Abstract
Background
While overall colorectal cancer (CRC) rates in the USA are declining, the incidence of early-onset CRC (eoCRC) under age 50 is increasing. The aim of this study was to examine the risk of a second primary malignancy (SPM) in individuals with eoCRC, and how this risk compares to those with late-onset CRC (loCRC).
Methods
We used data from the Surveillance, Epidemiology, and End Results Program database to examine the risk of SPM after a diagnosis of eoCRC. Standardized incidence ratios (SIR) were used to estimate the risk of SPM after eoCRC and loCRC in comparison with the risk of malignancy in the general population.
Results
Compared to the general population, individuals with eoCRC, but not loCRC, had an increased lifetime risk of SPM (SIR 1.42, 95% CI 1.37–1.48 and SIR 1.00, 95% CI 0.99–1.02, respectively), and locations at highest risk were the small intestine, ureter, rectum, and colon. The risk of SPM after eoCRC was similar in men and women, but higher in non-whites compared to whites and higher in those with a lower area-level median household income. The risk of SPM following eoCRC was high in the first 5 years after diagnosis (SIR 2.44, 95% CI 2.24–2.66) and, in a birth cohort analysis, was found to be increasing over time.
Conclusions
Individuals with eoCRC have a lifetime risk of SPM nearly 50% higher than the general population. The risk of SPM is highest in the first 5 years after diagnosis and is increasing over time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is the third most common cancer among men and women in the USA. Overall rates of CRC in the USA are declining, attributed in large part to increased use of screening methods such as colonoscopy. However, the incidence of early-onset CRC (eoCRC), defined as colon and rectal cancers diagnosed in individuals under 50 years of age, is on the rise. The incidence of eoCRC has increased by 1.5% per year in men and 1.6% per year in women since 1992, and the rate of increase is highest among individuals ages 20–29 [1]. Since the early 1990s, the proportion of rectal cancers diagnosed in individuals under the age of 55 has doubled from 14 to 29% [2]. One study estimated that by 2030, the incidence rates for colon and rectal cancers will increase by 90% and 124%, respectively, for patients aged 20–34 years [3]. Currently, the etiology behind this rise in eoCRC remains unknown.
An unanswered question is whether a diagnosis of eoCRC is associated with a higher risk for developing a second primary malignancy (SPM). Additionally, it is unknown whether this risk varies by sex, race, socioeconomic status, or location of index cancer along the colorectal tract. Understanding these questions could inform decisions about screening and surveillance for SPM in this population and potentially help our understanding of genetic or environmental factors that contribute to this phenomenon.
The aim of this study was to assess the risk of SPM after a diagnosis of eoCRC in comparison with those diagnosed at age 50 or older with CRC, defined as late-onset CRC (loCRC), using the Surveillance, Epidemiology, and End Results (SEER) Program database. We also aimed to identify changes in risk of SPM over time.
Methods
Data Source
The National Cancer Institute’s SEER database is a national cancer surveillance program which collects data from population-based cancer registries covering approximately 34% of the US population. The SEER Research Data, 9 Regs was used, which covers the geographic or metropolitan areas of San Francisco-Oakland, Connecticut, Detroit, Hawaii, Iowa, New Mexico, Seattle (Puget Sound), Utah, and Atlanta from 1975 to 2017 (Nov 2019 submission) [4]. Although it covers a smaller geographical area than other available datasets, this dataset has complete cancer records starting at an earlier date and was used to enable time trend analyses. All SEER records are publicly available and anonymized prior to submission, and permission was obtained to access the data for this study.
Study Population
The primary analysis included all individuals age 20 years and above who were diagnosed between the years of 1975 and 2017 with CRC (site record ICD-O-3/WHO 2008 variable limited to colon and rectum) who were also diagnosed with a subsequent primary malignancy (SPM). Histologic type ICD-O-3 was limited to adenocarcinoma (8140), and primary malignancies originating in the appendix were excluded from the analysis. The secondary analyses included individuals diagnosed during that timeframe with CRC and subsequently found to have metachronous CRC. Individuals with unknown age at diagnosis were excluded. Cancer behavior was limited to malignant cases only, and cases diagnosed by death certificate or autopsy only were excluded. The latency period between the index cancer and SPM was set at a minimum of 6 months to reduce inclusion of undiagnosed metastatic or synchronous disease.
