Summary
JI-101 is an oral multi-kinase inhibitor that targets vascular endothelial growth factor receptor type 2 (VEGFR-2), platelet derived growth factor receptor β (PDGFR-β), and ephrin type-B receptor 4 (EphB4). None of the currently approved angiogenesis inhibitors have been reported to inhibit EphB4, and therefore, JI-101 has a novel mechanism of action. We conducted a pilot trial to assess the pharmacokinetics (PK), tolerability, and efficacy of JI-101 in combination with everolimus in advanced cancers, and pharmacodynamics (PD), tolerability, and efficacy of JI-101 in ovarian cancer. This was the first clinical study assessing anti-tumor activity of JI-101 in a combinatorial regimen. In the PK cohort, four patients received single agent 10 mg everolimus on day 1, 10 mg everolimus and 200 mg JI-101 combination on day 8, and single agent 200 mg JI-101 on day 15. In the PD cohort, eleven patients received single agent JI-101 at 200 mg twice daily for 28 day treatment cycles. JI-101 was well tolerated as a single agent and in combination with everolimus. No serious adverse events were observed. Common adverse events were hypertension, nausea, and abdominal pain. JI-101 increased exposure of everolimus by approximately 22 %, suggestive of drug-drug interaction. The majority of patients had stable disease at their first set of restaging scans (two months), although no patients demonstrated a response to the drug per RECIST criteria. The novel mechanism of action of JI-101 is promising in ovarian cancer treatment and further prospective studies of this agent may be pursued in a less refractory patient population or in combination with cytotoxic chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
JI-101 is a novel oral multi-kinase inhibitor that targets three receptor tyrosine kinases: VEGF Receptor Type 2 (VEGFR-2), platelet-derived growth factor receptor beta (PDGFR-β) and Ephrin B4 (EphB4). By targeting multiple angiogenesis signaling pathways in tumor vessel beds, JI-101 has the potential to inhibit multiple stages of tumor angiogenesis and thus enhance anti-tumor efficacy. JI-101 was previously studied in a phase I trial in patients with advanced solid tumors to determine the maximum tolerated dose or optimal biologic dose of JI-101 [1]. JI-101 was given orally in 28-day cycles in doses up to 800 mg/day to determine the safety and tolerability of once daily and twice daily dosing with JI-101, using a continuous reassessment method for dose escalation. Doses of 100 mg/day, 200 mg/day, 400 mg/day, 200 mg twice daily, and 300 mg twice daily were studied in cohorts of 3, 3, 4, and 2 patients respectively (unpublished data). At the time of development of the current study protocol, the 200 mg twice daily dose level was declared as the maximum tolerated dose and appropriate for further development [2].
Everolimus is an FDA-approved drug for the treatment of advanced renal cell carcinoma and advanced neuroendocrine tumors. Everolimus down-regulates the mTOR (mammalian target of rapamycin) pathway and this can lead to direct anti-proliferative effects as well as decreased production of VEGF. mTOR is a key and highly conserved serine-threonine kinase, that is present in all cells and is a central regulator of protein synthesis and ultimately cell growth, cell proliferation, angiogenesis, and cell survival [3].
Given the non-overlapping mechanisms of action and the safety profiles of everolimus and of JI-101, the combination of these two agents may provide synergistic benefit to patients with certain solid tumors. JI-101 inhibits angiogenesis, and subsequently tumor growth, by inhibiting three receptor tyrosine kinases: VEGFR-2, PDGFR-β, and EphB4. The combination of everolimus and JI-101 would possibly provide enhanced anti-proliferative effects in solid tumors.
The challenge for a combination of these two agents is a potential for drug-drug interaction. Everolimus is a substrate of CYP3A4 and a substrate and a moderate inhibitor of the multi-drug efflux pump glycoprotein (P-gP, MDR1, ABCB1). Thus, its metabolism is sensitive to drugs that modify these enzymes (substrates, inducers, or inhibitors of these enzymes). Competitive inhibition could occur when everolimus is combined with drugs that are also CYP3A4 or P-glycoprotein substrates. Preliminary in vitro data suggests mild to moderate CYP3A4 inhibition by JI-101 [4]. The pharmacokinetic drug interaction phase of this study would characterize the effect on metabolism of the combination of JI-101 and everolimus.
