regulators of cancer cell replication and migration [1], since these proteins play an important role in the formation of the bipolar mitotic spindle and in the regulation of cell cycle progression [2]. The nuclear kinesin Eg5 (Kindle Spindle Protein/KSP/KIF11) is over-expressed in many solid tumors and covers an important part in tumorigenesis [3]. Monastrol was the first to be studied among these compounds [4], but also other small similar molecules, capable to arrest cell replication, have been more recently described [5, 6]. These agents cause arrest of mitosis by damaging monopolar spindle and consequently causing cell death [7]; some cells treated with kinesin inhibitors arrest in G1-like phase and die for apoptosis by a specific tetraploidy checkpoint [8]. The negative regulation of Eg5 is capable to arrest the replication of hepatocellular carcinoma [9], glioblastoma [10] and prostate cancer cells [11] in vitro, suggesting that Eg5 inhibition can be a potential target for anti-cancer therapy.
Several compounds that inhibit kinesins have been studied in clinical trials [12, 13], unfortunately with poor benefits, in opposite to successful results obtained in pre-clinical research on neoplastic cells [9–11]. Among Eg5-inhibitors, ispinesib has been shown to share the mode of action with monastrol [14], however its clinical utilization led to conflicting results. A group of 24 patients affected by different solid tumors was treated with ispinesib and docetaxel and 7 patients had a good response with a stable disease (SD) for ≥18 weeks in a phase I study [15]; but, at the same time, no patients with androgen-independent and docetaxel-resistant prostate cancer gave responses to ispinesib monotherapy in a phase II study [16].
Probably, the development of chemoresistance can limit the anti-neoplastic performance of kinesin inhibitors. For this reason, more studies are needed to understand why in vivo results are so disappointing; it should be explored whether a process of chemoresistance is triggered by Eg5-inhibiting compounds, as happens for taxanes. In fact, anti Eg5 agents can be considered novel taxanes, since both molecules target the microtubules of the cytoskeleton and lead to mitotic arrest and apoptosis [17]. Unfortunately, taxanes are characterized by many side effects, such as the neurotoxicity [18, 19], besides the chemoresistance, which determine the limitation of the use of these chemotherapeutics in all cancer patients. Therefore, there is an urgent unmet need to find anti-mitotic molecules that overcome taxane resistance; to this purpose, an inhibitor of Eg5 has demonstrated to overtake drug resistance in two taxane resistant cancer cell lines [20].
Our group has recently synthesized and screened a library of 1,3,4-thiadiazoline analogues, all characterized by the pharmacophoric structure of K858 [21], which is a newly discovered inhibitor of kinesin Eg5, capable to induce the mitotic arrest in several types of cancer cells [22]. In this previous research we screened the anti-proliferative activity of 103 derivatives of K858 on prostate cancer and melanoma cell lines and identified two compounds as the most effective, one corresponding to unmodified K858 and the other corresponding to a derivative where the methyl group in C5 of K858 was substituted with an ethyl group [21]. In the present work, we used the described compound corresponding to unchanged K858 and tested its efficacy in inducing cell death on different phenotypes of breast cancer. In parallel, since we recently demonstrated a significant correlation between resistance to taxane and up-regulation of survivin in the same cells [23], we evaluated the possible role of survivin in the establishment of chemoresistance to K858.
Materials and methods
Cell culture and treatments
The following four human breast cancer cell lines have been utilized: MCF7 (Luminal A: estrogen receptor-positive and progesterone receptor-positive, ErbB2-negative), BT474 (Luminal B: estrogen receptor/progesterone receptor/ErbB2-positive) SKBR3 (HER2 like: estrogen receptor-negative/progesterone receptor-negative, ErbB2-positive) and MDA-MB231 (Basal like: estrogen receptor/progesterone receptor/ErbB2-negative). All cell lines were grown in DMEM medium supplemented with 10 % fetal calf serum (FCS), 2 mM glutamine, 50 U/mL penicillin-streptomycin and starved, when necessary, in DMEM medium with 2 % dialyzed FCS.