Statistical Analysis
Multiple primary standard incidence ratios (MP-SIRs) were generated using SEER*Stat software to estimate the risk of SPM after initial CRC diagnosis [5]. MP-SIRs are standardized sessions used to evaluate cohorts of individuals who develop multiple primary cancers. The analyses compare the incidence of cancers in the study cohort to the incidence of cancer in the general population matched by age, sex, and calendar year. Risk is estimated using SIR values, which represent the ratio of observed cancer cases to the number of expected cases based on incidence rates for the general population. Confidence intervals were included for all SIR calculations, and statistical significance was set at p value < 0.05. Analyses were conducted to assess SIR values by gender, race, and area-level median household income in the past year. Household income data were available for the years 1990–2017. An additional sensitivity analysis was performed assessing SIR values by area-level median household income within each reported racial group. Chi-square tests of independence were performed to examine the relationship between age groups and study population characteristics. Index CRC locations were classified as proximal colon, comprised of cancers emerging from the cecum to the splenic flexure; distal colon, including cancers in the descending colon and sigmoid colon; and rectum, which also included cancers in the rectosigmoid junction.
Results
Study Population Characteristics
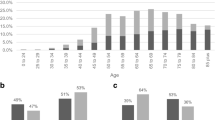
During the study period, a total of 356,899 CRC cases were diagnosed. There were 47,007 SPM detected amongst 37,043 individuals with a primary diagnosis of CRC at any age. Of this population, 2530 individuals were diagnosed with eoCRC and an SPM, while the remaining 34,513 were diagnosed with loCRC. Regardless of age at diagnosis with CRC, a greater proportion of individuals were male and the majority of index CRC cases were diagnosed in the right colon (Table 1). However, among those with eoCRC, a greater proportion were of black or American Indian/Alaska Native and Asian/Pacific Islander race than individuals with loCRC.
Risk of SPM by Age
As compared to the general population, individuals with eoCRC had a substantially increased lifetime risk of SPM (SIR 1.42, 95% CI 1.37–1.48) while those with loCRC have no increased risk (SIR 1.00, 95% CI 0.99–1.02) (Table 2). This was seen most strongly in individuals who were diagnosed with CRC under the age of 40. Individuals who developed eoCRC between the ages of 20–29 had more than a threefold risk over that of the general population of developing a second malignancy (SIR 3.85, 95% CI 3.07–4.78), while those between ages 30–39 had greater than twofold risk (SIR 2.14, 95% CI 1.96–2.33).
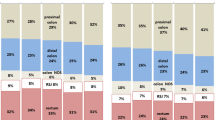
For individuals with eoCRC, the locations with the highest increase in risk of SPM were the small intestine (SIR 8.52, 95% CI 6.68–10.71), ureter (SIR 8.01, 95% CI 4.38–13.43), rectum (SIR 4.38, 95% CI 3.75–5.09), colon (SIR 4.30, 95% CI 3.91–4.7), and biliary system (SIR 4.30, 95% CI 3.06–5.87) (Fig. 1). Other areas with significantly increased risk of cancer were the stomach, pancreas, uterus, and bladder. The risk of subsequent melanoma, prostate, and oropharyngeal malignancies was significantly decreased in eoCRC as compared to the general population. For individuals with loCRC, the increase in risk of SPM was highest in the small intestine (SIR 2.75, 95% CI 2.48–3.05), the rectum (SIR 1.46, 95% CI 1.38–1.55), and the colon (SIR 1.46, 95% CI 1.42–1.5) (Fig. 2). Other sites with significantly elevated risk of SPM in this group included the vagina, ureter, biliary system, and uterus. The risk of SPM was significantly decreased in the liver, prostate, brain, ovaries, and for hematologic malignancies including leukemias, myeloma, and lymphomas.
Risk of SPM by Gender, Race, and Area-Level Household Income
The increase in risk of SPM after eoCRC was similar in men and women (SIR 1.44, 95% CI 1.36–1.521 and SIR 1.40, 95% CI 1.32–1.49, respectively). However, for individuals with loCRC, the risk of SPM was significantly increased for women (SIR 1.06, 95% CI 1.04–1.08) but significantly lower than the general population for men (SIR 0.97, 95% CI 0.95–0.98) (Table 2). The risk of SPM was higher in individuals of black or other non-white race, including American Indian/Alaska Native and Asian/Pacific Islander, than individuals of white race, regardless of age at CRC diagnosis. The greatest increase in risk was among American Indian/Alaska Native and Asian/Pacific Islander individuals with eoCRC (SIR 2.23, 95% CI 1.98–2.51).