In this pilot trial we sought to broaden the knowledge of the pharmacokinetic and pharmacodynamic properties of JI-101. We performed a pharmacokinetic drug-drug interaction study with everolimus to determine if common metabolic pathways would alter the plasma concentrations of either drug. This cohort was open to all solid tumor types. The second aim of this trial was to examine the pharmacodynamic properties of JI-101 and determine its efficacy in tumors that would most likely benefit from a drug with this unique mechanism of action. The pharmacodynamic cohort was intended to enroll patients with recurrent ovarian, colorectal and neuroendocrine tumors which had previously failed other therapies. We enrolled 11 ovarian patients on this pilot trial allowing for statistical analysis in this group.
Ovarian cancer is the most lethal gynecologic malignancy, with the majority of women initially presenting with advanced stage disease. Although ovarian cancer is the ninth most common cancer in women in the United States, it is the fifth leading cause of cancer death in women, with greater than 15,000 deaths per year [5, 6]. Despite advances in surgical care and systemic therapy, the median overall survival for patients diagnosed with advanced stage disease has not dramatically improved since the 1980s. Given the poor outcomes in ovarian cancer and the recent paradigm shift in oncology towards personalized approaches, novel strategies with targeted therapy are needed for these patients.
The use of anti-angiogenic agents has been an active area of research in ovarian cancer [7–9]. Studies have demonstrated VEGF overexpression and an association between tumor angiogenesis, progression, early recurrence, and prognosis [10]. A validated target for anti-angiogenic therapy is the receptor tyrosine kinases (RTKs). Of all the RTKs that are found in the human genome, the ephrin receptors constitute the largest subfamily of RTK proteins and all members share a similar structure, including a ligand binding extracellular domain, a single transmembrane domain, and an intracellular tyrosine kinase domain [11]. Ephrin type-B receptor 4 (EphB4) and its ligand, ephrin B2, are involved in endothelial cell interaction. Functional EphB4 is required for forming capillary networks during angiogenesis [12]. Interaction between ephrin B2 and EphB4 at the arterial-venous interface is thought to stimulate formation of new capillary sprouts. By targeting multiple angiogenesis signaling pathways, JI-101 has the potential advantage of inhibiting additional targets in tumor angiogenesis, in terms of inhibiting EphB4 [13, 14]. In addition, it is known that EphB4 overexpression portends a poor clinical outcome. Given this, EphB4 receptor down regulation in response to JI-101 may be an important mechanism of action of JI-101 in tumors that express EphB4.
The primary objective of the pharmacodynamic cohort was evaluation of progression free survival at two month intervals in this treatment refractory population. Secondary objective was evaluation of the tolerability of JI-101. Additionally, we assessed plasma VEGF levels on days 1, 8, and 15 of cycle one following administration of JI-101.
Materials and methods
Pharmacokinetic cohort
After obtaining study approval from the University of Utah institutional review board (IRB), four patients with advanced genitourinary malignancies were treated to determine the pharmacokinetic drug interactions between JI-101 and Everolimus. Study drugs were administered to the patient at study visits on days 1, 8, and 15. Patients were instructed to fast for a minimum of 8 hours prior to morning dosing and for 2 hours post dosing. Pharmacokinetics were measured on days 1, 8, and 15 as follows: Day 1: Patients were administered 10 mg of Everolimus orally. Blood draws were then performed pre-dose and at 0.5, 1, 2, 4, 6, 8, 10, and 24 hours post-dose. Day 8: Patients were administered 10 mg of Everolimus orally and 200 mg of JI-101 orally at the same time. Blood draws were performed pre-dose and at 0.5, 1, 2, 4, 6, 8, 10, and 24 hours post-dose. Day 15: Patients were administered 200 mg of JI-101 orally. Blood draws were performed prior to dosing and at 0.5, 1, 2, 4, 6, 8, 10, and 24 hours post-dose. From day 16 and onward, JI-101 was administered at a dose of 200 mg twice daily.
