Abstract
Purpose
To evaluate the physiology of the macular and whole retina after intravitreal aflibercept (IVAs) injections in patients with macular edema associated with a central retinal vein occlusion (CRVO) by electroretinography (ERG).
Methods
We studied 20 eyes of 20 patients with non-ischemic CRVO (72.0 ± 9.2 years). All patients were treated with monthly injections of IVA for the initial 3 months and then treated by the treat-and-extend (TAE) regimen for 12 months. The best-corrected visual acuity (BCVA), optical coherence tomographic images, focal macular ERGs (fmERGs), and full-field ERGs recorded before and after the treatment were compared. The fmERGs were elicited by a 15° white stimulus spot centered on the fovea. The full-field ERGs were recorded by a protocol recommended by International Society for Clinical Electrophysiology of Vision. The amplitudes and implicit times determined before and after the IVA were compared.
Results
The foveal thickness was significantly reduced accompanied by improvement of the BCVA after the treatments, and the improvements were maintained for at least 12 months. The amplitudes and implicit times of the fmERGs improved continuously for the 12 months. On the other hand, the reduced amplitudes of the full-field ERG, summed oscillatory potentials, and the photopic negative responses remained unchanged for the 12-month period. However, the implicit times of the maximum and cone responses were significantly shortened after the IVA.
Conclusions
IVA injections by the TAE regimen led to a continuous improvement of the macular function in patients with ME associated with a CRVO. However, the function of the whole retina changed differently than the macula after the treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A central retinal vein occlusion (CRVO) is a vascular disorder which affects approximately 0.3% of the Japanese population over the age of 40 years [1]. The visual prognosis and treatment protocol depend on whether it is the ischemic or non-ischemic type of CRVO. Because a transition from the non-ischemic type to the ischemic type often occurs [2], careful observations of the hemodynamics are required.
The macular edema (ME) associated with a CRVO is the major cause of the decreased vision, and it is associated with a poor visual prognosis. Because the ME frequently causes irreversible macular dysfunctions, prompt interventions are needed to resolve the ME. At present, intravitreal injections of anti-vascular endothelial growth factor (anti-VEGF) agents have become the first line treatment for the ME based on the results of large randomized clinical trials using ranibizumab [3, 4] or aflibercept [5, 6]. However, it is necessary to treat the eyes repeatedly with the intravitreal injections at short intervals [7].
In all of the clinical trials, the visual function has been mainly evaluated by the best-corrected visual acuity (BCVA) although a CRVO can affect wide areas of the retina. And, it has been assumed that retinal areas other than the fovea also had a functional recovery after the anti-VEGF treatments. In these earlier studies, the visual status was determined by the BCVA [5, 6], the retinal sensitivity [8], degree of metamorphopsia [9], and degree of aniseikonia [10]. Most of these assessments were subjective, and as best we know, there have been few reports using an objective assessment of the retinal physiology after an anti-VEGF treatment [11,12,13,14].
Electroretinography (ERG) can be used to assess the physiological status of the retina objectively, and it has been widely used in various eye disorders. The retinal function of the whole retina can be evaluated by full-field ERGs [15, 16], and the focal macular ERGs (fmERGs) developed by Miyake et al. [17, 18] can be used to evaluate the macular function in various retinal diseases [15].
CRVO is a retinal disorder which affects a wide area of the retina including the macula. Therefore, both the fmERGs [19] and the full-field ERGs are reduced, and the degree of reduction depends on the extent of the retina affected by the ischemia [13, 20, 21]. It has been reported that the reduced retinal physiology can partially recover after the administration of anti-VEGF agents [12]. However, it has not been determined whether the function of the macula and peripheral retina change to the same degree.
Thus, the aim of this study was to determine the physiological changes of the macula and whole retina in patients with ME due to a CRVO using fmERGs and full-field ERGs. The ERGs that were recorded before were compared to those recorded after the treatment with intravitreal aflibercept (IVA).
