Summary
Purpose LY2090314 (LY) is a glycogen synthase kinase 3 inhibitor with preclinical efficacy in xenograft models when combined with platinum regimens. A first-in-human phase 1 dose-escalation study evaluated the combination of LY with pemetrexed/carboplatin. Patients and Methods Forty-one patients with advanced solid tumors received single-dose LY monotherapy lead-in and 37 patients received LY (10–120 mg) plus pemetrexed/carboplatin (500 mg/m2 and 5–6 AUC, respectively) across 8 dose levels every 21 days. Primary objective was maximum tolerated dose (MTD) determination; secondary endpoints included safety, antitumor activity, pharmacokinetics, and beta-catenin pharmacodynamics. Results MTD of LY with pemetrexed/carboplatin was 40 mg. Eleven dose-limiting toxicities (DLTs) occurred in ten patients. DLTs during LY monotherapy occurred at ≥40 mg: grade 2 visual disturbance (n = 1) and grade 3/4 peri-infusional thoracic pain during or shortly post infusion (n = 4; chest, upper abdominal, and back pain). Ranitidine was added after de-escalation to 80 mg LY to minimize peri-infusional thoracic pain. Following LY with pemetrexed/carboplatin therapy, DLTs included grade 3/4 thrombocytopenia (n = 4) and grade 4 neutropenia (n = 1). Best overall response by RECIST included 5 confirmed partial responses (non-small cell lung cancer [n = 3], mesothelioma, and breast cancer) and 19 patients having stable disease. Systemic LY exposure was approximately linear over dose range studied. Transient upregulation of beta-catenin measured in peripheral blood mononuclear cells (PBMCs) occurred at 40 mg LY. Conclusions The initial safety profile of LY2090314 was established. MTD LY dose with pemetrexed/carboplatin is 40 mg IV every 3 weeks plus ranitidine. Efficacy of LY plus pemetrexed/carboplatin requires confirmation in randomized trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glycogen synthase kinase 3 (GSK3), a serine/threonine kinase, has emerged as an important therapeutic target in oncology, as it plays a regulatory role in a variety of pathways, including initiation of protein synthesis, cell proliferation, cell differentiation, and apoptosis [1, 2]. Pharmacologic inhibition of GSK3 in tumor cells causes loss of phosphorylation of key proteins in the WNT signaling pathway, specifically beta-catenin, causing aberrant stabilization of the substrate protein [3], which results in an increase in chemotherapy-induced p53-dependent [4] and -independent cell cycle arrest into apoptosis [5]. Inhibition of GSK3 activity leads to stabilization and accumulation of beta-catenin in cytoplasm [3]; thus, this protein is considered a potentially useful biomarker. By taking advantage of G1, S, or G2 cell cycle arrest caused by DNA damaging agents, tumor cells avoid immediate cell death [6]. Treatment with a GSK3 inhibitor in combination with a DNA damaging agent may prevent this arrest and subsequent DNA repair, thus bypassing 1 or more of these checkpoints, resulting in tumor cells being “pushed” into a defective cell division process that culminates in apoptosis of platinum-treated tumor cells [7].
LY2090314 (LY) is a potent competitive inhibitor of enzymatic activity of GSK3α (IC50 = 1.5 nM) and GSK3β (IC50 = 0.9 nM) with limited activity against additional kinases [8]. Inhibition in nanomolar ranges was noted against PRK2 and CDK5 with IC50 values of 18 and 79 nM, respectively (data on file, Eli Lilly and Company). While preclinical data with LY monotherapy suggests limited anti-tumor activity as a single-agent against solid-tumor-derived cancer cell lines in vitro and in xenograft models, its activity has been shown to enhance platinum-based chemotherapy in these cell lines (data on file, Eli Lilly and Company). Because the combination of LY with platinum-based therapy may be more effective than the GSK3 inhibitor alone, treatment with this combination deserves investigation in patients with solid tumors.
The primary objective of this multicenter first-in-human phase I study was to determine the maximum tolerated dose (MTD) of intravenous (IV) LY in combination with pemetrexed and carboplatin for patients with advanced/metastatic solid tumors. Secondary objectives included determination of the pharmacokinetics of LY, pemetrexed, and carboplatin; pharmacodynamics of LY (changes in beta-catenin levels in peripheral blood mononuclear cells [PBMCs]); and antitumor activity.
