Abstract
Background
Timely intervention can alter outcome in patients of infected pancreatic necrosis (IPN) but lacks adequate biomarker. Role of serum procalcitonin (PCT) in the management of IPN is understudied, and hence, this study was planned.
Methodology
All patients of acute pancreatitis with IPN without prior intervention were included. Baseline demographic, radiological and laboratory parameters were documented. PCT was measured at baseline, prior to intervention, and thereafter every 72 h. Patients were grouped into those having baseline PCT < 1.0 ng/mL and those with PCT ≥ 1.0 ng/mL and various outcome measures were compared.
Results
Of the 242 patients screened, 103 cases (66 males; 64.1%) with IPN were grouped into 2: PCT < 1.0 ng/mL (n = 29) and PCT ≥ 1.0 ng/mL (n = 74). Patients with baseline PCT ≥ 1.0 ng/mL had significantly more severe disease scores. 16 out of 19 patients with rise in PCT on day-7 post-intervention expired. PCT ≥ 1.0 ng/mL group had higher need for ICU (p = 0.001) and mortality (p = 0.044). PCT > 2.25 ng/mL (aOR 22.56; p = 0.013) at baseline and failure in reduction of PCT levels to < 60% of baseline at day-7 post-intervention (aOR 53.76; p = 0.001) were significant mortality predictors.
Conclusion
Baseline PCT > 1.0 ng/mL is associated with poor outcome. PCT > 2.25 ng/mL and failure in reduction of PCT levels to < 60% of its baseline at day-7 post-intervention can identify high-mortality risk patients.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute pancreatitis (AP) is an acute inflammatory disease of the pancreas due to different etiologies [1]. Severe AP (SAP) occurs in around 20% of cases with a high mortality of 20 to 40% [2]. Local complications such as pancreatic and peripancreatic necrosis, particularly infected necrosis, add to adverse outcomes [3, 4].
The dynamics of AP changes with the period of illness. In the first week of illness, there is sterile inflammation leading to systemic complications including organ failure (OF) termed as primary OF. This leads to early severe AP, and there is limited time frame or option to intervene and mainly entails supportive treatment. Later, however, infection of the necrotic pancreas may lead to sepsis and subsequently OF, termed as secondary OF [5]. This phase has a good window of opportunity and if timely intervened, can alter the final outcome. The chief strategy for infected pancreatic necrosis (IPN) and secondary OF is to control sepsis by antibiotics, percutaneous catheter drainage (PCD) of the infected collection and/or surgical debridement, using a ‘step-up’ approach.
Thus, for effective management of IPN, its early diagnosis and adequacy of response to the treatment is essential. Several biomarkers have been assessed for the early prediction of severe AP, and IL-6 has been found to have performed the best [6]. However, optimum biomarkers for prediction of IPN remain understudied. Additionally, a recent analysis has suggested that there is > 50% overuse of antibiotics in AP worldwide [7], and this is more than 80% in Asian countries. Serum levels of procalcitonin (PCT) have been found to be correlating with the presence of bacterial infection and sepsis [8]. Interestingly, PCT levels decrease after successful treatment [9]. Thus, not only it can act as a marker for infection, but also helps assessing the efficacy of the treatment and guide management decisions. This dynamics of PCT levels during the course of the management of IPN has not been studied earlier.
Hence, this study was planned to study the predictive capacity of elevated PCT levels and its dynamics during the course of IPN management on the overall outcome.
Materials and Methods
Patients
This is a prospective study conducted between January 2019 to June 2020 wherein all consecutive patients, with age more than 18 years, presenting to a tertiary care centre in North India with the diagnosis of AP were screened. Patients with a period of illness beyond 7 days having features suggestive of IPN were included in the study. Patients with a suspicion of IPN were initially subjected to routine sepsis screen investigations including chest X-ray and/or HRCT chest, urine culture, blood culture and tropical infections such as dengue/malaria etc. as part of the institute protocol. Once these investigations did not suggest any extra-pancreatic source of infection, they were included in the IPN cohort. Patients with prior PCD at the time of admission, patients who had undergone any prior endoscopic/radiological/surgical intervention for AP, pregnant females and known chronic pancreatitis were excluded from the study. The study was approved by the institute ethics committee (IEC no.: INT/IEC/2018/001869; Dated 21.11.2018). Written informed consent was taken from all patients.