Methods
Patients
We studied 20 eyes of 20 patients with a CRVO including 5 eyes with hemi-CRVO. There were 15 men and 5 women whose mean age was 72.0 ± 9.2 years (± standard deviation) with a range of 47 to 85 years. All patients underwent comprehensive ophthalmological examinations including measurements of the BCVA with a Snellen chart, slit-lamp biomicroscopy, and indirect ophthalmoscopy before and throughout the follow-up period. Fluorescein angiography (FAG) was performed before the treatments, and all eyes were classified as the non-ischemic type of CRVO based on the FAG. We excluded patients who required panretinal photocoagulation because of the transition to the ischemic type during the study period. Patients with a history of other retinal diseases treated with pharmacologic agents including anti-VEGF agents or steroid therapy, and with a history of vitrectomy or glaucoma surgery were excluded.
We also recorded focal macular and full-field ERGs with the same protocol from age-matched normal subjects with a mean age of 70.6 ± 9.1 years (n = 16) with a range from 43 to 84 years. The normal subjects consisted of 8 men and 7 women in which six had been treated with medication to control hypertension.
The Institutional Review Board of the Dokkyo Medical University approved the procedures used, and the procedures conformed to the tenets of the Declaration of Helsinki. An informed consent was obtained from all patients after a full explanation of the nature of the study and possible complications.
Intravitreal injections of aflibercept
A 30G needle was inserted through the pars plana to inject 0.5 mg/0.05 ml of aflibercept into the vitreous. The injections were made at monthly intervals for 3 months. Later, additional IVAs were given according to treat-and-extend (TAE) regimen for the following 9 months. The interval between the injections was extended by 1 month unless a recurrence was observed, and when a recurrence of exudation was observed, the interval of injection was shortened by 1 month.
Spectral-domain optical coherence tomography (SD-OCT)
The retinal morphology was evaluated by SD-OCT (RS-3000 advance, Nidek Corporation, Japan). The OCT scans passed through the fovea horizontally and vertically. The averaged foveal thickness was obtained by averaging the horizontal and vertical thickness at the fovea.
Focal macular ERG (fmERG) recordings
The pupils were dilated to approximately 8 mm in diameter by topical 0.5% tropicamide and 0.5% phenylephrine HCL. The fmERGs were recorded from the macular area by the methods developed by Miyake et al. [17, 18]. The stimulus system was integrated into an infrared fundus camera (ER-80, Kowa Company, Ltd., Aichi, Japan). The stimulus spot was 15° in diameter that was centered on the macula, and the position was confirmed by viewing the ocular fundus on a monitor of the fundus camera. The intensity of the white stimulus was 30 cd/m2 and that of the background was 1.5 cd/m2. The stimulus duration was 10 ms. The stimulus and background lights were produced by light-emitting diodes (LEDs). The fmERGs were picked-up by a Burian–Allen bipolar contact lens electrode (Hansen Ophthalmic Laboratories, Iowa City, IA), and a chlorided silver electrode was placed on the left ear lobe as the ground electrode. The responses were digitally band-pass filtered from 5 to 200 Hz for the a- and b-waves and the photopic negative response (PhNR) and from 50 to 500 Hz for the oscillatory potentials (OPs; PuREC, Mayo Corporation, Inazawa, Aichi, Japan). Approximately 300 responses elicited at a stimulation frequency of 5 Hz were averaged for the fmERGs.
The PhNR amplitude was measured from the baseline to the negative trough at 70 ms for the focal PhNR according to the findings in earlier studies [22]. The amplitudes of the OP1, OP2, and OP3 were measured and summed and are designated as the ΣOPs [22].