Patients and methods
Approval was obtained from the ethics committees at participating institutions and regulatory authorities. All patients provided informed consent. The study followed the Declaration of Helsinki and good clinical practice guidelines.
Patient population
Patients > 24 years old with histologic or cytologic diagnosis of advanced/metastatic solid tumor for which no proven curative therapy existed; adequate hematologic, hepatic, and renal function; and Eastern Cooperative Oncology Group performance status ≤ 2 were eligible. Because of reports of sternal cartilage degeneration in preclinical studies (data on file, Eli Lilly and Company), enrollment was restricted to adults > 24 years old to ensure that bone growth was complete. Patients previously treated with platinum and/or pemetrexed were allowed. Exclusion criteria included serious cardiac conditions or conduction abnormalities, concomitant medication that may prolong the corrected QT interval (QTc) or induce Torsades de Pointes, chronic atrial fibrillation and/or bradycardia, and symptomatic central nervous system malignancy or metastasis.
Study design and treatment
In the dose-escalation phase, the LY starting dose was 10 mg to ensure an appropriate margin of safety. The maximum dose was capped at 400 mg due to reversible QTc prolongation in dogs (data on file, Eli Lilly and Company). Using a standard 3+3 design, LY dose escalations were increased by 100 % increments up to 80 mg, then by 50 % increments up to 120 mg. Following identification of the MTD, the dose-confirmation phase was opened to include ≤ 10 patients.
The first cycle was 28 days; subsequent cycles were 21 days. On Cycle 1 Day 1 (C1D1), LY was infused intravenously over 60 min (all dose cohorts). Through the 20 mg LY dose cohort on C1D8, pemetrexed (500 mg/m2 10-min IV infusion) [9] was administered per label followed by carboplatin (AUC 5 [dose cohort 1] or AUC 6 mg/mL•min 30-min IV infusion) [10] to allow for pharmacokinetic investigation of pemetrexed/carboplatin alone. On C2D1 pemetrexed was administered, then carboplatin followed by the assigned LY dose (Fig. 1). After safety was established with the first three dose cohorts, the study design was amended to allow LY to be given alone on C1D1 and then in combination with pemetrexed/carboplatin on Day 8, followed by a 21-day recovery (Fig. 1). On C2D1, pemetrexed/carboplatin were then given without LY. For all cohorts, the pemetrexed/carboplatin/LY regimen was administered on C3D1 and beyond. Patients were allowed to continue treatment until disease progression, unacceptable toxicity, or withdrawal of consent.
Safety and efficacy assessments
Clinical and laboratory assessments, including electrocardiograms (ECG) and creatinine clearance using standard Cockroft-Gault estimation, were performed prior to treatment (baseline), weekly in Cycle 1, and then on Day 1 of subsequent cycles. Additional Holter monitoring occurred in Cycle 1 on Days 1, 2, 8, and 9. Slit-lamp examinations were performed for patients in the dose-confirmation phase due to visual disturbances noted during dose escalation. Treatment-emergent adverse events (TEAEs) were evaluated using National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE) v3.0. Dose-limiting toxicity (DLT) that was possibly related to study medication was defined per protocol as the following events during Cycle 1 and 2 (cohorts 1–3) or Cycle 1 (cohort 4 and beyond): grade ≥ 3 non-hematologic toxicity (except for nausea/vomiting without maximal symptomatic/prophylactic treatment), grade 4 hematologic toxicity of >5 days duration, febrile neutropenia, grade 4 thrombocytopenia of any duration, grade ≥ 2 thrombocytopenia plus bleeding, and grade ≥ 3 prolonged QTc interval. In addition, visual disturbance was also defined as a DLT by the investigators.
For patients with measureable disease, radiologic assessments were conducted every other cycle using RECIST version 1.0, and complete and partial responses required confirmation ≥ 4 weeks after the initial response [11, 12].