K858 was synthesized, characterized and utilized as previously described [21], K858 was solubilized in dimethylsulfoxide (DMSO) at 1 mM stock solution and utilized to final concentration of 10 μM. Wortmannin (Sigma) was solubilized in DMSO at 10 mM stock solution and used 100 nM.
Cytotoxicity assay
To determine cytotoxicity, sulforhodamine B colorimetric assay was performed: 1.5X104 cells were plated on 96 well plates, grown for 24 h (h) and treated with different concentrations of K858 (1 μM, 10 μM, 100 μM) for 24 and 48 h. Cells were then fixed with 50 % trichloroacetic acid for 1 h at 4 °C and stained for 30 min at room temperature (RT) with 0.4 % sulforhodamine B in 1 % acetic acid. Excess dye was removed by washing four times with 1 % acetic acid. Protein-bound dye was dissolved in 10 mM TRIS pH 10, and optical density (OD) was determined at 510 nm using a microplate reader.
Western blot
Cell lysates were obtained scraping the cells in lysis buffer 1 % Triton, 0.1 % SDS, 150 mM NaCl, 50 mM TRIS-HCl pH 7.4, 2 mM EDTA plus protease inhibitor cocktail tablet (Roche Applied Sciences) for 30 min at 4 °C, lysates were then centrifuged at 12,000 rpm for 15 min at 4 °C. Protein concentration was evaluated by Bio-Rad Protein Concentration Assay. Samples of lysate (50-100 μg) were separated by molecular weight on 10 or 12 % SDS-PAGE and then transferred into a nitrocellulose membrane. Blots were blocked for 1 h at RT in 5 % non-fat dry milk and then incubated with primary antibody, washed in TRIS-buffered saline with 0.1 % Tween 20 and then incubated with horseradish peroxidase conjugated anti-mouse or anti-rabbit antibodies (1:5000 diluted) (Sigma-Aldrich). The filters were then developed by enhanced chemiluminescence (Super Signal West Pico Chemiluminescent Substrate, Thermo Scientific) using Kodak X-Omat films. The densitometry quantitation of the bands was performed using Image J software.
The primary antibodies were the following: rabbit anti survivin (1:1000 diluted) (Novus Biological, Littleton, CO, USA); rabbit anti cleaved caspase-8 (1:500 diluted) (Cell Signaling); mouse anti cleaved caspase 9 (1:500 diluted) (Cell Signaling); mouse anti Parp1 (1:500 diluted) (Santa Cruz Biotechnology); mouse anti bcl2 (1:250 diluted); rabbit anti bax (1: 250 diluted) (BD Transduction Laboratories USA); mouse anti-actin (1:1000 diluted) (Sigma Aldrich).
Reverse transcriptase polymerase chain reaction (RT-PCR) assay
Total RNA from the cells was isolated using Trizol reagent (Invitrogen, Carlsband, CA) according to the manufacturer’s instructions; Moloney murine leukemia virus (M-MLV) reverse transcriptase (Biolab) was used to reverse 1 μg of total RNA into cDNA at 42 °C. 5 μg of each cDNA was then subjected to RT-PCR in a buffer containing 25 pmol of upstream and downstream and 1.25 U of Platinum Taq polymerase (Euroclone). The amount of amplified products, expressed in arbitrary optical density units, was normalized with glyceraldehyde-3-phosphate dehydrogenase (GADPH) as housekeeping gene. The amplification reaction was carried out in Piko-Thermal Cycler cyclers (Finnzymes Instrument). The resulting PCR products were separated in 2 % agarose gel and visualized with Gel-Red (Gel Red nucleic acid gel stain, Biotium). The sequences of human gene-specific primers (Sigma-Aldrich) with order of forward and reverse, the conditions of amplification as well as the amplified products size are the following: Gadph: 5-AGATGTTCCAATATGATTCC, 5′-TGGACTCCACGACGTACTCAG; 60 °C; 161 bp.
Bcl-2: GTGGAGGAGCTCTTCAGGGA, AGGCACCCAGGGTGATGCAA; 60 °C; 304 bp.