Patients continued to receive 200 mg of JI-101 at this dose until disease progression or until discontinuation from the study for any reason. Safety was assessed at baseline, immediately following administration of drug on days 1, 8, and 15, and at the time of the 24-hour post-dose sampling. Patients who continued on treatment with JI-101 had safety assessed at all subsequent clinic visits with disease assessments after completing two 28-day treatment cycles. Plasma everolimus and JI-101 concentrations were measured by LC-MS-MS. PK parameters were estimated by using non-compartmental analysis.
Pharmacodynamic cohort
This was a single center pilot study conducted at the Huntsman Cancer Institute at the University of Utah. After obtaining study approval from the University of Utah internal review board (IRB), eligible patients with refractory and/or recurrent ovarian cancer were identified and treated with JI-101 200 mg twice daily oral dosing in this phase one study. Patients were enrolled between October 2010 and October 2011. Patients were allowed to continue on therapy until disease progression, intolerable toxicities or at the discretion of the physican or patient.
Patients with histologically confirmed recurrent ovarian carcinoma were eligible. Patients required adequate bone marrow function, at least one measurable tumor as defined by RECIST (version 1.1), and an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2. Patients with pre-existing uncontrolled hypertension were excluded; those with hypertension controlled by anti-hypertensive therapies were eligible. Patients with unstable brain or central nervous system metastases, active peptic ulcer disease, inflammatory bowel disease, ulcerative colitis, or other gastrointestinal conditions with increased risk of perforation, history of abdominal fistula, and those with evidence of bleeding diathesis or coagulopathy or needing anticoagulation were excluded.
Safety assessments consisted of monitoring and recording all adverse events and serious adverse events, regular monitoring of hematology, chemistry, urine values, electrocardiograms, regular measurement of vital signs, including blood pressure monitoring, and performance of physical examinations. Safety and tolerability were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE version 4). Response was assessed using Response Evaluation Criteria in Solid Tumors (RECIST version 1.1). Baseline computed tomography (CT) scans were performed at baseline and repeated every two cycles until disease progression, intolerable toxicities, or at the discretion of the physician or patient.
Because of the effect of JI-101 on the angiogenesis pathway, we sought to examine the effect of inhibition VEGFR pathway by measuring serum VEGF levels. During the first cycle of the study, blood samples were collected from each patient to assess the level of plasma VEGF in patients on JI-101. Blood samples were collected before drug was given and again on days 8 and 15 after drug administration.
VEGF concentrations in plasma were measured with a quantitative chemiluminescent sandwich enzyme immunoassay (R&D Systems, QuantiGlo(r) ELISA Human VEGF Immunoassay, Minneapolis, Minnesota, USA) according to manufacturer recommendations. This assay is calibrated against recombinant human VEGF165 and recognizes both endogenous and recombinant human VEGF with an analytical measurement range of 6.4 to 20,000 pg/mL. Positive and negative controls were tested in parallel. All specimens were stored at -20C prior to testing and were assayed in duplicate.
RECIST criteria (version 1.1) were used as the basis for defining response and disease progression. Continuous data were summarized using median, minimum, and maximum value. Categorical data were presented in tables with frequencies and percentages. Confidence intervals were calculated at the 95 % level. Time dependent parameters were analyzed using the Kaplan-Meier method and 95 % confidence interval for the median. The primary and secondary efficacy analyses were performed on an intent-to-treat basis.
Results
Pharmacokinetic cohort
4 patients were enrolled in the pharmacokinetic cohort. All 4 patients were male. 3 patients were treated for prostate cancer and one patient was treated for renal cell carcinoma. Age range of patients was 65 to 80 years with a mean age of patients was 72. All 4 patients received single agent everolimus 10 mg on Day 1, combination everolimus 10 mg and JI-101 200 mg on day 8, and single agent JI-101 200 mg on day 15. All patients were determined to have progressive disease either at their first set of staging scans or as determined by clinical discretion of the enrolling physician.