The risk of SPM was also higher in young individuals with a lower area-level household income (Table 3). Those with eoCRC and an area-level median household income less than $45,000 had a higher risk of SPM than those with an area-level income greater than $75,000 (SIR 2.25, 95% CI 1.71–2.91 and SIR 1.62, 95% CI 1.49–1.75, respectively), whereas the risk of SPM was approximately equivalent across income levels in older individuals. An additional sensitivity analysis within racial groups indicated that area-level household income is linked to a substantially higher risk of SPM for those of minority race with eoCRC. Among black individuals, those with eoCRC and an area-level household income of less than $45,000 had a substantially higher risk of SPM than those with an area-level income greater than $75,000 (SIR 4.31, 95% CI 1.58–9.38 and SIR 1.46, 95% CI 1.12–1.87, respectively), with similar findings among American Indian/Alaska Native and Asian/Pacific Islander individuals (SIR 8.66, 95% CI 4.73–14.53 and SIR 2.19, 95% CI 1.77–2.66, respectively). However, for white individuals the risk of SPM after eoCRC was similar across area-level income groups (SIR 1.67, 95% CI 1.18–2.29 and SIR 1.56, 95% CI 1.41–1.71, respectively).
Risk of Metachronous CRC by Location in Colon
The risk of metachronous CRC diagnosis varied depending on location of index CRC and age at diagnosis. Overall, individuals with eoCRC had greater than four times the risk compared to the general population of developing a subsequent CRC (SIR 4.29, 95% CI 3.97–4.63) (Fig. 2). Individuals with loCRC had a much lower risk of metachronous CRC, although still nearly 50% greater than the general population (SIR 1.43, 95% CI 1.39–1.46). An index diagnosis of cancer in the proximal colon for individuals with eoCRC posed the highest increase in risk for metachronous CRC (SIR 5.39, 95% CI 4.77–6.08), and the subsequent location with the highest risk was the proximal CRC (SIR 6.17, 95% CI 5.10–7.41). However, for individuals with loCRC, the increase in risk of a metachronous CRC at any site was nearly equivalent whether the index malignancy was in the proximal (SIR 1.49, 95% CI 1.44–1.55) or distal colon (SIR 1.53, 95% CI 1.45–1.60). Individuals diagnosed with rectal cancer, regardless of age, had a lower risk of metachronous CRC than those diagnosed with index cancers in the colon, but the risk remained increased over the general population.
Latency Period of SPM After CRC
For individuals with eoCRC, the risk of SPM as compared to the general population was highest in the first 6–11 months after CRC diagnosis (SIR 2.91, 95% CI 2.33–3.58) (Fig. 3). The risk of SPM remained high in the first 1–5 years after eoCRC diagnosis (SIR 2.44, 95% CI 2.24–2.66), after which it decreased in a linear fashion. At 20 or more years after initial diagnosis with eoCRC, the risk of SPM was equivalent to the general population (SIR 1.05, 95% CI 0.98–1.13).
In comparison, the risk of SPM in after loCRC was slightly lower than that of the general population in the first 6–11 months after diagnosis (SIR 0.94, 95% CI 0.90–0.98). The risk of SPM rose slightly between 1 and 5 years after the onset of cancer (SIR 1.08, 95% CI 1.06–1.10), but subsequently decreased and at 20 or more years after diagnosis the risk of SPM was again lower than the general population (SIR 0.92, 95% CI 0.88–0.95).
Risk of SPM Over Time
The risk of a second malignancy after eoCRC has also increased over time (Fig. 4). When analyzing by birth year divided into 5-year increments, individuals diagnosed with eoCRC had a 69% greater risk than the general population of diagnosis with a SPM if they were born between 1950 and 1954 (SIR 1.69, 95% CI 1.51–1.87), whereas the risk was over double for those born between 1960 and 1964 (SIR 2.16, 95% CI 1.86–2.49) and more than fourfold for those born between 1970 and 1974 (SIR 4.55, 95% CI 3.38–6.00).
Discussion
In this study, we found that the risk of developing a SPM after eoCRC is nearly 50% greater than the risk of malignancy among the general population. These risks were highest among racial minorities and individuals diagnosed under the age of 40. The risk of SPM is highest within the first year after eoCRC diagnosis and remains high in the first 5 years after diagnosis.