Bax: GGCCCACCAGCTCTGAGCAGA, GCCACGTGGGCGTCCCAAAGT; 62 °C; 469 bp.
Survivin: CAGATTTGAATCGCGGGACCC, CCAAGTCTGGCTCGTTCTCAG; 60 °C; 206 bp.
Cell apoptosis assay
The cells were treated with 10 μM K858 for 24 h. Both detached and adherent cells were harvested by trypsin-EDTA and washed with cold phosphate buffered saline. The cells were double stained with AnnexinV-APC (allophycocyanin)/7AAD (7-amino-actinomycin) in a calcium binding buffer (BD Biosciences kit) and analyzed by a FACS scan cytofluorimeter (BD Biosciences).
Statistical analysis and graphic programs
All results were analyzed by ANOVA, and the significance was evaluated by the Tukey HSD post hoc test (Honestly Significant Difference). All figures were elaborated with Adobe Photoshop CS5 and all graphs with Graph Pad Prism 5.0.
Results
We first investigated the effects of K858 on cell viability and replication: MCF7, MDA-MB231, BT474 and SKBR3 cell lines were treated for 24 and 48 h with 1 μM, 10 μM, 100 μM K858. At 24 h of treatment, K858 determined a decrease of cell viability in all four cell lines; this decrease was significant at all three used concentrations for three cell lines, while MDA-MB231 had a decrease of cell viability significant at 10 and 100 μM, but not significant at 1 μM (Fig. 1). The described effects on cell viability were similar when the cells were treated with K858 for 48 h, without any significant variation as compared to 24 h of treatment (data not shown). Based on these data, K858 was used for the following experiments at the concentration of 10 μM for 24 h. In order to detect whether the described cell loss was due to apoptosis, K858 treated and untreated cells were evaluated for PARP (poly ADP-ribose polymerase), which is usually cleaved by caspase 3 during the last phases of apoptosis: K858 determined PARP cleavage in all four cell lines, as evidenced by the cleaved band present only in the treated cells by western blot analysis (Fig. 2); this result demonstrated that K858 was able to induce apoptosis. Following, the cleavage of caspase 8 and 9 was studied, since the presence of proteolytic fragments of caspases is an index of their activation and of consequent apoptosis: caspase 9 resulted cleaved in all four K858 treated cell lines, and caspase 8 was cleaved in BT474 and SKBR3 treated cell lines, while it was not expressed by MDA-MB231 and MCF7 cell lines (Fig. 2). These data suggested that MDA-MB231 and MCF7 cells followed an intrinsic pathway of apoptosis, characterized by the cleavage of caspase 9, while BT474 and SKBR3 had activated both intrinsic and extrinsic pathways.
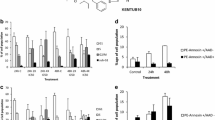
To better analyze the apoptosis determined by K858, the expression of Bax (an apoptosis inducer) and of Bcl2 (an apoptosis inhibitor) was then quantified, since an increase in Bax/Bcl2 ratio is often described during apoptosis. As expected, an increase of Bax/Bcl2 ratio was evident in all four K858 treated cell lines, as evaluated by western blot and subsequent densitometry analysis of the bands (Fig. 3a–b). In parallel, Bax and Bcl2 RNA expression was analyzed by RT-PCR and resulted that K858 enhanced Bax/Bcl2 RNA ratio in all four cell lines (Fig. 3c).
a Western blot of untreated and K858 treated (T) cell lines for Bax, Bcl2 and actin. b Histogram of Bax/Bcl2 protein ratio in untreated (C) and K858 treated (T) cells performed on the densitometry quantifications of Bax and Bcl2 protein bands upon normalization to actin. c Histogram of Bax/Bcl2 RNA ratio in untreated (C) and K858 treated (T) cells, performed on the densitometry quantifications of Bax and Bcl2 RT-PCR RNA bands upon normalization to GAPDH
In order to evaluate whether the cell loss induced by K858 was due also to necrosis, in addition to apoptosis, we double stained untreated and K858 treated cells with APC conjugated annexin V and with 7AAD and analyzed by fluorescence activated cell sorter (FACS): K858 treatment determined a high rate of annexin V staining in all four cell lines, indicative of apoptosis (Fig. 4). As positive control of apoptosis, all four cell lines were treated with staurosporine (an apoptosis inducer), and all staurosporine treated lines resulted highly positive for annexin V expression (data not shown).