Adverse events
No grade 4 adverse events were reported. Grade 3 treatment related adverse events were observed in two of the four patients: grade 3 hypertension in two patients, grade 3 fatigue in one of these two patients, and grade 3 diarrhea in one of these two patients.
Serum pharmacokinetics
Everolimus
An increase in the mean Cmax 24 hours after administration of JI-101 was seen in everolimus levels. Mean Cmax prior to JI-101 administration was 44.18 ng/mL on day 1; 24 hours after administration of JI-101 was 61.64 ng/mL on day 8. Subjects 1, 3, and 4 all had increased peak levels in everolimus, while subject 2 showed a decreased Cmax after JI-101 administration. However, all subjects still showed increased exposure of everolimus. Twenty-four hours after administration of JI-101 on day 8, increased exposure to everolimus was seen in all 4 patients (Table 1) as measured by AUC-inf in ng*hr/mL. The overall increase in mean exposure of everolimus with one dose of JI-101 in the studied population was 38 %. While subjects 1–3 exhibited nearly a 22 % increase in exposure to everolimus with a single dose of JI-101, subject # 4 exhibited a 199 % increase in exposure. Overall mean half-life across all patients was increased from 16 h to 23 h which represents an increase of 47 % as shown in Table 2.
JI-101
Max serum JI-101 levels (Cmax) increased in all 4 patients with increased dose of JI-101 on day 15 (Table 3). An increase in the mean Cmax from 196.95 to 287 which is a 46 % increase was observed. Similarly, increased exposure (AUC-inf in ng*hr/mL) to JI-101 was seen with co-administration of everolimus. Subjects 1, 3, and 4 all show an increase exposure to JI-101 in this study with co-administration of everolimus. Only subject 2 showed a decrease in exposure. The overall increase in mean exposure of JI-101 in presence of everolimus across all patients was 8960.3 ng*hr/mL to 11264.8 ng*hr/mL to which represents a 20 % increase in exposure. Overall half-life across all patients for JI-101 was increased from 23 hours to 42 h, representing an 82 % increase with co-administration of everolimus as shown in Table 4.
Pharmacodynamic cohort
Of the eleven ovarian cancer patients enrolled, eight patients were evaluable for disease response. Evaluable patients included only those that were able to complete two cycles of study drug and underwent assessment for disease response. Three patients were not evaluable for response assessment. One was withdrawn for disease related partial small bowel obstruction. The other two experienced toxicities considered possibly related to drug including one patient with grade 3 transaminitis and abdominal pain and one patient with intractable nausea and vomiting during cycle one. Patient characteristics are listed in Table 5. Of the eight evaluable patients, the age range of the patients was 52–76 with a median age of 58 years. All participants had undergone previous therapy prior to inclusion in the current study with 7 of 11 (64 %) of the enrolled patients having received three or more prior treatments.
Adverse effects
All eleven enrolled patients were assessed for drug related toxicity. Serious adverse events (Table 6) were uncommon with one grade 4 adverse event reported: hypertension. The most common treatment related grade 2 and 3 hematologic AE was grade 2 anemia (1) and grade 2 lymphopenia (1). The most common treatment related grade 2 and 3 non-hematologic AEs were grade 3 hypertension (8), grade 3 transaminitis (2), grade 3 bowel obstruction (2), grade 2 abdominal pain (3), grade 2 nausea (3), and grade 2 diarrhea (2). Additional toxicities noted included fatigue, headache, anorexia, and dehydration. Of the eight response -evaluable patients, four patients discontinued therapy due to progressive disease, two due to persistent nausea and vomiting, one due to persistent transaminitis, and one due to persistent diarrhea. Three patients were withdrawn from the study during the first cycle as noted above, and thus deemed non-evaluable for response.
Clinical response
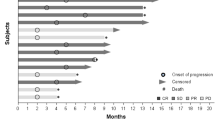
Eleven of the intent to treat patients with ovarian cancer, six patients had stable disease (SD) at two months and two had progressive disease (PD), three patients were removed from study prior to the first disease assessment evaluation as noted above. Best response was SD at four months. Two-month progression free survival was 71 % (95 % CI 45–99 %) and median PFS was 169 days (95 % CI 55-∞) (Fig. 1).