Prior studies have demonstrated that the risk of SPM is increased after a primary diagnosis of CRC at any age [6,7,8]. Liang et al. found that the risk of second malignancy was increased after eoCRC using a national cancer registry in Taiwan [9]. Another study looked at the risk of developing SPM after an initial cancer of any kind, which occurred in approximately 1 in 12 cancer survivors [10]. A study by He et al. looked at second primary malignancy after colorectal cancer using the SEER database, but did not look at risk by birth year or area-level household income [11]. Our study provides an updated analysis for the US population in the setting of increasing eoCRC in recent years. In addition, this is the first study that we know of to examine the risk of SPM by birth year and by area-level household income, which provides a new understanding of risk factors and the increase in risk of SPM over time, mirroring the increasing risk of eoCRC.
It has previously been demonstrated that eoCRC has distinct molecular and genetic features compared to cancers diagnosed at a later age. Young-onset colorectal tumors are more likely to be microsatellite-stable, diploid, have a higher likelihood of LINE-1 hypomethylation, and have a lower frequency of the CpG island methylator phenotype (CIMP) [12,13,14,15]. CIMP has been demonstrated in numerous other tumor types, including gliomas, leukemias, and breast cancer, but its significance in terms of cancer prognosis remains unknown [16]. Multiple studies have demonstrated that eoCRC is more likely to have mucinous and signet-ring histologic subtypes, which are linked to worse outcomes, and to be poorly differentiated and high-grade at presentation [17,18,19]. Whether these findings are linked to a higher risk of SPM remains unknown.
The risk of SPM after eoCRC was found to be nearly equivalent for women and men. This is in contrast to the study by Yang et al., which demonstrated the risk of SPM is higher for women compared to men after a diagnosis of CRC at any age [6]. Notably, we found that the risk of SPM after loCRC is higher for women than men, suggesting that these findings of increased malignancy risk are driven primarily by an older female population. The risk of SPM is higher among minorities regardless of age at CRC diagnosis, including for African-American, American Indian/Alaska Native, and Asian/Pacific Islander populations, which is similar to prior findings [7]. Notably, we found that the risk of SPM was greatly increased for those with an area-level median household income under $45,000, and the risk was highest for those who were also of black or other non-white race. However, minority individuals with eoCRC and an area-level median household income greater than $75,000 still carried a greater risk than both young white individuals and the general population of SPM. These findings suggest that the increased risk of SPM after eoCRC among minority groups may be partially mediated by socioeconomic class and reflect factors such as reduced access to healthcare [1, 14].
Individuals with eoCRC have a substantially increased risk of metachronous CRC compared to individuals first diagnosed with CRC at a later age, and the risk is highest for those diagnosed with eoCRC in the proximal colon. Other studies have similarly shown that the risk of metachronous CRC is highest after an index location in the proximal colon [6, 20]. For individuals with loCRC, the risk of metachronous CRC was approximately equivalent with an index cancer in the proximal or distal colon. This raises the question of whether there are potential genetic field defects leading to a higher risk of metachronous lesions in proximal colon eoCRC.
The risk of SPM is over two times that of the general population within the first 5 years after eoCRC diagnosis, while for loCRC the risk of SPM nearly equivalent to the general population during that time frame. This difference between the two groups suggests that the increased risk of SPM after eoCRC is not simply due to increased detection from the staging and surveillance testing patients undergo after an index cancer is diagnosed. Our findings demonstrate the need for a high level of surveillance within the first 5 years after a diagnosis of eoCRC, and particularly within the first year of diagnosis. Further studies, such as a prospective cohort study of eoCRC examining the yield of additional screening and surveillance, are needed to better inform decisions about optimal intervals and dedicated surveillance methods for SPM.
Although genetic syndromes such as Lynch syndrome are common in eoCRC, it is unlikely that a change in prevalence of germline mutations can account for the recent increase in eoCRC or the increasing risk of SPM. One study found germline mutations in only 16% of colorectal tumors diagnosed in individuals under the age of 50, with approximately half of those genetic alterations attributable to Lynch syndrome mutations [21]. It has therefore been suggested that only about 20% of eoCRCs can be explained by genetic alterations alone [14, 21]. Data on genetic syndrome prevalence are not available in the SEER database, which prevented additional sensitivity analyses. However, although there may be a higher contribution of familial cancer syndromes to the prevalence of CRC and SPM in those under 50 compared to an older population, this would not explain the recent rapid change in cancer incidence. Additionally, this further highlights the need for regular cancer surveillance among those with eoCRC.