Annexin V-APC and 7AAD expression evaluated by FACS. a The upper dot blots represent untreated cells (C) and the lower dot blots K858 treated cells (T). Quadrant location for the representative dot blots: lower left - living cells, lower and upper right - apoptotic cells, upper left - necrotic cells. b Each bar represents the percentage of apoptotic (grey) and necrotic (black) untreated (first column) and K858 treated (second column) cells expressed as mean +/−SD of three different experiments
Finally, we investigated the expression of survivin, which has been recently described as enhanced by monastrol, a kinesin-5 inhibitor molecule [24], in cancer cells. K858 treatment determined an up-regulation of survivin in all four cell lines, as evidenced by western blot, with a statistically significant increase (p = 0,002) (Fig. 5); these data were confirmed by RT-PCR, with an evident enhancement of survivin RNA levels in all four K858 treated cell lines (Fig. 5).
Apparently, this up-regulation of survivin was in contrast with the described apoptosis induced by K858; so, we then investigated whether survivin increased expression could negatively interfere with K858-dependent apoptotic response. Therefore, we inhibited survivin expression by treating MCF7 cells with 100 nM wortmannin, an inhibitor of phosphoinositide 3-kinase AKT (PI3K/AKT), since it is well known that Akt activation is required for survivin expression [25], and we recently demonstrated that wortmannin is able to negatively regulate survivin in the same cell lines [23]. As expected, the up-regulation of survivin determined by K858 treatment in MCF7 cells was totally reversed by wortmannin addition, as demonstrated by western blot (K858 treated versus K858 plus wortmannin treated cells) (Fig. 6). In the same experiment, the treatment with wortmannin together to K858 determined a significant increase of Bax/Bcl2 ratio and an increase of 41 % of cleaved PARP, as compared to the treatment with K858 alone, demonstrating an increased apoptosis rate contemporaneous to the inhibition of survivin (Fig. 6). These data suggested that, reducing survivin expression by wortmannin, tumor cells were more sensitized to K858-dependent apoptosis.
Discussion
Kinesin inhibitors have anti-proliferative properties and they can lead cancer cells to mitotic arrest and apoptosis, or in G1 phase by mitotic slippage until apoptosis, caused by a tetraploidy checkpoint [8]. Although promising results have been recently obtained on breast cancer cells in vitro [26, 27] and on breast cancer models in animals [28], limited results have been confirmed in clinical trials with cancer patients: for example, a phase I trial with ispinesib on 16 breast cancer patients obtained only 9 % of response rate, although it demonstrated a low range of toxicity of this therapy [29]. A recent work showed that a new compound with Eg5 inhibition activity was able to impair the proliferation of breast cancer cells and also to decrease the cancer stem cell number within the tumor [30].
Therefore, we have tested some effects of the kinesin inhibitor K858 on breast cancer cell lines, corresponding to four different molecular phenotypes, and found that all four phenotypes responded to K858 with a significant apoptosis rate. In particular, triple negative and luminal A cells followed mainly the intrinsic pathway of apoptosis, while luminal B and HER2 like cells had activated both intrinsic and extrinsic pathways. However, we found that K858 induced up-regulation of the anti-apoptotic molecule survivin; at this regard, we have recently demonstrated that an increase of survivin expression is one of the mechanisms at the basis of chemoresistance to taxane in breast cancer cells [23]. Another recent study reported that the over-expression of survivin in cancer cell lines treated with the kinesin-5 inhibitor monastrol increased the resistance to monastrol [24]. The implication of survivin in the chemoresistance process can be also supported by the description that its down-regulation is associated to mitotic slippage in the presence of spindle damage [31].