Full-field ERG recordings
For the full-field ERG recordings, the stimuli and background lights were produced by LEDs and were presented in a ganzfeld dome. The intensity and frequency of the stimuli were controlled by the UTAS Visual Testing System (LKC Technologies, Inc., Gaithersburg, MD, USA). According to the ISCEV protocol [16], the eyes were dark-adapted for at least 20 min, and the rod ERGs were recorded by white light at an intensity of 0.01 cd-s/m2. The maximum mixed cone-rod ERGs were elicited by a white stimulus of 9.5 cd-s/m2. After light-adaptation with a white background light of 34 cd/m2 for 10 min, the cone ERGs and 30-Hz flicker ERGs were recorded under the light-adaptation. The intensity of the white stimuli was 3 cd-s/m2 which was presented on the white background. The duration of the stimulus was 3 ms. The responses were digitally band-pass filtered from 0.5 to 500 Hz.
Statistical analyses
Kruskal–Wallis tests were used to determine the significance of the differences in the pretreatment and posttreatment values. Dunn’s post hoc tests were used for multiple comparisons among the groups. The statistical analyses were performed with GraphPad PRISM 7. A P < 0.05 was taken to be statistically significant.
Results
Representative case
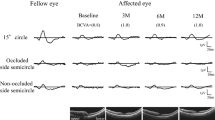
The findings in a representative 82-year-old man with a ME associated with a non-ischemic CRVO are shown in Fig. 1. The patient was treated initially with three monthly IVA injections followed by three additional injections during the 1 year TAE regimen (Fig. 1). FAG showed a late phase vascular leakage of dye from tortuous and dilated veins with pooling of dye in the macular area. Cross-sectional OCT images demonstrated a thickening of the macular area with intraretinal cysts. The cysts promptly disappeared after the first IVA without a recurrence for 12 months (Fig. 2). The amplitudes of the fmERGs were severely reduced in the affected eye at the baseline compared to the fellow eye. However, as the treatment continued, the fmERG amplitudes gradually increased. On the other hand, the amplitudes of the full-field ERG were mildly deteriorated in the affected eye at the baseline compared to the fellow eye and did not have any significant changes after the IVA injections. The average number of IVA injections was 5.9 ± 0.7 with a range from 5 to 8 throughout the study period.
Representive focal macular electroretinograms (fmERGs) and full-field ERGs recorded before and after beginning the IVA injections from a patient with ME associated with a CRVO. All components of the fmERGs were decreased in the affected eye at the baseline. However, the fmERG amplitudes gradually increased after beginning the IVA injections. In the full-field ERG, the b-wave amplitudes of the rod and maximum responses were reduced at the baseline and remained unchanged after starting the IVA injections. IVA, intravitreal injection of aflibercept; ME, macular edema; CRVO, central retinal vein occlusion
Changes of best-corrected visual acuity (BCVA) and foveal thickness
The BCVA was 0.59 ± 0.50 logMAR units at the baseline, and it was significantly improved to 0.33 ± 0.49 logMAR units at 3 months after the beginning the IVA (P < 0.005). At 12 months, the BCVA was 0.22 ± 0.34 logMAR units which was significantly better than that at the baseline (P < 0.0005).
The foveal thickness was 556 ± 145 μm at the baseline, and it was significantly reduced to 276 ± 47 μm at 3 months (P < 0.05 × 10−4). This significant recovery was maintained until month 12 (P < 0.00005–0.0001).
Changes of fmERGs following IVA injections
The changes in the amplitudes and implicit times of each component of the fmERGs before and after the IVA injections are shown along with data from the normal eyes in Fig. 3. At the baseline, the amplitudes of all components were significantly smaller in the affected eyes than those of the unaffected fellow eyes (Fig. 2a, d; P < 0.0001). In addition, the implicit times of the a- and b-waves were prolonged at the baseline compared to that of the unaffected eye (P < 0.0005–0.0001).