Biomarker studies
Blood samples for VEGF levels were collected during the first cycle at baseline then again on days 8 and 15 after drug administration. Seven of the eight response-evaluable patients had all three blood draws for biomarker studies. Baseline levels of VEGF were wide ranging between patients (16 – 482 pg/mL). We observed a general trend in the majority of patients (6 of 7 of the patients) that plasma VEGF levels were elevated over baseline seven days after JI-101 administration. Plasma VEGF levels declined from day 8 levels but generally stayed above baseline levels (Fig. 2). From our cohort of evaluable patients for disease assessment, 1 of the 2 patients that had disease progression after two cycles of drug showed decreased plasma VEGF at day 8 while all five patients with stable disease showed increases in plasma VEGF at day 8.
Discussion
In this report, we present the pharmacokinetic effects of JI-101 on everolimus and vice versa, in 4 patients treated with both drugs for advanced genitourinary malignancies. This is the first clinical study performed with JI-101 in combination with another anti-cancer therapy. In this study each patient was given a single clinically established dose of everolimus. Eight days later, JI-101 was administered in combination with everolimus. Interestingly, a single dose of JI-101 increased exposure, (AUC0-inf in ng*hr/mL), of everolimus by approximately 22 % in 3 patients. However, in the fourth patient (aged 80 years) exposure increased by 3-fold with a concomitant 2-fold increase in half-life of everolimus. Examination of PK data of JI-101 suggests that age may be a factor to explain the interaction in this patient. Additionally, everolimus is a substrate inhibitor of CYP3A4 and early in vitro data suggested mild to moderate CYP3A4 inhibition by JI-101. It is possible that subject 4 may have demonstrated such high exposure to everolimus after receiving JI-101 secondary to a higher propensity for innate CYP3A4 inhibition than the other 3 patients by virtue of genetic polymorphism or other unknown mechanisms.
Also unknown are the dietary effects on the GI absorption of JI-101. In the same subject with 3-fold increased exposure to everolimus after JI-101 dosing, JI-101 exposure was also the highest. However no consistent trends were observed in JI-101 pharmacokinetics in combination with everolimus.
Overall the combination of JI-101 and everolimus was well tolerated. The most consistent adverse event was hypertension requiring dose modification in one of the four patients. Hypertension is a well-known adverse effect of angiogenesis inhibitors as a class and when compared to the Phase I single agent data of JI-101, the combination with everolimus did not seem to increase the incidence. However, this is a very limited sample size. The only other adverse events reported during this trial were diarrhea and fatigue, which are known toxicities of both JI-101 and everolimus, and thus an expected toxicity. The combination of JI-101 and everolimus does not appear to have any added toxicity than from either agent alone, however, a formal Phase I study will need to be conducted to determine the dose and clinical safety of JI-101 with everolimus.
In conclusion, JI-101 consistently increased levels and exposure to everolimus when both drugs are used in combination to treat malignancy. However there remains great variability in this effect from patient to patient. The combination was also well tolerated with expected toxicities. Future trials with expanded patient accrual may provide further details on the pharmacokinetic and clinical effects of this potentially effective combination.
In our pharmacodynamic cohort, JI-101 as a single agent was well tolerated in patients with refractory or recurrent ovarian cancer with approximately three-fourths of the patients being progression free at two months with best response being stable disease at 4 months. There were no responses. The median progression free survival was 169 days (5.6 months). These results are comparable to previously published studies with single agent anti-angiogenic agents in women with recurrent, heavily pretreated ovarian cancer in regards to progression free survival [5, 15].