Pharmacokinetic assessments
Plasma LY concentrations were measured as previously described [8]. The pharmacokinetics of pemetrexed or carboplatin were assessed after LY co-administration. Pemetrexed was quantified by liquid chromatography tandem mass spectrometry; total and unbound platinum from carboplatin was measured by inductively coupled plasma mass spectrometry.
Pharmacodynamic assessments
Blood samples were obtained for measurement of beta-catenin in PBMCs using an enzyme-linked immunosorbent assay (ELISA). Due to technical difficulties with implementing this assay, an alternative fluorescence activated cell sorting (FACS) assay was developed and optimized for detection of beta-catenin in monocytes. Data using the FACS assay were collected from the last six patients during Cycle 1: predose, 4, 8, and 24 h post dose.
Briefly, whole blood was collected from patients pre- and post-administration of LY in cell preparation tubes (CPT™). Tubes were centrifuged following manufacturer’s instructions for PBMC isolation. Cells were stained for monocyte markers (CD-14) in the dark for 10 min at room temperature and red blood cells were lysed by quick exposure to deionized water, followed by the addition of fixation buffer (Cytofix™) and methanol. After repeated washing steps, cells were stained for beta-catenin and fixed in 1 % paraformaldehyde/phosphate bufftered saline (PBS). Beta-catenin assessment was performed using a BD FACSCanto™ II sorter by Esoterix (Brentwood, TN).
Data analysis
Analyses were descriptive and exploratory. For continuous variables, summary statistics included geometric mean, coefficient of variation, mean, median, standard deviation, minimum, and maximum values. Categorical endpoints were summarized using frequency and percentages. Missing data were not imputed. For the pharmacokinetic analysis, parameters were determined using non-compartmental methods (WinNonlin Enterprise v. 5.3; Pharsight, Cary, NC).
Results
Patient population
Forty-one patients were enrolled between November 2007 and February 2011 (median age 59 years, 56 % male). The most common tumor types were non-small cell lung cancer (NSCLC, 24 %) and mesothelioma (22 %) (Table 1). Thirty-five (85.4 %) patients reported receiving ≥ 1 prior systemic therapy with ≥3 prior regimens reported in 17 (41.5 %) patients. Twenty-two (53.7 %), nine (22.0 %), and eight (19.5 %) patients were previously treated with carboplatin, cisplatin, and pemetrexed, respectively.
Dose-escalation and MTD
Forty-one patients received single-dose LY monotherapy lead-in and 37 patients received LY plus pemetrexed/carboplatin across 9 levels (8 dose-escalation levels, 1 dose-confirmation). Ten patients experienced 11 DLTs (Table 2). The first DLT was grade 3 chest wall pain that occurred after treatment with pemetrexed/carboplatin alone (10 mg LY/AUC 6 carboplatin dose level). Escalation proceeded through the LY 20 mg dose without a DLT. The second DLT, occurring after triple-combination therapy, was grade 4 thrombocytopenia at LY 40 mg.
While no DLTs were observed in the LY 80 mg cohort, 1 patient experienced a grade 1 visual impairment described as flashing lights/floater on Day 6 after triple-combination therapy. Subsequently, 3 of 4 patients in the LY 120 mg cohort experienced 3 DLTs during LY monotherapy: grade 2 visual disturbance (blurred vision) and 2 events of grade 3 peri-infusional thoracic pain that included chest, back, and upper abdominal pain, which typically started during or within 1 h of completion of LY infusion. In all DLT cases of peri-infusional thoracic pain, myocardial infarction was ruled out by a thorough cardiology assessment that included serial cardiac enzyme and troponin assessments, electrocardiograms, and observation. These peri-infusional thoracic pain events could not be attributed to any identifiable external cause (e.g., change in drug formulation, lot numbers).