Graphs showing the averaged amplitudes and implicit times of the fmERGs recorded from normal subjects and patients with ME associated with a CRVO before and after the IVA injections. The averaged amplitudes of the a- (a) and b-waves (b), PhNR (c) and ΣOPs (d) were decreased in the affected eyes at the baseline. After beginning the IVAs, the amplitudes of these ERG components significantly increased. Although the averaged implicit times of the a- (e) and b-waves (f) were prolonged at the baseline, they significantly shortened after starting the IVAs. fmERG, focal macular electroretinogram; ME, macular edema; CRVO, central retinal vein occlusion; IVA, intravitreal injection of aflibercept; PhNR, photopic negative response; OPs, oscillatory potentials. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
At 3 months after beginning the treatment, the amplitudes of the fmERGs except for the a-wave were significantly increased compared to the baseline (P < 0.05). The implicit times of the a- and b-waves were significantly shortened after the IVA (P < 0.0005). The amplitudes appeared to increase with a continuation of the treatment. Even at the month 12, the amplitudes and implicit time of each component of the fmERGs remained improved compared to the baseline (P < 0.0005–0.05). Although the implicit times returned to values of the unaffected contralateral eyes, the amplitudes of all components were still reduced at 12 months compared to those of the unaffected eyes.
Changes of full-field ERGs following IVA injections
The averaged amplitudes and implicit times of the dark-adapted ERGs at the baseline and after the IVA injections are plotted along with data from the normal eyes in Fig. 4. At the baseline, the amplitude of the full-field rod b-wave of the affected eyes was significantly smaller than that of the unaffected eyes (P < 0.001). The rod b-wave amplitude remained unchanged or slightly reduced at 12 months after the TAE regimen.
Averaged amplitudes and implicit times of the rod and maximum responses of the full-field ERG recorded from normal subjects and CRVO patients with ME before and after IVAs. The amplitudes of the rod b-wave (a) and ΣOPs of the maximum response (d) significantly decreased in the affected eyes at the baseline (before the treatment) without consequent improvement after the treatment. The a- (b) and b-wave (c) amplitudes of the maximum response remained unchanged in the affected eyes at the baseline. Although the implicit times of the a- (e) and b-waves (f) of the maximum response were significantly prolonged at the baseline, they shortened after the IVA injections. ERGs, electroretinograms; CRVO, central retinal vein occlusion; ME, macular edema; IVA, intravitreal injection of aflibercept; OPs, oscillatory potentials. *P < 0.05, **P < 0.01, ***P < 0.001
The averaged ΣOPs amplitudes of the maximum responses of the full-field ERGs were significantly reduced at the baseline compared to those of the contralateral unaffected eyes (P < 0.001). However, the a- and b-waves were not significantly different from that of the fellow normal eyes (Fig. 4b–d). The ΣOPs amplitudes showed a trend of improvement after the IVA, but the improvement was not significant. Although the implicit times of the a- and b-waves were significantly prolonged at the baseline (P < 0.05 for a-wave, P < 0.001 for b-wave), they returned to values that were not significantly different from that of the contralateral unaffected eyes at 3 months (Fig. 3e, f). The b/a-wave amplitude ratios at the baseline were 1.33 ± 0.35 and 1.35 ± 0.43 for affected and unaffected fellow eyes, respectively. The value of the normal eyes was 1.45 ± 0.37. These findings suggest that the retinal function might be subclinically impaired even in the unaffected fellow eyes.
The averaged amplitudes and implicit times of the light-adapted cone responses of the affected and unaffected contralateral eyes are plotted along with data from the normal eyes in Fig. 5. Although the a- and b-wave amplitudes were not reduced in the affected eyes, the PhNR and ΣOPs amplitudes were significantly smaller at the baseline compared to the unaffected eyes (P < 0.01 for PhNR, P < 0.05 for ΣOPs). Despite the IVA injections, the PhNR and ΣOPs amplitudes remained unchanged at 12 months. The implicit times of the a- and b-waves of the cone response were significantly prolonged at the baseline compared to the unaffected eyes (P < 0.05 for a-waves, P < 0.001 for b-waves), but they shortened to the values of the contralateral eyes at 3 months (P < 0.05).