Definitions Used
-
(i)
Organ failure: OF was defined using the modified Marshall Scoring system [2].
-
(ii)
IPN: IPN was defined when (a) patients with necrotising pancreatitis developed fever > 38 °C after the first week of illness with leucocytosis/rising CRP, deteriorating clinical condition and/or new onset or worsening OF, without any other focus of infection and culture negativity; (b) culture positivity of blood/tissue without extra-pancreatic source of infection; (c) and/or presence of air in acute necrotic collection on CT imaging [10].
Severity Assessment
Severity assessment was done as per the revised Atlanta Classification [2] into mild, moderately severe and severe. Severity parameters such as Acute Physiology and Chronic Health evaluation (APACHE)-II [11], Systemic Inflammatory Response Syndrome (SIRS) and Bedside index for severity in AP (BISAP) [12] were calculated. CT severity index (CTSI) was calculated for patients undergoing CT. Necrosis was defined using the CTSI score [13].
Parameters Monitored
All the baseline parameters, both clinical and objective were recorded and blood samples were collected for total leukocyte count, cultures (bacterial and fungal), C-reactive protein and PCT at baseline (pre-intervention). A CECT abdomen was done for the enrolled patients at day 5–7 of illness for number and location of necrotic collections, extent of pancreatic necrosis, CTSI and the presence of gas in the collections. Intra-abdominal pressure (IAP) was expressed as mm of Hg or cm of water (1 mm of Hg = 1.36 cm of H2O). As per the consensus document, intra-abdominal hypertension (IAH) was defined by a persistent or repeated elevation of IAP over 12 mmHg [14].
PCT Measurement
PCT was measured at baseline when the diagnosis of IPN was made, before the initiation of antibiotics and/or PCD. After the initiation of intervention, antibiotics and/or PCD placement, patients were followed up for clinical improvement and serial PCT levels were measured every 72 h.
Management
Patients were managed as per the standard recommendations [15], including adequate fluid resuscitation, organ system support, pain management and nutritional support (enteral or parenteral) [16, 17].
Intervention
For management of IPN, the patients were managed as per the step-up approach [18]. Extra-pancreatic infections and infected pancreatic necrosis (IPN) were initially managed with antibiotics. In case of the absence of any definite culture positivity, the patients were started on antibiotics as per the prevailing bacterial growth and sensitivity pattern of the hospital. After the initiation of antibiotics, patients were followed up for clinical and biochemical parameter improvement. PCT levels at baseline or otherwise were not considered for management decisions as that were not part of the institute protocol during the study. In cases of non-improvement within 72–96 h, the fluid collections were drained (USG/CT guided). A dedicated unit comprising gastroenterologists and an interventional radiologist decided upon the site and route of drainage based upon the location, type and extent of collection. We have already established, in our previous study that the outcomes of endoscopic drainage under endosonographic guidance differ from that of PCD [19]. Thus, we have included only patients who were drained using only PCD to have a homogenous outcome cohort. The need for additional PCDs and or upgradation of the existing ones as well as change of antibiotics were optimised through multi-disciplinary team discussion. Patients not showing improvement were subsequently taken up for surgical necrosectomy.
Outcome Measures
Outcome measures evaluated included the duration of hospital stay, requirement of organ support (mechanical ventilation and dialysis), need for PCD, surgical necrosectomy, intensive care (ICU) admission and in-hospital mortality.