The LY dose was de-escalated to 80 mg and ranitidine IV was added (80+R) prophylactically as a premedication intended to minimize peri-infusional thoracic pain because the symptoms were most consistent with a gastrointestinal spasm or irritation. Despite premedication and de-escalation, 2 of 3 patients who received LY 80+R experienced transient peri-infusional thoracic pain during Cycle 1 with LY monotherapy (grade 1 and grade 4). The further de-escalated dose of LY 60+R was also intolerable with DLTs of grade 3 peri-infusional thoracic pain after LY monotherapy and grade 3 thrombocytopenia with bleeding and grade 4 thrombocytopenia occurring after triple-combination therapy. Thus, the MTD was identified as LY 40 mg, pemetrexed 500 mg/m2, and carboplatin AUC 6 with ranitidine premedication. For this dose cohort, an additional 5 patients (dose-confirmation cohort) were enrolled, wherein 1 patient experienced 2 DLT-equivalent events after receiving triple-combination therapy (grade 4 neutropenia and grade 4 thrombocytopenia with bleeding).
Safety
LY monotherapy and LY plus pemetrexed/carboplatin were well tolerated at the MTD and rates of drug-related TEAEs were similar across the dose cohorts (Table 3). Four patients received LY monotherapy only and 37 patients received a median of 3 cycles of triple-combination treatment. No deaths occurred during the study period.
LY monotherapy lead-in
TEAEs related to LY monotherapy were observed in 26 (63.4 %) patients (Table 3). The most frequent LY-related TEAEs (≥10 %) were nausea (9 [22 %]), fatigue (7 [17.1 %]), gastrointestinal pain (6 [14.6 %]), vomiting (5 [12.2 %]), and peri-infusional thoracic pain (4 [9.8 %]). Five patients (12.2 %) had at least 1 drug-related grade 3/4 TEAE (peri-infusional thoracic pain [n = 2], and 1 each of prolonged QTc, nausea, vomiting, hyperglycemia, and gastrointestinal pain). Three (7.3 %) patients discontinued treatment due to drug-related TEAEs after LY monotherapy: peri-infusional thoracic pain (n = 2; both LY 120) and hypertension (LY 60+R). Dose reductions in LY were reported for two patients after only receiving LY, both due to peri-infusional thoracic pain (120 and 80+R cohorts).
LY with pemetrexed/carboplatin
Thirty-six (97.3 %) patients experienced TEAEs related to LY plus pemetrexed/carboplatin. Hematologic TEAEs included thrombocytopenia (20 [54.1 %]), neutropenia (20 [54.1 %]), leukopenia (14 [37.8 %]), and anemia (12 [32.4 %]) (Table 3). There were no events of febrile neutropenia. Seven patients received growth factor support with darbepoietin or erythropoietin for anemia and two patients received pegfilgrastim for leukopenia. The most common non-hematologic toxicities (≥10 %) were nausea (14 [37.8 %]), anorexia (7 [18.9 %]), vomiting (7 [18.9 %]), fatigue (7 [18.9 %]), dyspepsia (5 [13.5 %]) and mucositis (4 [10.8 %]). At least 1 drug-related grade 3/4 TEAE was reported in 22 (59.5 %) patients, which were primarily hematologic (Table 3).
Eight (19.5 %) patients discontinued treatment due to drug-related TEAEs: carboplatin hypersensitivity/infusion reactions (n = 4), fatigue (n = 2), and 1 each of sensory neuropathy and thrombocytopenia. The most common reason for dose modification for all therapies was thrombocytopenia. The second most common reason for LY dose reduction was peri-infusional thoracic pain.
QTc changes
Among 39 patients with baseline and post-baseline Fridericia-corrected readings, maximum post-baseline QTc change was <30 ms for 16 (41.0 %) patients, 30 to 60 ms for 16 (41.0 %) patients, and >60 ms for 7 (17.9 %) patients. QTc interval changes were observed at all dose levels of LY > 20 mg with changes more pronounced at higher doses of LY (>60 mg). Most QTc prolongations occurred within 30 min of end of infusion and essentially resolved within 2 to 4 h. No measurements > 500 msec were reported. QTc results were deemed clinically insignificant.
Efficacy
Thirty-five (85.4 %) patients who received LY with pemetrexed/carboplatin were evaluable for efficacy including 5 partial responses, 19 stable disease, and 11 progressive disease. Six patients were not evaluable for efficacy by RECIST because all were discontinued during Cycle 1 before obtaining mandatory follow-up scan. The best overall percent change in tumor size from baseline is shown in Fig. 2 for 30 patients with measureable disease.