Averaged amplitudes and implicit times of the cone ERGs of the full-field ERGs recorded from normal subjects and patients with ME associated with a CRVO before and after the IVA injections. The amplitudes of the a- (a) and b-waves and ΣOPs (c) remained unchanged in the affected eyes at the baseline, and the PhNR amplitude was significantly reduced without a subsequent improvement by the treatment (d). Although the implicit times of the a- (e) and b-waves (f) are significantly prolonged at the baseline, they shortened after the treatment. ERG, electroretinogram; ME, macular edema; CRVO, central retinal vein occlusion; OPs, oscillatory potentials; PhNR, photopic negative response. *P < 0.05, **P < 0.01, ***P < 0.001
The averaged implicit times and amplitudes of the 30 Hz flicker ERGs before and after the IVA injections for the affected and unaffected contralateral eyes are plotted along with data from the normal eyes in Fig. 6. Although the amplitudes of the affected eyes at the baseline were not different from those of the unaffected contralateral eyes, the implicit times of the affected eyes at the baseline were prolonged significantly compared to the unaffected eyes (P < 0.001). It was significantly shorter at 6 and 12 months after treatment compared to the baseline (P < 0.05).
Averaged amplitudes (a) and implicit times (b) of the full-field 30 Hz flicker ERGs recorded from normal subjects and patients with ME associated with a CRVO before and after IVA injections. Although the amplitudes of the flicker response remained unchanged in the affected eyes, the implicit times were prolonged with subsequent recovery following the IVA injections. ME, macular edema; CRVO, central retinal vein occlusion. *P < 0.05, **P < 0.001
Correlation between mfERGs at baseline and final visual acuity
The foveal thickness at the baseline measured by OCT was not significantly correlated with the BCVA at month 12. However, the amplitudes of the a- and b-waves and the ΣOPs of the fmERG were significantly correlated with the BCVA at month 12 (a-wave, P < 0.05; b-wave, P < 0.0001; ΣOPs, P < 0.05). These findings suggest that the fmERGs could predict a visual prognosis after anti-VEGF therapy.
Discussion
The results showed that the macular physiology assessed by the fmERGs is depressed by the ME associated with a CRVO, and it was improved by IVA injections. By continuing the IVA injections by the TAE regimen, further improvements of the macular function were obtained. On the other hand, the whole retinal function was only partially recovered. The amplitudes of the rod b-wave, OPs of the maximum and cone full-field ERGs, and the PhNR were reduced in the CRVO eyes, and it remained unchanged despite the IVAs. The implicit times of the a- and b-waves of maximum and cone responses and the 30 Hz flicker responses were improved soon after beginning the IVA injection. These findings indicate that the function of the whole retina changes differently from that of the macula after the IVA injections.
Partial but sustained recovery of macular function after IVA
Ogino et al. [19] reported that the amplitudes and implicit times of the fmERG were reduced in eyes with ME associated with a CRVO. Our results showed that the implicit times promptly recovered to the normal level after beginning the IVA injections. On the other hand, the amplitudes gradually increased for 12 months, but they did not reach the value of the unaffected contralateral eyes. This indicated that the surviving retinal cells had some functional recovery from the treatment, but there was some irreversible damage to the macula due to the ME.
All retinal layers are affected by ME
CRVO is a retinal vascular disorder that mainly affects the inner and middle layers of the retina. However, all components of the fmERGs and full-field ERGs including the a-wave implicit times were decreased which indicated that the photoreceptors were also affected. Serous macular detachments are often seen in patients with severe ME. Examination of the OCT images shows abnormal configurations of the ellipsoid zones in these eyes which were found to be a sign of limited visual recovery [23, 24]. These structural changes of the outer retina could explain the abnormal a-wave amplitudes in our CRVO patients.
The amplitudes of the inner retinal components of the full-field ERG, such as the OPs and PhNR amplitudes, were predominantly affected by the CRVO. Despite the treatment, the depressed inner retinal function did not have any recovery. This indicated that even in cases of non-ischemic CRVO there is permanent damage to the amacrine cells and retinal ganglion cells (RGCs).
