Abstract
Background
Chronic pancreatitis (CP) is a risk factor for pancreatic ductal adenocarcinoma (PDAC); nevertheless, the true incidence of PDAC in CP patients in the United States remains unclear.
Aims
We evaluated the risk of developing PDAC two or more years after a new diagnosis of CP.
Methods
Retrospective study of veterans from September 1999 to October 2015. A three-year washout period was applied to exclude patients with preexisting CP and PDAC. PDAC risk was evaluated in patients with new-diagnosis CP and compared with controls without CP using Cox-proportional hazards model. CP, PDAC, and other covariates were extracted using ICD-9 codes.
Results
After exclusions, we identified 7,883,893 patients [new-diagnosis CP − 21,765 (0.28%)]. PDAC was diagnosed in 226 (1.04%) patients in the CP group and 15,858 (0.20%) patients in the control group (p < 0.001). CP patients had a significantly higher PDAC risk compared to controls > 2 years [adjusted hazard ratio (HR) 4.28, 95% confidence interval (CI) 3.74–4.89, p < 0.001], 5 years (adjusted HR 3.32, 95% CI 2.75–4.00, p < 0.001) and 10 years of follow-up (adjusted HR 3.14, 95% CI 1.99–4.93, p < 0.001), respectively. By multivariable analysis, age (odds ratio 1.02, 95% CI 1.00–1.03, p = 0.03), current smoker (odds ratio 1.67, 95% CI 1.02–2.74, p = 0.042), current smoker + alcoholic (odds ratio 2.29, 95% CI 1.41–3.52, p < 0.001), and diabetes (odds ratio 1.51, 95% CI 1.14–1.99, p = 0.004) were the independent risk factors for PDAC.
Conclusion
Our data show that after controlling for etiology of CP and other cofactors, the risk of PDAC increased in CP patients after two years of follow-up, and risk was consistent and sustained beyond 5 years and 10 years of follow-up.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Outcomes for pancreatic ductal adenocarcinoma (PDAC) continue to be dismal despite recent advances in imaging techniques. Early detection and identifying at-risk groups are critical for improving survival rates. The known risk factors for PDAC include family history, age, tobacco smoking, obesity, diabetes, and chronic pancreatitis (CP) [1]. Recurring pancreatic injury leading to prolonged inflammation has been thought to play a critical role in the initiation and development of pancreatic cancer [1].
Evidence from epidemiological studies also supports long-standing CP as a strong risk factor for PDAC. In a multicenter study, Lowenfels et al. [2] reported a 16-fold risk of PDAC in patients with CP. Subsequent studies from Europe, Japan, and China showed higher risk of PDAC in CP patients [3,4,5,6,7,8]. Nevertheless, the true incidence and risk of PDAC in CP patients in the United States (US) remain unclear due to limitations of prior studies such as misclassification of PDAC and CP, confounding bias, and the use of smaller study cohorts.
In this study, we sought to evaluate the risk of developing PDAC two or more years after a new diagnosis of CP using a large cohort of veteran’s administration (VA) patients and to also identify patient characteristics that are associated with higher risk of PDAC in CP patients.
Methods
Data Source
This is a retrospective study of VA patients from September 1999 (beginning of the electronic data) to October 2015 (study end period) using national VA medical care datasets electronically maintained by the VA healthcare system. Data (inpatient and outpatient) were extracted using International Classification of Diseases, ninth Revision Clinical Modification (ICD 9) codes.
Variables Used in the Study
Primary Predictor
The primary predictor variable of interest was CP, defined based on one or more primary or secondary diagnoses codes (inpatient or outpatient, ICD 9 code 577.1). The date of first ICD 9 diagnosis code was used as the CP diagnosis date.
Primary Outcome
The primary outcome for the study was PDAC, defined based on primary or secondary diagnoses codes (≥ 1 code) for adenocarcinoma of pancreas (inpatient or outpatient, ICD 9 codes 157.0–157.4, 157.8 and 157.9). The date of first ICD 9 diagnosis code was used as the PDAC diagnosis date.
Etiology of CP
Using personal history of smoking (current/past/nonsmoker) and alcohol history (ICD 9 codes 303, 303.0, 303.9, 305.0), CP patients were classified into following etiologies: current smokers, alcoholics, current smokers and alcoholics, past smokers, past smokers and alcoholics, and all other causes of CP.