Data Analysis
PCT-based algorithms for management of critical illness use a cut-off of > 1.0 ng/mL [9] and use of antibiotics is “strongly recommended” for PCT > 1.0 ng/mL. Hence, the patients were grouped as per the PCT levels at initial inclusion in the study into those with PCT < 1.0 ng/mL and those with PCT ≥ 1.0 ng/mL. Severity parameters were compared between the two groups. The parameters studied were SIRS, BISAP and APACHE II scores and the severity of AP as per revised Atlanta classification. Serial values of PCT were measured prior to intervention and sequentially thereafter as described earlier. The values were compared to evaluate the trends in their levels and its impact on the outcome. In our previously published data, 82.4% of IPN had PCT > 1.0 ng/mL [20]. Thus, assuming that 80% of the IPN would have PCT > 1.0 ng/mL, we derived that a sample size of 97 would be required with 10% precision relative to the expected proportion.
Statistical Analysis
All data were entered in Microsoft Excel 2010. The data were analysed using SPSS software (version 22.0, IBM, USA). The data were checked for normal distribution by Kolmogorov–Smirnov test. For normally distributed data, Student's t test was used for continuous variables while for skewed data, non-parametric tests were used. Dichotomous variables were compared using Chi square test.
Logistic regression analysis was performed to identify the characteristics that significantly determined mortality among the selected pool of patients. A Kaplan–Meir Survival curve analysis was carried out for patients with/without decline in PCT levels post-intervention. The p value of less than 0.05 was taken as statistically significant.
Results
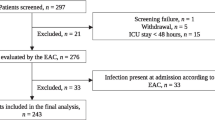
During the study period, 276 patients of AP were admitted of whom 242 had ANP. Of these 242 patients, 103 had IPN, beyond 7 days of illness and formed the study group (Fig. 1). The commonest aetiology of AP was alcohol (55; 53.4%) followed by gallstone disease (27; 26.2%). Of the total cohort, 8 patients (7.77%) were treated with antibiotics alone, while the remaining (95; 92.2%) underwent PCD placement within 72–96 h of starting of antibiotics. The median time between the date of pain and PCD placement was 19.0 (IQR 13.0) days.
The IPN cohort was divided into those with PCT < 1.0 ng/mL (n = 29) and those with PCT ≥ 1.0 ng/mL (n = 74). The baseline characteristics were similar between the two groups (Table 1). The pre-PCD collection size and the change in the volume of the collection were similar between the two groups.
Comparison of Severity Between Those With/Without Raised PCT Levels
Patients with baseline PCT ≥ 1.0 ng/mL had significantly more severe disease scores compared to those without (Table 1). SIRS (p = 0.021), BISAP (p = 0.002), APACHE II (p < 0.0001) and modified Marshall score (p < 0.0001) were significantly higher in the raised PCT group. PCT ≥ 1.0 ng/mL group had more severe disease (89.% vs 44.8%; p < 0.0001) as per the revised Atlanta classification.
PCT ≥ 1.0 ng/mL group had more multiple OF (51.5% vs 15.4%; p = 0.03) with greater proportion of patients with shock (28.4% vs 6.9%; p = 0.019). IAH was also seen in higher proportion of patients in the raised PCT group (62.2% vs 20.7%; p < 0.0001).
Evidently, PCT ≥ 1.0 ng/mL group had more patients with bacterial blood culture positivity (24.7% vs 13.6%; p = 0.216) although it did not reach statistical significance.
Comparison of Outcome Measures Between Those With/Without Raised PCT Levels in Terms of Intervention
PCT ≥ 1.0 ng/mL group had significantly higher requirements for PCD (98.6% vs 75.9%; p = 0.001) (Table 2) placement. Patients with PCT ≥ 1.0 ng/mL had significantly greater need for ICU admission (56.8% vs 20.7%; p = 0.001), ventilator support (43.2% vs 10.3%; p = 0.001) and prolonged hospital stay (24.9 ± 16.4 days vs 18.12 ± 13.6 days; p = 0.05). Mortality was higher in the PCT ≥ 1.0 ng/mL group (24.3% vs 6.9%; p = 0.044).