Although the maximum b-wave amplitude of the full-field ERGs was preserved in the affected eyes, the rod b-wave amplitude was decreased indicating that the rod-bipolar cells lose their sensitivity, viz., the stimulus intensity to obtain one-half of the maximum b-wave amplitude was reduced. In addition, this functional change remained unchanged or even worsened despite continuing the treatment which suggested an irreversible damage to the rod-bipolar cells.
While the rod-bipolar cell function was permanently affected, the cone b-wave and 30 Hz flicker response amplitudes were preserved but with prolonged implicit times which were restored by the treatment. This suggests that the function of the cone-bipolar cells is less affected by CRVO than the rod-bipolar cells. It was reported that the cone b-wave amplitudes and the 30-Hz flicker response became even supernormal with prolonged implicit times in CRVO patients with mild degree of ischemia [11, 25], which is similar to our results that the cone b-wave and 30 Hz flicker ERG amplitudes were not affected by non-ischemic type CRVO. We have reported that the predominant alternation was the rod-bipolar cell function in patients with central retinal artery occlusion [26] although further studies are needed to draw a solid conclusion that retinal circulator disturbance predominantly affects the function of the rod-bipolar cell rather than the cone-bipolar cell. Alternatively, intraretinal hemorrhage may predominantly affect the ERG elicited by low intensity stimuli, such as the rod response, because it can act like a neutral density filter to absorb the stimuli which reach the photoreceptor.
Recovery of whole retina function is different from that of macular function after IVA treatment
Although the amplitudes of all components of the fmERG showed partial recovery, the rod b-wave, Ops, and PhNR amplitudes of the full-field ERG remained unchanged during and after the treatment. The FA demonstrated that the dye leakage from the retinal vessels occurred in the macula rather than the peripheral retina (see Fig. 1). This suggested that the macular lesion was caused by circulatory disturbances and vascular leakages, while the peripheral lesion was mainly by circulatory disturbances in eyes with a non-ischemic CRVO. Suppression of the intraocular VEGF by anti-VEGF agents decreased the vascular leakage leading to the functional recovery of the macula but not the circulatory disturbances. Therefore, the recovery of the whole retinal function was minimal in contrast to the recovery of the macular function.
Implicit times of 30 Hz flicker ERGs
It has been reported that the implicit times of the 30 Hz flicker ERGs are good indicators for predicting the development of neovascular glaucoma (NVG) [27]. The cutoff value of the implicit time for this discrimination has been reported to be 35.0 ms. In our cohort, the implicit time was shortened from 33.1 ± 3.3 to 32.3 ± 2.6 ms by the IVA injections, indicating that the risk for NVG development was lowered by the IVA injections. In fact, none of the patients developed NVG during the follow-up period.
Limitations of study
First, it was difficult to eliminate the possibility of a spontaneous recovery of the ERGs because it has been reported that CRVOs can resolve spontaneously during its natural course [28]. Second, we did not include ischemic CRVO which requires laser photocoagulation to prevent the development of NVG [29] because the laser photocoagulation affects the amplitudes of the full-field ERGs. Ischemic CRVO possibly has different changes of the retinal function after the treatment. Third, we treated patients with the TAE regimen, while most of the CRVO patients with ME are treated with the pro re nata regimen in clinical practice. Therefore, number of injections and recurrence rates may differ from our results, which could affect the functional changes of the macula and whole retina differently.
Conclusions
Administration of IVAs with the TAE regimen for patients with ME associated with a CRVO leads to a continuous improvement of the macular function as assessed by the fmERGs. However, the function of the whole retina changed differently than the macula after the treatment leaving a permanent depression of the rod-bipolar, amacrine, and RGCs responses. These findings suggest that irreversible and widespread damage to the retina occurs even in cases of non-ischemic CRVO.