Individual best overall % change in tumor size from baseline for patients with measureable target lesions (n = 30). Eleven patients were excluded from this plot, including 2 with incomplete data (best overall response not calculated), 3 with non-target lesions only, and 6 who were discontinued from study prior to the second scan. Partial response (PR) indicated by *. Two patients appeared to have a complete response (CR) (100 %), but were deemed a PR due to lack of confirmatory scans. One of six patients with PR was not confirmed. Type of cancer, previous treatment with pemetrexed (P) or carboplatin (C), and number of cycles (cyc) in patients who received at least 6 cyc indicated above bars. Aden NOS adenocarcinoma not otherwise specified, Colon adenocarcinoma colon, Lung adenocarcinoma lung, Esophageal adenocarcinoma esophagus, Unsp NOS unspecified not otherwise specified, Gastric gastric adenocarcinoma, Leio-EPI leiomyosarcoma-epitheliod, Leio-Uterine leiomyosarcoma-uterine, NSCLC non-small cell lung cancer, SCLC small cell lung cancer, SCC small cell cancer
Thirteen (31.7 %) patients who received ≥ 6 cycles had an extended clinical benefit (4 partial responses + 9 stable disease). Prior carboplatin, cisplatin, and pemetrexed therapy was received by 4, 2, and 1 patients, respectively. Nine of 13 patients with clinical benefit received an LY dose that was ≤40 mg.
Pharmacokinetics
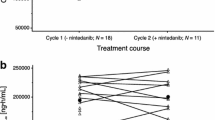
The pharmacokinetic data for this phase I study were previously published [8]. In brief, LY concentrations reached maximal levels at completion of infusion followed by rapid decline in the first hour, more slowly through 8 h post-dose, and then slowly thereafter at 40 mg (Fig. 3). Visual inspection and statistical analysis of the data suggest approximate linearity over the dose range studied (ratios of dose-normalized geometric means were approximately 1 for most dose comparisons; however, 90 % CIs suggested high variability). There were no significant pharmacokinetic interactions when LY was administered in combination with pemetrexed/carboplatin versus when given alone. Pemetrexed/carboplatin pharmacokinetics were similar when given with and without LY (data not shown).
Mean LY2090314 concentration-time profiles after 40 mg (n = 4) on linear scale (shown in red). Mean beta-catenin response over time (n = 4) was measured in peripheral blood mononuclear cells (PBMCs) using the flow assay (shown in black). Reported as fluorescent units. LY209314 concentrations and beta-catenin responses were assessed on Cycle 1 day 1 only
Pharmacodynamics and beta-catenin
Figure 3 summarizes mean beta-catenin response over time following administration of LY 40 mg alone using the FACS-based assay in four patients. Data were collected in the last four patients enrolled in the study; therefore limiting the sample size. A transient increase from baseline in beta-catenin was observed 4 to 8 h after administration, which returned to baseline levels by 24 h post-dose.
Discussion
LY is a GSK3 inhibitor being studied in cancer patients. In this first-in-human trial, the MTD of LY was 40 mg when given in combination with pemetrexed/carboplatin plus ranitidine on a 21-day cycle. Dose-escalation of LY above 40 mg was limited by peri-infusional thoracic pain and visual disturbances.
Although peri-infusional thoracic pain presented in a variety of ways (chest, back, and upper abdominal pain) within 1 h of LY dosing and was associated with negative cardiac evaluations including serial troponin and ECG monitoring, symptoms were potentially due to gastrointestinal spasm or irritation. Addition of IV ranitidine as a premedication to the triple-combination regimen appeared to reduce the frequency and intensity of the pain, although the number of patients treated was low. Whether peri-infusional thoracic pain is due to inhibition of GSK3 or an off-target effect remains unknown. Ranitidine prophylaxis should be evaluated in future trials.