Raised PCT Compared to Other Severity Score for Prediction of Mortality
An ROC curve analysis (Fig. 2) showed that PCT level > 2.25 ng/mL had a greater prediction capacity for mortality compared to APACHE II at admission with a larger AUC (0.738 vs 0.654).
Predictors of Mortality in Suspected/Proven IPN
Multiple factors were assessed on univariate analysis and significant factors (p < 0.20) were then analysed in a multivariate model for prediction of mortality. On multivariable logistic regression analysis (Online resource Table 1), PCT > 2.25 ng/mL (aOR 22.56; p = 0.013) and failure in reduction of PCT levels to < 60% of its baseline even after 7 days of intervention (aOR 53.76; p = 0.001) emerged as significant predictors of mortality, even after adjusting for age, comorbidities, APACHE II at admission, Marshall score at admission and the presence of organ failure at onset.
Figure 3 shows the survival curve analysis for patients having decline in PCT levels to > 60% of baseline value after 7 days post-intervention compared to those who did not.
Dynamics of PCT Levels
Serial PCT measurements for those cases not requiring PCD placement (n = 8) showed a steady decline in the values. Serial PCT measurements for those undergoing PCD placement showed that 88 patients (85.4%) had a fall in the PCT values on day 3 of post-PCD, while 15 (14.6%) showed a rise in PCT values. Day-7 post-PCD values showed that 19 (18.4%) patients had a rise in the PCT levels, compared to baseline. Out of these, 16 patients expired and a significant difference in the serial PCT pattern was noted between those who survived and those who did not (p < 0.0001) (Fig. 4).
Discussion
This prospective study evaluated the prediction capacity of raised PCT levels on the outcome of AP patients with IPN. We found that raised PCT levels at baseline were associated with higher disease severity scores and higher number of OF. IAH was noted in a greater proportion of patients having PCT ≥ 1.0 ng/mL. IPN cases with higher PCT values had greater requirement of ICU admission and ventilator support and higher mortality compared to those without.
The two main determinants governing outcome in AP are OF and IPN [21]. OF can be early due to inflammatory burst, or late due to infective complications. Treatment options for early OF seen in early severe AP are limited and carries a high mortality [22]. On the other hand, the late OF due to sepsis and IPN can be managed effectively if intervened timely. While multiple early biomarkers for predicting the severity of AP have been extensively studied [6], limited data exist on the prediction of infective complications. Some studies have looked into various predictive markers of pancreatic necrosis such as CRP, PCT and lactate dehydrogenase [23], but with mixed results. Early values of PCT have been able to predict the risk of future development of IPN in some studies [24,25,26], while others differed [27]. However, the role of PCT as a marker of bacterial infection is well established.
PCT is a precursor of calcitonin and released from hepatocytes, thyroid and peripheral monocytes [8, 28]. It acts as a surrogate marker of bacterial infection and sepsis [8, 26] and is released when induced by the bacterial endotoxin [29]. In fact, it has better sensitivity and specificity than CRP for differentiating bacterial from inflammatory conditions. Hence, PCT has been used in various critical care settings as a tool for guiding therapy [30]. In fact, recent recommendations suggest use of PCT rather than leucocytes or CRP [7] for antibiotic use in AP. However, the cut-off for PCT as marker of sepsis in IPN is not adequately defined. While Mofidi et al. [25] have reported a meta-analysis with a cut-off of > 0.5 ng/mL, PCT-based algorithms for management of critical illness use a cut-off of > 1.0 ng/mL [9, 31, 32]. In fact, the decision for antibiotic use is “encouraged” for values > 0.5 ng/mL but “strongly recommended’ for > 1.0 ng/mL. Since the current study describes IPN cases only, we have divided the cohort using a cut-off of 1.0 ng/mL to compare those expected to require more aggressive treatment, based on PCT values.