History of smoking is obtained from the VA Health Factors smoking data and consists of text values representing an answer to a clinical reminder or question a healthcare provider has asked a patient. Using an algorithm, these text value entries were classified into never, former/past, or current smoking status.
Covariates
Diabetes mellitus (DM, ICD 9 code 250), presence of gallstones (ICD codes 574, 574.1, 574.3, 574.5, 574.7, 574.8, 574.9), and demographic variables (age, gender, race) were included as covariates in the analysis. The date of first ICD 9 diagnosis code for DM in the VA system was used as the DM diagnosis date and the timing of DM in relation to CP and PDAC was evaluated.
Inclusion and Exclusion Criteria (Fig. 1)
VA patients followed for at least three years in the VA system (time from first entry into the database and the last visit to the VA or end of study period) were selected for the study (n = 9,842,401). To identify a new diagnosis of CP, we applied a washout period and excluded CP patients diagnosed less than three years from time of entry into the database (n = 29,025). Thus, all patients included in the study were observed for a minimum of three years and no patients in the final cohort had CP or PDAC before entry into the study period. Based on the ICD codes for CP, VA patients were then classified into two groups: CP group and control group (rest of the patients in the database without CP). For patients in CP group, date of the initial CP diagnosis was designated as the time of cohort entry and for controls, the first outpatient or inpatient encounter after the three years of washout period was designated as the time of cohort entry.
As patients with pancreatic cysts have an increased long-term risk of PDAC [9], pancreatic cysts without an associated CP diagnosis (n = 15,238) and patients with pancreatic cyst diagnosis before the CP diagnosis (n = 2416) were also excluded. We further excluded patients < 18 years of age (n = 599,065) as the risk of PDAC is extremely low in this age-group. As we evaluated the risk of developing PDAC two or more years after a new diagnosis of CP, CP patients with less than two years of follow-up after CP diagnosis (n = 6920) and patients without CP with less than 2 years of follow-up after study entry (n = 1,301,102) were also excluded from this study. Follow-up time for the study participants who developed PDAC ended on the date of first diagnosis of PDAC, and for those who did not develop PDAC it ended at the time of death, or end of the study period.
Statistical Analysis
Patient characteristics were reported using frequencies (n, %) and age using median ± interquartile range (IQR). Demographic and clinical characteristics of CP patients and controls were compared using Chi-squared test, univariate logistic regression analysis, and Kruskal–Wallis test where appropriate. Proportion of patients who were subsequently diagnosed to have PDAC was also calculated. The incidence rates for PDAC (per 1000 person-years) were estimated, and Hazard ratios (unadjusted and after adjusting for demographic factors, etiology of CP, and other covariates) with 95% confidence intervals (CIs) were evaluated using Cox regression for the following times: greater than two years, greater than five years, and greater than 10 years.
For calculating incidence rate and HRs for PDAC risk more than 5 years and 10 years, patients with < 5 years of follow-up (n = 2,630,877) and < 10 years of follow-up (n = 5,403,451) were excluded, respectively.
Time interval (lag period) between the initial CP diagnosis and subsequent diagnosis of PDAC (after two years of initial CP diagnosis) was calculated. Finally, we also determined the independent predictors of PDAC developing two years after the CP diagnosis.
All analyses were performed using SAS version 9.3 (SAS Inc, Cary, NC). Significant tests were done by using two-tailed hypothesis, and the level of significance (α) was set to 0.05. This study was approved by the Veterans Affairs Saint Louis Medical Center.
Results
Patient Characteristics
After exclusions, the total number of patients included in the study was 7,883,893 (Fig. 1). During the study period, 21,765 veterans (0.28%) were diagnosed with CP and 16,804 veterans (0.20%) were diagnosed with PDAC [226 in the CP group (1.04%) and the remaining 15,858 (0.20%) in the controls]. Median duration of follow-up and median time to PDAC for the CP group were 5.5 years and 4.0 years, and for the controls were 8.7 years and 6.2 years, respectively.
Demographic characteristics of patients with and without CP are outlined in Table 1. Patients in the CP group had a significantly higher proportion of men (94.7% vs. 91.9%), African American race (28.6% vs. 15.4%), those with a history of alcohol use alone (9.7% vs 4.3%), combined smokers and alcoholics (42.6% vs 10.1%), diabetes (53.8% vs 29.5%), and gallstone disease (24.8% vs 3.1%) compared to controls (all p < 0.001) (Table 1).