LY was also associated with visual disturbances and QTc prolongation. Two of the visual effects occurred at doses above the MTD. Data from a preclinical study in pigmented rats examining tissue distribution of [14C]LY2090314 demonstrated that the highest radioactivity exposure was in the eye uveal tract with an extended tissue half-life (380–461 h); melanin binding is at least partially responsible for high LY uveal distribution (data on file, Eli Lilly and Company). Additionally, GSK3 expression has been reported in the retina [13]. However, in a retinal-ischemia mouse model, moderate levels of GSK3 inhibition improved neovascularization and retinal hypoxia [14]. Thus, it is possible that visual disturbances at high doses of LY are related to high and extended drug exposure in the uveal tract. Preclinical modeling in dogs provided early evidence that QTc prolongation might influence selection of the LY dose to be used in humans. In this phase I study, QTc interval changes became more pronounced at higher doses of LY (>60 mg) suggesting a relationship between QTc prolongation and dose/concentration. In all patients with QTc prolongation, results were deemed not clinically significant.
The toxicity profile for LY in combination with pemetrexed/carboplatin in this study was generally similar to that reported for pemetrexed/cisplatin [9]. However, grade 3/4 hematologic event rates reported for pemetrexed/cisplatin in subjects with NSCLC and mesothelioma were somewhat lower compared to those reported in this study (anemia: 4 to 6 % vs. 13.5 %, neutropenia: 15 to 23 % vs. 37.8 %, leukopenia: 5 to 15 % vs. 13.5 %, and thrombocytopenia: 4 to 5 % vs. 37.8 %) [9]. Accordingly, the addition of LY to pemetrexed/platinum therapy possibly contributed to the increase in grade 3/4 hematologic events of anemia, neutropenia, and thrombocytopenia, although the small sample size in this study prevents firm conclusions. In addition, the events of peri-infusional thoracic pain and visual disturbance seen with LY in combination with pemetrexed/carboplatin are more likely attributable to LY alone as these events are not commonly reported with pemetrexed/platinum combinations [9].
Preclinical and clinical LY pharmacokinetics were reported previously [8]. Following the 60-min IV infusion, LY declined rapidly from its maximal level in a multi-exponential manner with a mean t1/2 of approximately 3 h at the MTD. Statistical evaluation of LY pharmacokinetics suggests that exposure based on Cmax and AUC was approximately linear over the dose range studied (10 to 120 mg) and associated with moderate to high variability. LY pharmacokinetics were not affected by pemetrexed/carboplatin co-administration, nor were the pharmacokinetics of pemetrexed or carboplatin altered when given with LY (data on file, Eli Lilly and Company).
In this first-in-human study, responses following LY plus pemetrexed/carboplatin treatment were seen among patients with mesothelioma, NSCLC, and breast cancer. As pemetrexed/carboplatin is a recommended NCCN regimen for mesothelioma and non-squamous NSCLC [15, 16], any potential additional efficacy associated with LY therapy would require randomized studies. Interestingly, 73 % of patients enrolled in this trial received prior platinum therapy and 4 of 13 patients with extended clinical benefit had received previous carboplatin, cisplatin, or pemetrexed therapy, suggesting a possible additional benefit of GSK3 blockade may have influenced the clinical benefit observed not only in platinum-naïve patients but also in those with prior platinum exposure. These data corroborate in vitro and xenograft model observations wherein platinum-based regimens exhibited more potent preclinical activity when combined with LY (data on file, Eli Lilly and Company). Transient beta-catenin elevation was demonstrated in PBMCs via flow cytometry from a subset of patients following LY 40 mg monotherapy. Inhibition of GSK3 leads to non-activated/non-phosphorylated beta-catenin that accumulates in the cell, thereby permitting measurement of target engagement with increases in beta-catenin. Previous in-vitro data have also shown that loss of GSK3 by siRNA knockdown results in loss of cell viability [17]. In addition to the observed transient pharmacodynamic effect, inhibition of GSK3 (a downstream target of AKT) by LY [18] may also lead to down-regulation of the PI3K/AKT pathway, a sentinel-signaling pathway for both breast cancer and NSCLC tumorgenesis [19, 20]. AKT up-regulation has been identified as a potential mechanism that can lead to chemotherapy resistance, including to platinum agents [21–23]. GSK3 has also been implicated as a mediator of epithelial to mesenchymal transition [24, 25]. The specific mode(s) of action by which LY exerts its effects when such a complex pathway is being targeted and whether the duration of target inhibition was sufficient to enhance clinical activity of carboplatin and pemetrexed is unknown. A randomized trial is required to fully evaluate whether inhibition of GSK3 truly enhances the clinical effects associated with carboplatin and pemetrexed.