The risk of SPM after eoCRC has been increasing over time, mirroring the increasing incidence of eoCRC alone in birth cohort analyses [14]. It has previously been suggested that this increase may be linked to clinical factors such as obesity, tobacco use, or changes in diet. However, our findings did not support a strong link between obesity or tobacco use and other associated malignancies after eoCRC [22]. In this study, individuals with eoCRC had a risk no greater than the general population of developing cancers strongly associated with obesity, such as esophageal, ovarian, or breast cancer, and also demonstrated a lower overall risk of prostate cancer. Similarly, cancers linked to tobacco use, such as oropharyngeal, esophageal, and lung, showed no greater risk of occurrence after eoCRC than among the general population. Furthermore, the delay of approximately 30 years between smoking and onset of carcinogenesis and the decreasing rates of smoking among young adult individuals would not explain this increase in malignancy risk over time [23,24,25]. These findings also cannot be attributed solely to sequelae of cancer treatments, such as radiotherapy-induced malignancy, which typically require a minimum of 10 years to develop [26]. Although some malignancies, particularly metachronous CRC, may be partially explained by a history of CRC and prior pelvic radiation, this would not explain the increase in other types of malignancies seen in this study. Other potential environmental contributors such as diet, childhood antibiotic exposure, or decreasing levels of physical activity are difficult to assess using these data and require further studies to explore.
Limitations of this study include that the database covers only certain metropolitan and geographic areas, which may not be representative of the US population as a whole. Additionally, the limited demographic information of the SEER database precludes close analyses of potential risk factors such as family history, obesity, tobacco use, or other habits. The area-level median household income data are only available for the years 1990–2017 and reflect 2018 census data, limiting additional longitudinal information for this analysis. Some of the second primary cancers included in this analysis may represent late metastases and not a de novo malignancy, making it similarly difficult to interpret risk factors for those malignancies. Similarly, the findings for metachronous CRCs may represent a recurrence of the initial malignancy and not a second primary, which cannot be clarified with the current data. Finally, younger people with cancer diagnoses are inherently more likely than older individuals to develop a second cancer during their lifetimes, due to a greater number of total person-years and carcinogen exposures, which is a limitation on comparisons between eoCRC and loCRC.
In this study, we found that the risk of a SPM after eoCRC is substantially higher than after loCRC and increased over the general population. The risk is highest for racial minorities, those with a low area-level household income, and individuals diagnosed under the age of 40, and particular attention must be paid to these populations for both screening and surveillance for SPM. The risk is highest within the first 5 years after diagnosis with eoCRC and has been increasing over time. Greater consideration should be given to optimal screening and surveillance methods for such populations at highest risk of metachronous CRC and SPM.
References
Siegel RL, Jemal A, Ward EM. Increase in incidence of colorectal cancer among young men and women in the United States. Cancer Epidemiol Biomark Prev. 2009;18:1695–1698. https://doi.org/10.1158/1055-9965.Epi-09-0186.
Siegel RL, Fedewa SA, Anderson WF et al. Colorectal cancer incidence patterns in the United States, 1974–2013. J Natl Cancer Inst. 2017;109:djw322. https://doi.org/10.1093/jnci/djw322.
Bailey CE, Hu C-Y, You YN et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975–2010. JAMA Surg. 2015;150:17–22. https://doi.org/10.1001/jamasurg.2014.1756.
Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence—SEER Research Data, 9 Registrties, Nov 2019 Sub (1975–2017)—Linked to County Attributes—Time Dependent (1990–2017) Income/Rurality, 1969–2017 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released Apreil 2020, based on the November 2019 submission.
Surveillance Research Program. National Cancer Institute SEER*Stat software (seer.cancer.gov/seerstat) version 8.3.8.
Yang J, Li S, Lv M et al. Risk of subsequent primary malignancies among patients with prior colorectal cancer: a population-based cohort study. Onco Targets Ther. 2017;10:1535–1548. https://doi.org/10.2147/OTT.S129220.
Guan X, Jin Y, Chen Y et al. The incidence characteristics of second primary malignancy after diagnosis of primary colon and rectal cancer: a population based study. PLoS ONE. 2015;10:e0143067. https://doi.org/10.1371/journal.pone.0143067.
Hemminki K, Li X, Dong C. Second primary cancers after sporadic and familial colorectal cancer. Cancer Epidemiol Biomark Prev 2001;10:793–798.
Liang Y-H, Shao Y-Y, Chen H-M et al. Young patients with colorectal cancer have increased risk of second primary cancers. Jpn J Clin Oncol. 2015;45:1029–1035. https://doi.org/10.1093/jjco/hyv137.