References
Arakawa S, Yasuda M, Nagata M, Ninomiya T, Hirakawa Y, Doi Y, Kiyohara Y, Ishibashi T (2011) Nine-year incidence and risk factors for retinal vein occlusion in a general Japanese population: the Hisayama Study. Invest Ophthalmol Vis Sci 52:5905–5909
The Central Vein Occlusion Study Group (1997) Natural history and clinical management of central retinal vein occlusion. The Central Vein Occlusion Study Group. Arch Ophthalmol 115:486–491
Brown DM, Campochiaro PA, Singh RP, Li Z, Gray S, Saroj N, Rundle AC, Rubio RG, Murahashi WY, Investigators CRUISE (2010) Ranibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology 117:1124–1133
Campochiaro PA, Brown DM, Awh CC, Lee SY, Gray S, Saroj N, Murahashi WY, Rubio RG (2011) Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: twelve-month outcomes of a phase III study. Ophthalmology 118:2041–2049
Brown DM, Heier JS, Clark WL, Boyer DS, Vitti R, Berliner AJ, Zeitz O, Sandbrink R, Zhu X, Haller JA (2013) Intravitreal aflibercept injection for macular edema secondary to central retinal vein occlusion: 1-year results from the phase 3 COPERNICUS study. Am J Ophthalmol 155:429–437
Ogura Y, Roider J, Korobelnik JF, Holz FG, Simader C, Schmidt-Erfurth U, Vitti R, Berliner AJ, Hiemeyer F, Stemper B, Zeitz O, Sandbrink R, GALILEO Study Group (2014) Intravitreal aflibercept for macular edema secondary to central retinal vein occlusion: 18-month results of the phase 3 GALILEO study. Am J Ophthalmol 158:1032–1038
Heier JS, Campochiaro PA, Yau L, Li Z, Saroj N, Rubio RG, Lai P (2012) Ranibizumab for macular edema due to retinal vein occlusions: long-term follow-up in the HORIZON trial. Ophthalmology 119:802–809
Ota M, Tsujikawa A, Ojima Y, Miyamoto K, Murakami T, Ogino K, Akagi-Kurashige Y, Muraoka Y, Yoshimura N (2012) Retinal sensitivity after resolution of the macular edema associated with retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol 250:635–644
Manabe K, Osaka R, Nakano Y, Takasago Y, Fujita T, Shiragami C, Hirooka K, Muraoka Y, Tsujikawa A (2017) Metamorphopsia associated with central retinal vein occlusion. PLoS ONE 12:e0186737
Okamoto F, Sugiura Y, Okamoto Y, Hiraoka T, Oshika T (2017) Aniseikonia in various retinal disorders. Graefes Arch Clin Exp Ophthalmol 255:1063–1071
Miyata R, Kondo M, Kato K, Sugimoto M, Matsubara H, Ikesugi K, Ueno S, Yasuda S, Terasaki H (2018) Supernormal flicker ERGs in eyes with central retinal vein occlusion: clinical characteristics, prognosis, and effects of anti-VEGF agent. Invest Ophthalmol Vis Sci 59:5854–5861
Yasuda S, Kachi S, Ueno S, Piao CH, Terasaki H (2015) Flicker electroretinograms before and after intravitreal ranibizumab injection in eyes with central retinal vein occlusion. Acta Ophthalmol 93:e465–e468
Gardašević Topčić I, Šuštar M, Brecelj J, Hawlina M, Jaki Mekjavić P (2014) Morphological and electrophysiological outcome in prospective intravitreal bevacizumab treatment of macular edema secondary to central retinal vein occlusion. Doc Ophthalmol 129:27–38
Moschos MM, Moschos M (2008) Intraocular bevacizumab for macular edema due to CRVO. A multifocal-ERG and OCT study. Doc Ophthalmol 116:147–152
Miyake Y (2006) Electrodiagnosis of retinal diseases. Springer, Tokyo
McCulloch DL, Marmor MF, Brigell MG, Hamilton R, Holder GE, Tzekov R, Bach M (2015) ISCEV Standard for full-field clinical electroretinography (2015 update). Doc Ophthalmol 130:1–12
Miyake Y, Yanagida K, Kondo K, Ota I (1981) Subjective scotometry and recording of local electroretinogram and visual evoked response. System with television monitor of the fundus. Jpn J Ophthalmol 25:439–448