Pancreatic Cancer Characteristics in Chronic Pancreatitis and Controls (Table 2)
The median age at which PDAC was diagnosed was 64 years in CP patients, and 71 years in controls (p < 0.001). CP patients who developed PDAC had a higher proportion of women (6.2% vs 2.6%, p < 0.001), African American race (29.8% vs 16.9%, p < 0.001), those with the history of alcohol use alone (7.5% vs 4.4%, p < 0.001), combined smokers and alcoholics (49.6% vs 13.6%, p < 0.001), diabetes (63.2% vs 54.7%, p = 0.002), and gallstone disease (27.9% vs 11.5%, p < 0.001) compared to controls.
Incidence and Risk of Pancreatic Cancer in Chronic Pancreatitis and Controls
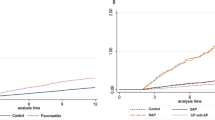
Table 3 summarizes the number of patients diagnosed with PDAC during the follow-up, the incidence rates, and hazard ratio of PDAC diagnosis for the CP and control groups. After adjusting for age, sex, race, etiology of CP, gallstones, and diabetes, the risk of PDAC was significantly higher in CP group than in controls after two years (adjusted HR 4.28, 95% CI 3.74–4.89, p < 0.001), 5 years (adjusted HR 3.32, 95% CI 2.75–4.00, p < 0.001), and 10 years of follow-up (adjusted HR 3.14, 95% CI 1.99–4.93, p < 0.001). The cumulative incidence curves for the PDAC for CP and controls are further illustrated in Fig. 2. The results of the Cox regression analysis comparing the risk (HR) of PDAC diagnosis after 2 years of follow-up for the CP and controls are summarized in Supplementary Table 1.
Cumulative incidence of pancreatic cancer in controls and CP patients. CP chronic pancreatitis, Controls rest of the patients in the database without chronic pancreatitis, CI confidence intervals, HR hazard ratio. Note Hazard ratios presented on the figures are unadjusted hazard ratios from Cox-proportional model. Adjusted HRs with 95% CI from Cox-proportional model and the corresponding P values are listed in the appropriate sections of the manuscript. Gray K-sample test P value is for comparing the cumulative incidence between the groups
Pancreatic Cancer Patients in Chronic Pancreatitis by Diabetes Status
Approximately 63% (143/226) of the CP patients who developed subsequent PDAC had a diagnosis of DM during the study period, of which 90 (90/143, 62.9%) patients had DM diagnosis prior to CP diagnosis, and 53 patients after the diagnosis of CP. Additionally, of the total 143 CP patients with PDAC who had diabetes, 29 patients (20.3%, 29/143) had diabetes diagnosed within three years of PDAC diagnosis (new-onset diabetes), while 104 patients (72.7%, 104/143) had a diagnosis of diabetes more than three years prior to the diagnosis of PDAC (preexisting DM), and five patients had a diagnosis of diabetes three years after the diagnosis of PDAC (Table 4).
Independent Predictors of Pancreatic Cancer Among Patients with Chronic Pancreatitis
Supplementary Figure 1 illustrates the number of patients with PDAC diagnosed each year after CP diagnosis. Among the 21,765 CP patients, the independent predictors of PDAC were evaluated using multivariate logistic regression analysis. Age (OR 1.02, 95% CI 1.00–1.03, p = 0.03), current smoker (OR 1.67, 95% CI 1.02–2.74, p = 0.042), current smoker + alcoholic (OR 2.29, 95% CI 1.41–3.52, p < 0.001), and DM (OR 1.51, 95% CI 1.14–1.99, p = 0.004) were determined to be the only independent risk factors for PDAC. Supplementary Figure 2 illustrates that overall, among CP patients there was no significant difference in the cumulative incidence of PDAC by etiology of CP (p = 0.07), though the number of PDAC cases was more in smokers and alcoholics. However, on multivariate logistic regression analysis, current smoker and current smoker + alcoholic etiologies were found to have a significant risk of PDAC.