Donin N, Filson C, Drakaki A et al. Risk of second primary malignancies among cancer survivors in the United States, 1992 through 2008. Cancer. 2016;122:3075–3086. https://doi.org/10.1002/cncr.30164.
He X, Wu W, Ye Ding, Li Y, Si J, Sun L. Excessive risk of second primary cancers in young-onset colorectal cancer survivors. Cancer Med. 2018;2018:1201–1210. https://doi.org/10.1002/cam4.1437.
Boardman LA, Johnson RA, Petersen GM et al. Higher frequency of diploidy in young-onset microsatellite-stable colorectal cancer. Clin Cancer Res 2007;13:2323–2328. https://doi.org/10.1158/1078-0432.Ccr-06-2739.
Antelo M, Balaguer F, Shia J et al. A High Degree of LINE-1 hypomethylation is a unique feature of early-onset colorectal cancer. PLoS ONE. 2012;7:e45357. https://doi.org/10.1371/journal.pone.0045357.
Dwyer AJ, Murphy CC, Boland CR et al. A summary of the fight colorectal cancer working meeting: exploring risk factors and etiology of sporadic early-age onset colorectal cancer. Gastroenterology. 2019;157:280–288. https://doi.org/10.1053/j.gastro.2019.04.049.
Ballester V, Rashtak S, Boardman L. Clinical and molecular features of young-onset colorectal cancer. World J Gastroenterol. 2016;22:1736–1744. https://doi.org/10.3748/wjg.v22.i5.1736.
Hughes LAE, Melotte V, de Schrijver J et al. The CpG island methylator phenotype: what’s in a name? Cancer Research 2013;73:5858–5868. https://doi.org/10.1158/0008-5472.Can-12-4306.
You YN, Xing Y, Feig BW, Chang GJ, Cormier JN. Young-onset colorectal cancer: is it time to pay attention? JAMA Intern Med. 2012;172:287–289. https://doi.org/10.1001/archinternmed.2011.602.
O’Connell JB, Maggard MA, Liu JH, Etzioni DA, Livingston EH, Ko CY. Do young colon cancer patients have worse outcomes? World Journal of Surgery. 2004;28:558–562. https://doi.org/10.1007/s00268-004-7306-7.
Yeo H, Betel D, Abelson JS, Zheng XE, Yantiss R, Shah MA. Early-onset colorectal cancer is distinct from traditional colorectal cancer. Clin Colorectal Cancer. 2017;16:e6. https://doi.org/10.1016/j.clcc.2017.06.002.
Gervaz P, Bucher P, Neyroud-Caspar I, Soravia C, Morel P. Proximal location of colon cancer is a risk factor for development of metachronous colorectal cancer: a population-based study. Dis Colon Rectum. 2005;48:227–232. https://doi.org/10.1007/s10350-004-0805-7.
Pearlman R, Frankel WL, Swanson B et al. Prevalence and spectrum of germline cancer susceptibility gene mutations among patients with early-onset colorectal cancer. JAMA Oncol. 2017;3:464–471. https://doi.org/10.1001/jamaoncol.2016.5194.
De Pergola G, Silvestris F. Obesity as a major risk factor for cancer. J Obes 2013;2013:291546. https://doi.org/10.1155/2013/291546.
Botteri E, Iodice S, Bagnardi V, Raimondi S, Lowenfels AB, Maisonneuve P. Smoking and colorectal cancer: a meta-analysis. JAMA. 2008;300:2765–2778. https://doi.org/10.1001/jama.2008.839.
Nelson DE, Mowery P, Asman K et al. Long-term trends in adolescent and young adult smoking in the United States: metapatterns and implications. Am J Public Health. 2008;98:905–915. https://doi.org/10.2105/ajph.2007.115931.
Pierce JP, Fiore MC, Novotny TE, Hatziandreu EJ, Davis RM. Trends in cigarette smoking in the United States: educational differences are increasing. JAMA. 1989;261:56–60. https://doi.org/10.1001/jama.1989.03420010066034.
Ng J, Shuryak I. Minimizing second cancer risk following radiotherapy: current perspectives. Cancer Manag Res. 2014;7:1–11.
Funding
None.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors. For this type of study, formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tiritilli, A., Ko, C. Patients with Early-Onset Colorectal Cancer Have an Increased Risk of Second Primary Malignancy. Dig Dis Sci 67, 1328–1336 (2022). https://doi.org/10.1007/s10620-021-06971-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-021-06971-x