The results of this pilot trial suggest that in heavily pre-treated patients with refractory disease, JI-101 is relatively well tolerated with the majority of adverse events related to those which would be expected based on the known mechanism of JI-101 and its inhibition of anti-angiogenesis signaling pathways. The most common treated related adverse event was hypertension, including grade 4 in 1 patient (9 %) and grade 3 in 8 patients (73 %). This was an expected toxicity and medically managed with anti-hypertensive medication and blood pressure control improved during the course of the clinical trial. These patients were able to continue on the study drug. We did see gastrointestinal toxicities in our patients as well including nausea, vomiting, diarrhea, which are commonly seen with other anti-VEGF agents. These toxicities were managed medically as well. We did have two patients of the 11 total with partial small bowel obstruction. This complication has also been reported with anti-VEGF agents though typically rare. While this is possibly drug related, intestinal obstructions are frequently seen in recurrent ovarian cancer patients given the nature of their abdominal disease.
The novel mechanism of action of JI-101 with inhibition of EphB4 makes this an attractive agent in ovarian cancer because EphB4 expression is expressed in 86 % of ovarian cancers [16]. Assessment of a biomarker for ephB4 levels and ephrinB2 ligand would be ideal but the sensitivity of the ephB4 levels and ephrinB2 levels we obtained were not adequate for a blood biomarker. We did look at plasma VEGF levels after exposure to JI-101 and all five patients with stable disease had increased VEGF levels at day 8. This is a small number of patients and it is difficult to ascertain the clinical significance of these data. However, this would be interesting to correlate VEGF levels over a longer period of time and correlate this with disease response in a larger cohort of patients.
With continued interest in the use of targeted anti-angiogenesis inhibitors in patients with refractory ovarian cancer, JI-101 represents a novel triple-kinase inhibitor with anti-cancer activity. Although no patient demonstrated a response according to RECIST criteria, the progression free survival and tolerability of this agent in the ovarian cohort in this trial may warrant further studies of JI-101 in patients with advanced ovarian cancer. Given the relative tolerability in dosing and schedule, use of JI-101 alone and studies in combination with cytotoxic chemotherapy should be considered.
References
Velleca M, Brittelli D, Elkin L, et al. (2009) Preclinical and preliminary phase 1 trial results of JI-101: A novel, oral tyrosine kinase inhibitor that selectively targets VEGFR2, EphB4, and PDGFRβ. NCI - EORTC Int Conf Molec Targ Cancer Therapeut. November 15–19, Boston, MA USA, Abstract no: B11
Sharma S, Miller L, Gordon M, et al. (2010) Phase I study of JI-101, a novel oral tyrosine kinase inhibitor that selectively targets EphB4, VEGFR2 and PDGFRß. Poster presentation at 22nd EORTC-NCI-AACR Symp Molec Targ Cancer Therapeut. November 16–19, Berlin, Abstract no: 400
Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, Grunwald V, Thompson JA, Figlin RA, Hollaender N, Urbanowitz G, Berg WJ, Kay A, Lebwohl D, Ravaud A, Group R-S (2008) Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet 372(9637):449–456. doi:10.1016/S0140-6736(08)61039-9
Gurav SD, Gilibili RR, Jeniffer S, Mohd Z, Giri S, Govindarajan R, Srinivas NR, Mullangi R (2012) Pharmacokinetics, tissue distribution and identification of putative metabolites of JI-101 - a novel triple kinase inhibitor in rats. Arzneimittelforschung 62(1):27–34. doi:10.1055/s-0031-1295427
Burger RA (2007) Experience with bevacizumab in the management of epithelial ovarian cancer. J Clin Oncol 25(20):2902–2908. doi:10.1200/JCO.2007.12.1509