While PCT is a predominant sepsis marker, multiple studies have evaluated its utility for AP severity prediction. While some studies found it useful [26, 33], others found it to be of limited use [34]. Our study entailed only moderately severe and severe AP cases. We found that PCT > 1.0 ng/mL was associated with greater severity scores and more number of OF. Severe AP will have a tendency to develop more necrosis and in turn higher risk of infective complications. Thus, this above finding could as well be an epiphenomenon. Interestingly, we found that PCT > 1.0 ng/mL was associated with greater proportion of IAH. We have already established previously that IAH is associated with raised inflammatory markers [35]. Thus, this association with PCT could be part of this inflammatory phenomenon. Similar findings of raised PCT with raised intra-abdominal pressure were noted by Bezmarevic et al. [34]
As already discussed, IPN, in itself, is harbinger of poor outcome. Our study showed that pre-intervention PCT > 1.0 ng/mL in patient with IPN was associated with still poorer outcome. This group of patients had greater requirement of PCD placement, ICU admission and ventilator support. Mortality was 24% in the group with PCT > 1.0 ng/mL compared to just 7% in the lower PCT group. Mortality prediction by PCT has been demonstrated in other infectious conditions [36], but its role in specific sub-cohort of IPN has not been studied earlier. IPN is a difficult-to-treat cohort and early predictive marker is essential to optimise therapy.
Our study showed that in cases of IPN, if there is ongoing rise in the PCT levels, in spite of intervention, on day 3 or day 7, then the risk of mortality increases manifold. In fact, 16/19 patients (84.2%) expired when PCT level failed to show decline even after 7 days of PCD placement. Additionally, it was noted that PCT > 2.25 ng/mL was a strong predictor of mortality with a 23 times higher risk of death. APACHE II at admission is one of the established robust scoring systems for severity and prognosis of AP. However, AP is a dynamic disease, and thus, scores calculated in the first week might not reflect the true picture of the prognosis in the ensuing weeks. We found that, for IPN, PCT > 2.25 ng/mL was a better predictor of mortality compared to APACHE II and could be used as a marker for requirement of early intervention to possibly modify disease course.
Once we diagnose a condition, objective assessment of the initiated treatment’s efficacy is key to further decide and prognosticate. Hence, dynamic measurement of markers is more helpful in gauging the clinical course than a single-point assessment. It has been established that decline in the inflammatory markers after placement of PCD in fluid collections [37] or ascites [38] in AP leads to favourable outcomes. PCT is a surrogate marker of the endotoxin load, and thus, decline in PCT should corroborate with resolution of sepsis [9]. This, however, has not been studied earlier for AP or IPN. We objectively established that failure in reduction of the PCT levels to < 60% of the baseline value even after 7 days of intervention was a predictor of mortality. Thus, this sub-cohort of patients should be more aggressively treated and optimally prognosticated. This finding underlines the significance of serial measurement of PCT for IPN management.
This is the first study of its kind to specifically analyse the role of PCT in the management of IPN. The strengths of the study are (i) inclusion of homogenous cohort of IPN cases; (ii) measurement of PCT prior to intervention rather than first week; and (iii) serial measurement of PCT to associate with the disease dynamics. There are some limitations of the study as well. This is a single tertiary care centre study with a modest sample size. The arm of study with PCT < 1.0 ng/mL has lower number of patients compared to the other, and this might skew the results to some extent. Thus, the risk analysis calculated should be interpreted cautiously as larger number of patients would be needed to validate these data. Ours is a specialised referral centre and consequently, we have a very high rate of ANP and eventually IPN as compared to other centres and follow a stringent PCD protocol. Thus, the results may lack generalisability. Correlation with other biomarkers would possibly give better perspective of the IPN dynamics. Large prospective multi-centre studies would be needed to validate the findings of the current study.
Conclusion
Serum PCT is an effective marker to prognosticate the outcome in patients with IPN. Baseline PCT > 1.0 ng/mL is associated with poor outcome. PCT > 2.25 ng/mL and failure in reduction of PCT levels to < 60% of its baseline even after 7 days of intervention can identify patients who have high mortality, and who, thus, may benefit from a more aggressive intervention.