This trial established that LY, either alone or in combination with pemetrexed/carboplatin, can be dosed at a safe and tolerable level in patients with cancer, thus paving the way for further investigation of this agent in solid tumors and hematologic malignancies. The MTD and recommended phase 2 dose of LY in combination with pemetrexed/carboplatin was identified as 40 mg with ranitidine. At doses above 40 mg, we observed an increased rate of peri-infusional thoracic pain and visual disturbance. At doses greater than or equal to the MTD, beta-catenin transiently accumulated in PBMCs, suggesting proof of concept. Antitumor activity following LY plus pemetrexed/carboplatin was observed, and clinical benefit was durable in a subset of patients. The contribution of LY to the clinical benefit observed in this study requires further clinical evaluation.
References
Doble BW, Woodgett JR (2003) GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci 116:1175–1186
Cohen P, Goedert M (2004) GSK3 inhibitors: development and therapeutic potential. Nat Rev Drug Discov 3:479–487
Derksen PW, Tjin E, Meijer HP, Klok MD, MacGillavry HD, van Oers MH, Lokhorst HM, Bloem AC, Clevers H, Nusse R, van der Neut R, Spaargaren M, Pals ST (2004) Illegitimate WNT signaling promotes proliferation of multiple myeloma cells. Proc Natl Acad Sci 101:6122–6127
Tan J, Zhuang L, Leong HS, Iyer NG, Liu ET, Yu Q (2005) Pharmacologic modulation of glycogen synthase kinase-3beta promotes p53-dependent apoptosis through a direct Bax-mediated mitochondrial pathway in colorectal cancer cells. Cancer Res 65:9012–9020
Shimura T, Kakuda S, Ochiai Y, Kuwahara Y, Takai Y, Fukumoto M (2011) Targeting the AKT/GSK3β/cyclin D1/Cdk4 survival signaling pathway for eradication of tumor radioresistance acquired by fractionated radiotherapy. Int J Radiat Oncol Biol Phys 80:540–548
Kho PS, Wang Z, Zhuang L, Li Y, Chew J-L, Ng H-H, Liu ET, Yu Q (2004) p53-regulated transcriptional program associated with genotoxic stress-induced apoptosis. J Biol Chem 279:21183–21192
Ghosh JC, Altieri DC (2005) Activation of p53-dependent apoptosis by acute ablation of glycogen synthase kinase-3β in colorectal cancer cells. Clin Cancer Res 1:4580–4588
Zamek-Gliszczynski MJ, Abraham TL, Alberts JJ, Kulanthaivel P, Jackson KA, Chow KH, McCann DJ, Hu H, Anderson S, Furr NA, Barbuch RJ, Cassidy KC (2013) Pharmacokinetics, metabolism, and excretion of the GSK-3 inhibitor LY2090314 in rats, dogs, and humans: a case study in rapid clearance by extensive metabolism with low circulating metabolite exposures. Drug Metab Dispos 41:714–726
Alimta® pemetrexed for injection Product Information, Lilly USA, LLC, January 2013
Paraplatin (carboplatin aqueous solution) Injection. Bristol-Myers Squibb Company, July, 2010
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Byrne MJ, Nowak AK (2004) Modified RECIST criteria for assessment of response in malignant pleural mesothelioma. Ann Oncol 15:257–260
Kumar A, Hou X, Lee C, Li Y, Maminishkis A, Tang Z, Zhang F, Langer HF, Arjunan P, Dong L, Wu Z, Zhu LY, Wang L, Min W, Colosi P, Chavakis T, Li X (2010) Platelet-derived growth factor-DD targeting arrests pathological angiogenesis by modulating glycogen synthase kinase-3beta phosphorylation. J Biol Chem 285:15500–15510
Hoang MV, Smith LE, Senger DR (2010) Moderate GSK-3β inhibition improves neovascular architecture, reduces vascular leakage, and reduces retinal hypoxia in a model of ischemic retinopathy. Angiogenesis 13:269–277
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Malignant pleural mesothelioma. Version 1: 2013. National Comprehensive Cancer Network, Inc. Available at http://www.nccn.com. Accessed 01 April 2015