Survivin expression is described in several tumors, and its up-regulation usually correlates with the worst prognosis in breast cancer, in fact survivin is a key molecule among the inhibitor of apoptosis (IAP) family of proteins [32]. The main activation pathway of survivin in breast cancer cells is PI3K/AKT/mitogen-activated protein kinase MAPK, which may cross-react and strengthen other pathways, such as the estrogen pathway (PI3K/AKT/mammalian target of rapamycin mTOR) [25, 33], reinforcing the signal transduction mediated by hormone receptors, with the onset of continuous stimulation on hormone-responsive tumor cells. For this reason we have utilized wortmannin, an inhibitor of PI3K/AKT, which negatively regulates survivin, trying to reduce chemoresistance and to improve the efficacy of K858 treatment. In fact, we found that, turning off the survivin pathway, breast cancer cells were more sensitized to K858-mediated apoptosis.
Our data substantially confirm the role played by survivin in inducing resistance to kinesin inhibitors, and suggest that the efficacy of anti-cancer treatment with K858 can be improved by silencing survivin expression. These findings underline a potential synergic activity of kinesin inhibitors with AKT inhibitor molecules, with the target to overcome chemoresistance in breast cancer cells.
Although taxanes and kinesin inhibitors share the mechanisms of action in blocking tumor growth, an important clinical aspect differentiates one agent from the other, since taxane can cause neuropathy [18], while kinesin inhibitors present a good tolerability without neurotoxicity [16, 29, 34]. Unfortunately, neuropathy is very common (accounting for more than 80 %) in breast cancer patients treated with taxanes and neurotoxicity is an important dose limiting side effect of taxanes, frequently causing the discontinuation of docetaxel or paclitaxel treatment in cancer patients. So, it is important to find alternative molecules among anti-mitotic agents microtubule-targeted, which exert a significant anti-neoplastic activity without serious side effects.
More investigations are needed to study in deep the efficiency of K858 treatment on breast cancer cells, with the aim to find out the mechanisms capable to negatively regulate survivin expression on multiple fronts and to improve kinesin inhibitors as potential not neurotoxic but effective new anti-cancer drugs.
Conclusion
Our experiments demonstrate that K858 displays anti-proliferative activity through the induction of apoptosis in breast cancer cells but, at the same time, unfortunately, K858 exerts a stimulation of survivin expression, which finally leads to resistance. These data suggest that K858 can have a potential role as anti-cancer agent, probably improvable by the addition of survivin/AKT inhibitors. This information is supportive to future clinical investigations on the utilization of kinesin inhibitors for the treatment of breast cancer.
Change history
30 August 2024
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1007/s10637-024-01469-5
References
Rath O, Kozielski F (2012) Kinesins and cancer. Nat Rev Cancer 12:527–539
Enos AP, Morris NR (1990) Mutation of a gene that encodes a kinesin-like protein blocks nuclear division in A. nidulans. Cell 60:1019–1027
Castillo A, Morse HC 3rd, Godfrey VL, Naeem R, Justice MJ (2007) Overexpression of Eg5 causes genomic instability and tumor formation in mice. Cancer Res 67:10138–10147
Mayer TU, Kapoor TM, Haggarty SJ, King RW, Schreiber SL, Mitchison TJ (1999) Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science 286:971–974