References
Samanta J, Dhaka N, Gupta P et al. Comparative study of the outcome between alcohol and gallstone pancreatitis in a high-volume tertiary care center. JGH Open 2019;3:338–343.
Banks PA, Bollen TL, Dervenis C et al. Classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013;62:102–111.
Boxhoorn L, Voermans RP, Bouwense SA et al. Acute pancreatitis. Lancet 2020;396:726–734.
Padhan RK, Jain S, Agarwal S et al. Primary and secondary organ failures cause mortality differentially in acute pancreatitis and should be distinguished. Pancreas 2018;47:302–307.
Garg PK, Singh VP. Organ failure due to systemic injury in acute pancreatitis. Gastroenterology 2019;156:2008–2023.
van den Berg FF, de Bruijn AC, van Santvoort HC et al. Early laboratory biomarkers for severity in acute pancreatitis A systematic review and meta-analysis. Pancreatology 2020;20:1302–1311.
Párniczky A, Lantos T, Tóth EM et al. Antibiotic therapy in acute pancreatitis: from global overuse to evidence based recommendations. Pancreatology 2019;19:488–499.
Simon L, Gauvin F, Amre DK et al. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clin Infect Dis 2004;39:206–217.
Schuetz P, Chiappa V, Briel M, Greenwald JL. Procalcitonin algorithms for antibiotic therapy decisions: a systematic review of randomized controlled trials and recommendations for clinical algorithms. Arch Intern Med 2011;171:1322–1331.
Jain S, Mahapatra SJ, Gupta S et al. Infected pancreatic necrosis due to multidrug-resistant organisms and persistent organ failure predict mortality in acute pancreatitis. Clin Transl Gastroenterol 2018;9:190.
Larvin M, McMahon MJ. APACHE-II score for assessment and monitoring of acute pancreatitis. Lancet 1989;2:201–205.
Wu BU, Johannes RS, Sun X et al. The early prediction of mortality in acute pancreatitis: a large population-based study. Gut 2008;57:1698–1703.
Balthazar EJ, Robinson DL, Megibow AJ, Ranson JH. Acute pancreatitis: value of CT in establishing prognosis. Radiology 1990;174:331–336.
Kirkpatrick AW, Roberts DJ, De Waele J et al. Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive care Med 2013;39:1190–1206.
Mallick B, Dhaka N, Gupta P et al. An audit of percutaneous drainage for acute necrotic collections and walled off necrosis in patients with acute pancreatitis. Pancreatology 2018;18:727–733.
Dellinger RP, Levy MM, Rhodes A et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Critical Care Med 2013;41:580–637.
Hasibeder WR, Torgersen C, Rieger M, Dunser M. Critical care of the patient with acute pancreatitis. Anaesthesia Intensive Care 2009;37:190–206.
van Santvoort HC, Besselink MG, Bakker OJ et al. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med 2010;362:1491–1502.
Samanta J, Dhar J, Muktesh G et al. Endoscopic drainage versus percutaneous drainage for the management of infected walled-off necrosis: a comparative analysis. Expert Rev Gastroenterol Hepatol. 2022;16:297–305.
Singh AK, Samanta J, Shukla J et al. Impact of different patterns of organ failure on mortality in acute necrotizing pancreatitis. Pancreas. 2021;50:1030–1036.
Petrov MS, Shanbhag S, Chakraborty M et al. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology 2010;139:813–820.
Sharma M, Banerjee D, Garg PK. Characterization of newer subgroups of fulminant and subfulminant pancreatitis associated with a high early mortality. Am J Gastroenterol 2007;102:2688–2695.
Komolafe O, Pereira SP, Davidson BR, Gurusamy KS. Serum C-reactive protein, procalcitonin, and lactate dehydrogenase for the diagnosis of pancreatic necrosis. Cochrane Database Syst Rev 2017;4:CD012645.