Discussion
Using the nationwide VA database, we found an increased risk of PDAC in patients with a new diagnosis of CP who were followed for more than two years in the system. The increased risk of PDAC in CP patients persisted beyond five years and 10 years of follow-up, even after controlling for demographic and etiological factors. The overall incidence of PDAC in the CP cohort was 1.04%. The median age of PDAC diagnosis was significantly lower in CP patients compared to patients without CP.
CP is an established risk factor for PDAC. Activation of immune system and release of pro-inflammatory mediators may enhance the risk of PDAC in CP [7, 10]. Pancreatic stellate cells which form the principal source of fibrotic response in CP contribute to the extracellular matrix of the pancreatic tumor stroma [11, 12]. Several epidemiological studies including recent meta-analysis [13] confirmed the increased risk of PDAC in CP patients. However, the true incidence of PDAC within the CP cohort in the US is still unclear. In 1993, Lowenfels et al. [2] reported a 16-fold risk of PDAC in patients with CP utilizing a multicenter and multinational cohort. Within the subset of patients from the US, four out of 1024 CP patients (0.39%) developed PDAC after 2 years of follow-up, and three out of 626 (0.48%) developed PDAC after 5 years of follow-up. Subsequently, Bansal and Sonneberg [3] performed a case–control study using US veterans and concluded that CP constituted a significant risk for PDAC development with an odds ratio of 2.23 (95% CI 1.43–3.49). However, as it was a case–control study, the temporal relationship between CP and PDAC could not be evaluated. Many other studies [4,5,6,7,8, 13, 14] (from Europe, Japan, and China) demonstrated a wide variability in the risk of PDAC in CP patients either due to nonuse or in use of different lag periods (one year vs two years) leading to misclassification of PDAC as CP and confounding bias, and furthermore, these aforementioned studies used smaller study cohorts.
In this study, we evaluated the risk of PDAC after observing all patients in the system for at least three years from the entry into the database to identify and exclude preexisting CP and PDAC. Therefore, no patients in the final cohort had CP or PDAC before entry into the study period. We further excluded PDAC diagnosed within two years of CP diagnosis to avoid possible inflation of risk due to PDAC being misdiagnosed as CP [15].
Although we agree that the diagnosis of CP is often overlooked for several months [14, 16], once a diagnosis of CP is made, a lag period of five years from the diagnosis of CP to the diagnosis of PDAC, as suggested by the Kirkegard et al. [14], may be too long and it is unlikely that cancers diagnosed within years three to five after CP are entirely slowly progressive tumors that were not captured at the time of diagnosis. These are more likely to be tumors where the growth was enhanced by CP as suggested by previous studies [2]. Our data show that the number of PDACs diagnosed each year after CP was stable and the adjusted risk of PDAC after two years, five years, and 10 years of follow-up was significantly higher for the CP group when compared to the controls, and more importantly, the PDAC risk did not fluctuate drastically. Moreover, our data also show that the median age of PDAC presentation in CP patients was six years earlier compared to controls suggesting a possible role of chronic inflammation in the development of PDAC [1, 17]
We also evaluated the role of diabetes in relation to CP and PDAC. CP patients with DM had a higher proportion of PDAC, and after adjusting for other factors, it was still statistically significant. Prior studies showed that patients who are 50 years and older and who developed new-onset diabetes are considered as a high-risk group for developing PDAC [18, 19]. However, the potential interaction and additive role of diabetes in the context of CP should be further examined.
Some of the limitations of our study include retrospective design and use of administrative data using ICD 9 codes for PDAC, CP, and other covariates. ICD 9 code 577.1 used for CP is also used for recurrent pancreatitis and relapsing pancreatitis. Unfortunately, due to the nature of our large cohort and database, we do not have the packs per year use of smoking and years of drinking for the veterans. However, Health Factors smoking data we had used for identifying smoking history of veterans have been validated previously by comparing it to two different sources of patient self-reported smoking survey data and the prevalence of alcohol history in our cohort is consistent with the previous VA studies [20,21,22]. Our data (which include 92% males) are derived from VA database which limits the generalization. We did not validate the ICD 9 codes used for CP, PDAC, and other covariates in the database; however, prior studies have validated the use of ICD 9 codes for PDAC in the VA system [23]. Also, a recent systematic review and meta-analysis study by Amy et al. [24] showed that using ICD-9 codes yielded a positive predictive value of 0.67 on sensitivity analysis and pooled estimates of sensitivity and specificity of ICD codes (derived from two studies only) for CP were 0.75 and 0.94, respectively. To evaluate the strength of the association between CP and PDAC, we conducted a sensitivity analyses using two ICD 9 diagnosis codes (within 12-month period) as a criterion for defining PDAC and CP diagnosis (for both inpatient and outpatient diagnoses). Although the number of PDAC cases decreased in the CP and control groups, the overall finding of increased risk of PDAC in CP group for > 2 years (adjusted HR 4.30, 95% CI 3.62–5.10, p < 0.001), > 5 years (adjusted HR 3.38, 95% CI 2.66–4.30, p < 0.001) and > 10 years (adjusted HR 2.99, 95% CI 1.65–5.43, p < 0.001) is consistent with the data presented in the manuscript.