Spannuth WA, Mangala LS, Stone RL, Carroll AR, Nishimura M, Shahzad MM, Lee SJ, Moreno-Smith M, Nick AM, Liu R, Jennings NB, Lin YG, Merritt WM, Coleman RL, Vivas-Mejia PE, Zhou Y, Krasnoperov V, Lopez-Berestein G, Gill PS, Sood AK (2010) Converging evidence for efficacy from parallel EphB4-targeted approaches in ovarian carcinoma. Mol Cancer Ther 9(8):2377–2388. doi:10.1158/1535-7163.MCT-10-0200
Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, Mannel RS, Homesley HD, Fowler J, Greer BE, Boente M, Birrer MJ, Liang SX, Gynecologic Oncology G (2011) Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med 365(26):2473–2483. doi:10.1056/NEJMoa1104390
Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, Carey MS, Beale P, Cervantes A, Kurzeder C, du Bois A, Sehouli J, Kimmig R, Stahle A, Collinson F, Essapen S, Gourley C, Lortholary A, Selle F, Mirza MR, Leminen A, Plante M, Stark D, Qian W, Parmar MK, Oza AM, Investigators I (2011) A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med 365(26):2484–2496. doi:10.1056/NEJMoa1103799
Monk BJ, Sill MW, Burger RA, Gray HJ, Buekers TE, Roman LD (2009) Phase II trial of bevacizumab in the treatment of persistent or recurrent squamous cell carcinoma of the cervix: a gynecologic oncology group study. J Clin Oncol : Off J Am Soc Clin Oncol 27(7):1069–1074. doi:10.1200/JCO.2008.18.9043
Dvorak HF (2002) Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol : Off J Am Soc Clin Oncol 20(21):4368–4380
Alam SM, Fujimoto J, Jahan I, Sato E, Tamaya T (2008) Coexpression of EphB4 and ephrinB2 in tumour advancement of ovarian cancers. Br J Cancer 98(4):845–851. doi:10.1038/sj.bjc.6604216
Pasquale EB (2008) Eph-ephrin bidirectional signaling in physiology and disease. Cell 133(1):38–52. doi:10.1016/j.cell.2008.03.011
DeGeorge JJ, Ahn CH, Andrews PA, Brower ME, Giorgio DW, Goheer MA, Lee-Ham DY, McGuinn WD, Schmidt W, Sun CJ, Tripathi SC (1998) Regulatory considerations for preclinical development of anticancer drugs. Cancer Chemother Pharmacol 41(3):173–185
Tang XX, Brodeur GM, Campling BG, Ikegaki N (1999) Coexpression of transcripts encoding EPHB receptor protein tyrosine kinases and their ephrin-B ligands in human small cell lung carcinoma. J Clin Oncol : Off J Am Soc Clin Oncol 5(2):455–460
Burger RA, Sill MW, Monk BJ, Greer BE, Sorosky JI (2007) Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a gynecologic oncology group study (2007). J Clin Oncol 25(3):5165–5171
Kumar SR, Masood R, Spannuth WA, Singh J, Scehnet J, Kleiber G, Jennings N, Deavers M, Krasnoperov V, Dubeau L, Weaver FA, Sood AK, Gill PS (2007) The receptor tyrosine kinase EphB4 is overexpressed in ovarian cancer, provides survival signals and predicts poor outcome. Br J Cancer 96(7):1083–1091. doi:10.1038/sj.bjc.6603642
Acknowledgments
The authors would like to thank the study participants and their families, as well as the research coordinators. We would like to acknowledge Joely Straseski, PhD, who analyzed the plasma VEGF samples.
Funding
This work was supported by a grant from Vanthys Pharmaceutical Development.
Conflict of interest
Neeraj Agarwal: Research support to the institution from Amgen, Bayer, Bristol Myers Squibb, GlaxoSmithKline, Imclone, Medivation, Takeda, Novartis, Pfizer.
Sunil Sharma: Research support to the institution from Novartis, Spectrum, GlaxoSmithKline, Merrimack, Millennium, Takeda, Amgen, LSK Biopartners, Mirati, MedImmune, Johnson & Johnson, Gilead Sciences, Plexxikon, Celgene, Sanofi, Onyx, Bayer; and financial interest in Beta Cat Pharmaceuticals, Salarius, and ConverGene.
Theresa L. Werner: Research support to the institution from Abbvie, Amgen, Bayer, GlaxoSmithKline, Novartis, Roche-Genentech, Pfizer.
The other authors have declared no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Werner, T.L., Wade, M.L., Agarwal, N. et al. A pilot study of JI-101, an inhibitor of VEGFR-2, PDGFR-β, and EphB4 receptors, in combination with everolimus and as a single agent in an ovarian cancer expansion cohort. Invest New Drugs 33, 1217–1224 (2015). https://doi.org/10.1007/s10637-015-0288-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-015-0288-5