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Non-small cell lung cancer. Version 2: 2013. National Comprehensive Cancer Network, Inc. Available at http://www.nccn.com. Accessed 01 April 2015
Naito S, Bilim V, Yuuki K, Ugolkov A, Motoyama T, Nagaoka A, Kato T, Tomita Y (2010) Glycogen synthase kinase-3beta: a prognostic marker and a potential therapeutic target in human bladder cancer. Clin Cancer Res 16:5124–5132
Manning BD, Cantley LC (2007) AKT/PKB signaling: navigating downstream. Cell 129:1261–1274
Wong KK, Engelman JA, Cantley LC (2010) Targeting the PI3K signaling pathway in cancer. Curr Opin Genet Dev 20:87–90
David O, Jett J, LeBeau H, Dy G, Hughes J, Friedman M, Brody AR (2004) Phospho-Akt overexpression in non-small cell lung cancer confers significant stage-independent survival disadvantage. Clin Cancer Res 10:6865–6871
Kim D, Dan HC, Park S, Yang L, Liu Q, Kaneko S, Ning J, He L, Yang H, Sun M, Nicosia SV, Cheng JQ (2005) AKT/PKB signaling mechanisms in cancer and chemoresistance. Front Biosci 10:975–987
Abedini MR, Muller EJ, Bergeron R, Gray DA, Tsang BK (2010) Akt promotes chemoresistance in human ovarian cancer cells by modulating cisplatin-induced, p53-dependent ubiquitination of FLICE-like inhibitory protein. Oncogene 29:11–25
Girouard J, Lafleur MJ, Parent S, Leblanc V, Asselin E (2013) Involvement of Akt isoforms in chemoresistance of endometrial carcinoma cells. Gynecol Oncol 128:335–343
Bachelder RE, Yoon SO, Franci C, de Herreros AG, Mercurio AM (2005) Glycogen synthase kinase-3 is an endogenous inhibitor of Snail transcription: implications for the epithelial-mesenchymal transition. J Cell Biol 168:29–33
Zhou BP, Deng J, Xia W, Xu J, Li YM, Gunduz M, Hung MC (2004) Dual regulation of Snail by GSK-3 beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol 6:931–940
Acknowledgments
The authors would like to thank the following co-investigators and study personnel:
Charles C. Williams MD and Susan Minton, DO (Moffitt Cancer Center co-investigators) and Rasa Hamilton (Moffitt Cancer Center) for her editorial services.
Declaration of personal interests
Drs. Jhanelle Gray, Jeffrey Infante, George Simon and Howard Burris have served as consultants/advisors for Eli Lilly and Company and received research funding from Eli Lilly and Company as investigators for this study. Jennifer Cooksey and Suzanne Jones have no conflicts to report. Adeline Yeo was an employee of InVentiv Health at the time this study was conducted and was under contract with Eli Lilly and Company to provide statistical support. At the time this study was conducted, Les Brail, Daphne Farrington, Kimberley Jackson, Kay Chow, and Maciej Zamek-Gliszczynski were all employees and stockholders of Eli Lilly and Company.
Declaration of funding interests
These studies and the preparation of this paper were funded in full by Eli Lilly and Company. Data analyses were undertaken by Eli Lilly and Company. Writing support was provided by Teresa Tartaglione, PharmD of ClinGenuity, LLC, and funded by Eli Lilly and Company.
Financial support
Work was supported by Eli Lilly and Company, Indianapolis, Indiana, USA.
Author information
Authors and Affiliations
Corresponding author
Additional information
Registered under ClinicalTrials.gov (http://clinicaltrials.gov) identifier: NCT01287520.
Rights and permissions
About this article
Cite this article
Gray, J.E., Infante, J.R., Brail, L.H. et al. A first-in-human phase I dose-escalation, pharmacokinetic, and pharmacodynamic evaluation of intravenous LY2090314, a glycogen synthase kinase 3 inhibitor, administered in combination with pemetrexed and carboplatin. Invest New Drugs 33, 1187–1196 (2015). https://doi.org/10.1007/s10637-015-0278-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-015-0278-7