Miyake Y (1988) Studies on local macular ERG. Acta Soc Ophthalmol Jpn 92:1419–1449
Ogino K, Tsujikawa A, Nakamura H, Miyamoto K, Murakami T, Muraoka Y, Kurashige Y, Yoshimura N (2011) Focal macular electroretinogram in macular edema secondary to central retinal vein occlusion. Invest Ophthalmol Vis Sci 52:3514–3520
Yasuda S, Kachi S, Kondo M, Ushida H, Uetani R, Terui T, Piao CH, Terasaki H (2011) Significant correlation between electroretinogram parameters and ocular vascular endothelial growth factor concentration in central retinal vein occlusion eyes. Invest Ophthalmol Vis Sci 52:5737–5742
Chen H, Wu D, Huang S, Yan H (2006) The photopic negative response of the flash electroretinogram in retinal vein occlusion. Doc Ophthalmol 113:53–59
Machida S, Toba Y, Ohtaki A, Gotoh Y, Kaneko M, Kurosaka D (2008) Photopic negative response of focal electroretinogram in glaucomatous eyes. Invest Ophthalmol Vis Sci 49:5636–5644
Lima VC, Yeung L, Castro LC, Landa G, Rosen RB (2011) Correlation between spectral domain optical coherence tomography findings and visual outcomes in central retinal vein occlusion. Clin Ophthalmol 5:299–305
Hasegawa T, Ueda T, Okamoto M, Ogata N (2014) Presence of foveal bulge in optical coherence tomographic images in eyes with macular edema associated with branch retinal vein occlusion. Am J Ophthalmol 157:390–396
Gouras P, MacKay CJ (1992) Supernormal cone electroretinograms in central retinal vein occlusion. Invest Ophthalmol Vis Sci 33:508–515
Machida S, Gotoh Y, Tanaka M, Tazawa Y (2004) Predominant loss of the photopic negative response in central retinal artery occlusion. Am J Ophthalmol 37:938–940
Kjeka O, Bredrup C, Krohn J (2007) Photopic 30 Hz flicker electroretinography predicts ocular neovascularization in central retinal vein occlusion. Acta Ophthalmol Scand 85:640–643
McIntosh RL, Rogers SL, Lim L, Cheung N, Wang JJ, Mitchell P, Kowalski JW, Nguyen HP, Wong TY (2010) Natural history of central retinal vein occlusion: an evidence-based systematic review. Ophthalmology 117:1113–1123
The Central Vein Occlusion Study Group (1995) A randomized clinical trial of early panretinal photocoagulation for ischemic central vein occlusion. The Central Vein Occlusion Study Group N report. Ophthalmology 102:1434–1444
Acknowledgements
We thank to Dr. Duco Hamasaki for editing the manuscript. The study was registered in the University Hospital Information Network Clinical Trial Registry prior to study commencement (Umin000027917).
Funding
This study was supported by JSPS KAKENHI Grant Number 18K09420 and Dokkyo Medical University Grant 2016.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Shigeki Machida has received research grants from Bayer Yakuhin, Ltd. (Bayer).
Statement of human rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of Dokkyo Medical University Saitama Medical Center and with the 1964 Declaration of Helsinki and later amendments or comparable ethical standards.
Statement of the welfare of animals
No animals were used in this study.
Informed consent
Informed consent was obtained from all individual participants included in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nishimura, T., Machida, S. & Hara, Y. Changes of focal macular and full-field electroretinograms after intravitreal aflibercept in patients with central retinal vein occlusion. Doc Ophthalmol 141, 169–179 (2020). https://doi.org/10.1007/s10633-020-09762-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-020-09762-3