Chen HZ, Ji L, Li L et al. Early prediction of infected pancreatic necrosis secondary to necrotizing pancreatitis. Medicine (Baltimore) 2017;96:e7487.
Mofidi R, Suttie SA, Patil PV et al. The value of procalcitonin at predicting the severity of acute pancreatitis and development of infected pancreatic necrosis: systematic review. Surgery 2009;146:72–81.
Rau BM, Kemppainen EA, Gumbs AA et al. Early assessment of pancreatic infections and overall prognosis in severe acute pancreatitis by procalcitonin (PCT): a prospective international multicenter study. Ann Surg 2007;245:745–754.
Müller CA, Uhl W, Printzen G et al. Role of procalcitonin and granulocyte colony stimulating factor in the early prediction of infected necrosis in severe acute pancreatitis. Gut 2000;46:233–238.
Staubli SM, Oertli D, Nebiker CA. Laboratory markers predicting severity of acute pancreatitis. Crit Rev Clin Lab Sci 2015;52:273–283.
Dandona P, Nix D, Wilson MF et al. Procalcitonin increase after endotoxin injection in normal subjects. J Clin Endocrinol Metab 1994;79:1605–1608.
Sager R, Kutz A, Mueller B, Schuetz P. Procalcitonin-guided diagnosis and antibiotic stewardship revisited. BMC Med 2017;15:15.
Schroeder S, Hochreiter M, Koehler T et al. Procalcitonin (PCT)-guided algorithm reduces length of antibiotic treatment in surgical intensive care patients with severe sepsis: results of a prospective randomized study. Langenbecks Arch Surg 2009;394:221–226.
Siriwardena AK, Jegatheeswaran S, Mason JM et al. PROCalcitonin-based algorithm for antibiotic use in Acute Pancreatitis (PROCAP): study protocol for a randomised controlled trial. Trials 2019;20:463.
Gurda-Duda A, Kusnierz-Cabala B, Nowak W et al. Assessment of the prognostic value of certain acute-phase proteins and procalcitonin in the prognosis of acute pancreatitis. Pancreas 2008;37:449–453.
Bezmarevic M, Mirkovic D, Soldatovic I et al. Correlation between procalcitonin and intra-abdominal pressure and their role in prediction of the severity of acute pancreatitis. Pancreatology 2012;12:337–343.
Singh AK, Samanta J, Dawra S et al. Reduction of intra-abdominal pressure after percutaneous catheter drainage of pancreatic fluid collection predicts survival. Pancreatology 2020;20:772–777.
Leroux P, De Ruffi S, Ramont L et al. Clinical outcome predictive value of procalcitonin in patients suspected with infection in the emergency department. Emerg Med Int 2021;2021:2344212.
Mallick B, Tomer S, Arora SK et al. Change in serum levels of inflammatory markers reflects response of percutaneous catheter drainage in symptomatic fluid collections in patients with acute pancreatitis. JGH Open 2019;3:295–301.
Samanta J, Rana A, Dhaka N et al. Ascites in acute pancreatitis: not a silent bystander. Pancreatology 2019;19:646–652.
Acknowledgments
Nil.
Funding
No funding.
Author information
Authors and Affiliations
Contributions
Guarantor of the article: JS. JS and JD: conception and design, data interpretation, data analysis, drafting the work, patient care and final approval. CLB, PG: data interpretation, patient care, intellectual review of the work and final approval. TDY, VG: data interpretation, intellectual review of the work, patient care and final approval. SKS, RK: data interpretation, critical review of the work and final approval. All authors have approved the final version of the article including the authorship list.
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare no potential conflicts of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (DOCX 19 KB)
Online Resource table 1: Factors predicting mortality in patients with acute pancreatitis
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Samanta, J., Dhar, J., Birda, C.L. et al. Dynamics of Serum Procalcitonin Can Predict Outcome in Patients of Infected Pancreatic Necrosis: A Prospective Analysis. Dig Dis Sci 68, 2080–2089 (2023). https://doi.org/10.1007/s10620-022-07758-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-022-07758-4