Sarli V, Giannis A (2008) Targeting the kinesin spindle protein: basic principles and clinical implications. Clin Cancer Res 14:7583–7587
El-Nassan HB (2013) Advances in the discovery of kinesin spindle protein (Eg5) inhibitors as antitumor agents. Eur J Med Chem 62:614–631
Leizerman I, Avunie-Masala R, Elkabets M, Fich A, Gheber L (2004) Differential effects of monastrol in two human cell lines. Cell Mol Life Sci 61:2060–2070
Silkworth WT, Cimini D (2012) Transient defects of mitotic spindle geometry and chromosome segregation errors. Cell Div 7:19
Gasnereau I, Boissan M, Margall-Ducos G, Couchy G, Wendum D, Bourgain-Guglielmetti F, Desdouets C, Lacombe ML, Zucman-Rossi J, Sobczak-Thepot J (2012) KIF20A and its gene product MKlp2 are increased during hepatocyte proliferation and hepatocarcinogenesis. Am J Pathol 180:131–140
Muller C, Gross D, Sarli V, Gartner M, Giannis A, Bernhardt G, Buschauer A (2007) Inhibitors of kinesin Eg5: antiproliferative activity of monastrol analogues against human glioblastoma cells. Cancer Chemother Pharmacol 59:157–164
Xing ND, Ding ST, Saito R, Nishizawa K, Kobayashi T, Inoue T, Oishi S, Fujii N, Lv JJ, Ogawa O, Nishiyama H (2011) A potent chemotherapeutic strategy in prostate cancer: S-methoxytrityl-L-cysteine, a novel Eg5 inhibitor. Asian J Androl 13:236–241
Wojcik EJ, Buckley RS, Richard J, Liu L, Huckaba TM, Kim S (2013) Kinesin-5: cross-bridging mechanism to targeted clinical therapy. Gene 531:133–149
Mitchison TJ (2012) The proliferation rate paradox in antimitotic chemotherapy. MolBiol Cell 23:1–6
Lad L, Luo L, Carson JD, Wood KW, Hartman JJ, Copeland RA, Sakowicz R (2008) Mechanism of inhibition of human KSP by ispinesib. Biochemistry 47:3576–3385
Blagden SP, Molife LR, Seebaran A, Payne M, Reid AH, Protheroe AS, Vasist LS, Williams DD, Bowen C, Kathman SJ, Hodge JP, Dar MM, de Bono JS, Middleton MR (2008) A phase I trial of ispinesib, a kinesin spindle protein inhibitor, with docetaxel in patients with advanced solid tumours. Br J Cancer 98:894–899
Beer TM, Goldman B, Synold TW, Ryan CW, Vasist LS, Van Veldhuizen PJJ, Dakhil SR, Lara, PN Jr. , Drelichman A, Hussain MH, Crawford ED (2008) Southwest Oncology Group phase II study of ispinesib in androgen-independent prostate cancer previously treated with taxanes. Clin Genitourin Cancer 6: 103–109.
Sikov WM (2015) Assessing the role of platinum agents in aggressive breast cancers. Curr Oncol Rep 17:3
De Iuliis F, Taglieri L, Salerno G, Lanza R, Scarpa S (2015) Taxane induced neuropathy in patients affected by breast cancer: literature review. Crit Rev Oncol Hematol 96:34–45
Hershman DL, Weimer LH, Wang A, Kranwinkel G, Brafman L, Fuentes D, Awad D, Crew KD (2011) Association between patient reported outcomes and quantitative sensory tests for measuring long-term neurotoxicity in breast cancer survivors treated with adjuvant paclitaxel chemotherapy. Breast Cancer Res Treat 125:767–774
Marcus AI, Peters U, Thomas SL, Garret S, Zelnak A, Kapoor TM, Giannakakou P (2005) Mitotic inhibitors induce mitotic arrest and cell death in Taxol-resistant and sensitive cancer cells. J Biol Chem 280:11569–11577
De Monte C, Carradori S, Secci D, D'Ascenzio M, Guglielmi P, Mollica A, Morrone S, Scarpa S, Aglianò AM, Giantulli S, Silvestri I (2015) Synthesis and pharmacological screening of a large library of 1,3,4-thiadiazolines as innovative therapeutic tools for the treatment of prostate cancer and melanoma. Eur J Med Chem 105:245–262