Strengths of our study include the large sample size of the cohort and long duration of follow-up (> 10 years), using a three-year washout period to exclude preexisting CP and PDAC diagnosis, controlling for smoking (current and past smokers), and heavy alcohol use, besides also evaluating the risk of PDAC in the context of diabetes. Furthermore, we also excluded patients in both groups with pancreatic cysts without coexistent diagnosis CP, as they may have a higher risk of PDAC [9] and therefore would have confounded the results. As we had used ICD 9 codes for identifying patients with cysts, the etiologic distribution of the cysts was not known and therefore the cysts that were excluded were not only the mucinous cysts (mucinous cystic neoplasm and side branch IPMN), but also others without significant malignant potential such as simple cyst, serous cystadenoma. Our dataset is derived from one of the largest databases in the USA, spanning over 16 years with large number of CP and PDAC patients. This is the first study to evaluate the risk of PDAC in CP cohort beyond 10 years and is also more robust due to utilization of strict exclusion criteria. Hence, our results may reflect the more accurate incidence of PDAC in CP.
In conclusion, our study shows increased risk of PDAC in CP patients after 2 years of follow-up and risk is sustained beyond five years and 10 years of follow-up, after controlling for demographics and etiological factors. CP patients also presented with earlier age of onset of PDAC compared to rest of the patients without CP. Although the overall incidence is 1%, identifying and selecting high-risk patients within the CP cohort for PDAC surveillance may confer benefit to aiding in earlier diagnosis and improved health outcomes.
References
Momi N, Kaur S, Krishn SR, Batra SK. Discovering the route from inflammation to pancreatic cancer. Minerva gastroenterologica e dietologica 2012;58:283–297
Lowenfels AB, Maisonneuve P, Cavallini G et al. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med. 1993;328:1433–1437
Bansal P, Sonnenberg A. Pancreatitis is a risk factor for pancreatic cancer. Gastroenterology 1995;109:247–251
Ekbom A, McLaughlin JK, Karlsson BM et al. Pancreatitis and pancreatic cancer: a population-based study. J. Natl. Cancer Inst. 1994;86:625–627
Malka D, Hammel P, Maire F et al. Risk of pancreatic adenocarcinoma in chronic pancreatitis. Gut 2002;51:849–852
Talamini G, Falconi M, Bassi C et al. Incidence of cancer in the course of chronic pancreatitis. Am J Gastroenterol. 1999;94:1253–1260
Tong GX, Geng QQ, Chai J et al. Association between pancreatitis and subsequent risk of pancreatic cancer: a systematic review of epidemiological studies. Asian Pac J Cancer Prev. 2014;15:5029–5034
Wang W, Liao Z, Li G et al. Incidence of pancreatic cancer in chinese patients with chronic pancreatitis. Pancreatology 2011;11:16–23
Munigala S, Gelrud A, Agarwal B. Risk of pancreatic cancer in patients with pancreatic cyst. Gastrointest Endosc. 2016;84:81–86. https://doi.org/10.1016/j.gie.2015.10.030.