Nakai R, Iida S, Takahashi T, Tsujita T, Okamoto S, Takada C, Akasaka K, Ichikawa S, Ishida H, Kusaka H, Akinaga S, Murakata C, Honda S, Nitta M, Saya H, Yamashita Y (2009) K858, a novel inhibitor of mitotic kinesin Eg5 and antitumor agent, induces cell death in cancer cells. Cancer Res 69:3901–3909
De Iuliis F, Salerno G, Giuffrida A, Milana B, Taglieri L, Rubinacci G, Giantulli S, Terella F, Silvestri I, Scarpa S (2015) Breast cancer cells respond differently to docetaxel depending on their phenotype and on survivin upregulation. Tumor Biol. doi:10.1007/s13277-015-4075-x
Asraf H, Avunie-Masala R, Hershfinkel M, Gheber L (2015) Mitotic slippage and expression of survivin are linked to differential sensitivity of human cancer cell-lines to the kinesin-5 inhibitor monastrol. PLoS One 10:e0129255
Asanuma H, Torigoe T, Kamiguchi K, Hirohashi Y, Ohmura T, Hirata K, Sato M, Sato N (2005) Survivin expression is regulated by coexpression of human epidermal growth factor receptor2 and epidermal growth factor receptor via phosphatidylinositol 3-kinase/AKT signaling pathway in breast cancer cells. Cancer Res 65:11018–11025
Wang H, Lu C, Li Q, Xie J, Chen T, Tan Y, Wu C, Jiang J (2014) The role of kif4A in doxorubicin-induced apoptosis in breast cancer cells. Mol Cells 37:812–818
Li Y, Lu W, Chen D, Boohaker RJ, Zhai L, Padmalayam I, Wennerberg K, Xu B, Zhang W (2015) KIFC1 is a novel potential therapeutic target for breast cancer. Cancer Biol Ther 16:1316–1322
Purcell JW, Davis J, Reddy M, Martin S, Samayoa K, Vo H, Thomsen K, Bean P, Kuo WL, Ziyad S, Billig J, Feiler HS, Gray JW, Wood KW, Cases S (2010) Activity of the kinesis spindle protein inhibitor ispinesib (SB-715992) in models of breast cancer. Clin Cancer Res 16:566–576
Gomez HL, Philco M, Pimentel P, Kiyan M, Monsalvo ML, Conlan MG, Saikali KG, Chen MM, Seroogy JJ, Wolff AA, Escandon RD (2012) Phase I dose-escalation and pharmacokinetic study of ispinesib, a kinesin spindle protein inhibitor, administered on days 1 and 15 of a 28-day schedule in patients with no prior treatment for advanced breast cancer. Anti-Cancer Drugs 23:335–341
Guido BC, Ramos LM, Nolasco DO, Nobrega CC, Andrade BY, Pic-Taylor A, Neto BA, Corrêa JR (2015) Impact of kinesin Eg5 inhibition by 3,4-dihydropyrimidin-2 (1H)-one derivatives on various breast cancer cell features. BMC Cancer 15:283
Lens SM, Wolthuis RM, Klompmaker R, Kauw J, Agami R, Brummelkamp T, Kops G, Medema RH (2003) Survivin is required for a sustained spindle checkpoint arrest in response to lack of tension. Embo J 22:2934–2347
Lv YG, Yu F, Yao Q, Chen JH, Wang L (2010) The role of survivin in diagnosis, prognosis and treatment of breast cancer. J Thorac Dis 2:100–110
Siddiqa A, Long LM, Li L, Marciniak RA, Kazhdan I (2008) Expression of HER-2 in MCF-7 breast cancer cells modulates anti-apoptotic protein survivin and bcl-2 via the extracellular signal-related kinase (ERK) and phosphoinositide-3 kinase (PI3K) signaling pathways. BMC Cancer 8:129
Lo Russo PM, Goncalves PH, Casetta L, Carter JA, Litwiler K, Roseberry D, Rush S, Schreiber J, Simmons HM, Ptaszynski M, Sausville EA (2015) First in human phase 1 study of filanesib, a kinesis spindle protein inhibitor, in patients with advanced solid tumors. Investig New Drugs 33:440–449
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1007/s10637-024-01469-5
About this article
Cite this article
De Iuliis, F., Taglieri, L., Salerno, G. et al. RETRACTED ARTICLE: The kinesin Eg5 inhibitor K858 induces apoptosis but also survivin-related chemoresistance in breast cancer cells. Invest New Drugs 34, 399–406 (2016). https://doi.org/10.1007/s10637-016-0345-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-016-0345-8