Duell EJ, Casella DP, Burk RD, Kelsey KT, Holly EA. Inflammation, genetic polymorphisms in proinflammatory genes TNF-A, RANTES, and CCR5, and risk of pancreatic adenocarcinoma. Cancer Epidemiol Biomark Prev. 2006;15:726–731
Apte MV, Park S, Phillips PA et al. Desmoplastic reaction in pancreatic cancer: role of pancreatic stellate cells. Pancreas 2004;29:179–187
Haber PS, Keogh GW, Apte MV et al. Activation of pancreatic stellate cells in human and experimental pancreatic fibrosis. Am J Pathol. 1999;155:1087–1095
Karlson BM, Ekbom A, Josefsson S, McLaughlin JK, Fraumeni JF Jr, Nyren O. The risk of pancreatic cancer following pancreatitis: an association due to confounding? Gastroenterology 1997;113:587–592
Kirkegard J, Mortensen FV, Cronin-Fenton D. Chronic pancreatitis and pancreatic cancer risk: a systematic review and meta-analysis. Am J Gastroenterol. 2017;112:1366–1372
Munigala S, Kanwal F, Xian H, Agarwal B. New diagnosis of chronic pancreatitis: risk of missing an underlying pancreatic cancer. Am J Gastroenterol. 2014;109:1824–1830
Majumder S, Chari ST. Chronic pancreatitis. Lancet 2016;387:1957–1966
Ueda J, Tanaka M, Ohtsuka T, Tokunaga S, Shimosegawa T. Surgery for chronic pancreatitis decreases the risk for pancreatic cancer: a multicenter retrospective analysis. Surgery 2013;153:357–364
Chari ST, Leibson CL, Rabe KG, Ransom J, de Andrade M, Petersen GM. Probability of pancreatic cancer following diabetes: a population-based study. Gastroenterology 2005;129:504–511. https://doi.org/10.1016/j.gastro.2005.05.007.
Maitra A, Sharma A, Brand RE et al. A prospective study to establish a new-onset diabetes cohort: from the consortium for the study of chronic pancreatitis, diabetes, and pancreatic cancer. Pancreas 2018;47:1244–1248
McGinnis KA, Brandt CA, Skanderson M et al. Validating smoking data from the Veteran’s Affairs Health Factors dataset, an electronic data source. Nicotine Tob Res. 2011;13:1233–1239
Golden SE, Hooker ER, Shull S et al. Validity of Veterans Health Administration structured data to determine accurate smoking status. Health Inform J. 2020;26:1507–1515
Hoggatt KJ, Lehavot K, Krenek M, Schweizer CA, Simpson T. Prevalence of substance misuse among US veterans in the general population. Am J Addict. 2017;26:357–365
El-Serag HB, Engels EA, Landgren O et al. Risk of hepatobiliary and pancreatic cancers after hepatitis C virus infection: a population-based study of US veterans. Hepatology 2009;49:116–123
Xiao AY, Tan ML, Plana MN, Yadav D, Zamora J, Petrov MS. The use of international classification of diseases codes to identify patients with pancreatitis: a systematic review and meta-analysis of diagnostic accuracy studies. Clin Transl Gastroenterol. 2018;9:191
Acknowledgment
This material is the result of work supported with resources and the use of facilities at the VA Saint Louis Health Care System. The contents do not represent the views of the U.S. Department of Veterans Affairs or the Untied Stated Government.
Funding
None.
Author information
Authors and Affiliations
Contributions
SM contributed to study concept and design, statistical analysis, and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content and approved the final draft of the manuscript. DSS was involved in critical revision of the manuscript for important intellectual content and approved the final draft of the manuscript. DPS was involved in critical revision of the manuscript for important intellectual content and approved the final draft of the manuscript. TEB was involved in critical revision of the manuscript for important intellectual content and approved the final draft of the manuscript. DLC was involved in critical revision of the manuscript for important intellectual content and approved the final draft of the manuscript. SS contributed to study concept and design, interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content and approved the final draft of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors report no conflict of interest in relation to this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
10620_2021_6886_MOESM1_ESM.tiff
Supplementary Figure 1. Pancreatic cancer cases diagnosed among chronic pancreatitis patients, by year of follow-up (TIFF 943 kb)
10620_2021_6886_MOESM3_ESM.docx
Supplemental Table 1. Predictors of pancreatic cancer among chronic pancreatitis patients (after 2 years of follow-up) (DOCX 15 kb)
Rights and permissions
About this article
Cite this article
Munigala, S., Subramaniam, D.S., Subramaniam, D.P. et al. Incidence and Risk of Pancreatic Cancer in Patients with a New Diagnosis of Chronic Pancreatitis. Dig Dis Sci 67, 708–715 (2022). https://doi.org/10.1007/s10620-021-06886-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-021-06886-7