Abstract
Background
Since HLA-G is an immune checkpoint molecule and since Crohn’s disease (CD) and ulcerative colitis (UC) exhibit deregulated immune-mediated mechanisms, we aimed to evaluate intestinal HLA-G expression and soluble HLA-G (sHLA-G) levels in CD/UC patients stratified according to the CD phenotype/localization and UC extension.
Methods
HLA-G tissue expression was assessed by immunohistochemistry in biopsies collected from 151 patients (90 CD, 61 UC) and in surgical resection specimens (28 CD, 12 UC). Surgical material from 24 healthy controls was also assessed. Plasma sHLA-G levels (97 CD, 81 UC, and 120 controls) were evaluated using ELISA.
Results
HLA-G expression was similarly observed in the intestinal epithelial cells of control and CD/UC specimens. However, in biopsies, the plasma cells/lymphocytes infiltrating the lamina propria in CD/UC presented (1) increased HLA-G expression compared to controls (P < 0.0001), (2) greater cell staining in UC cells than in CD cells irrespective of disease extent (P = 0.0011), and (3) an increased number of infiltrating cells in the inflammatory CD phenotype compared to that in the stenosing and fistulizing phenotypes (P = 0.0407). In surgical specimens, CD/UC patients exhibited higher infiltrating cell HLA-G expression in lesion areas than in margins. sHLA-G levels were higher in UC/CD patients (P < 0.0001) than in controls, but no difference was observed between diseases.
Conclusions
Increased infiltrating cell HLA-G expression associated with increased sHLA-G levels in CD/UC patients may reflect ongoing host strategies to suppress chronic inflammation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammatory bowel diseases (IBDs), comprised mainly of ulcerative colitis (UC) and Crohn’s disease (CD), are a group of chronic, recurrent inflammatory diseases thought to be caused by deregulated immune-mediated mechanisms in genetically susceptible individuals. Despite recent advances, the molecular immunopathogenic mechanisms involved in IBDs remain poorly understood [1, 2].
Although exhibiting some shared pathogenic mechanisms, UC and CD may differ in several aspects. In UC, inflammation is restricted to the large bowel mucosa, and the disease is stratified according to the extension of colon involvement into proctitis, left colitis (up to the splenic flexure), or pancolitis (extending beyond the splenic flexure). The rectum and the sigmoid colon are the most commonly affected areas [1, 3]. In contrast, CD can affect any segment of the gastrointestinal tract, especially the terminal ileum and the proximal colon, and typically involves not only the mucosa but also deeper gut layers (transmural lesions). This may produce different disease behaviors (inflammatory, stenosing, penetrating, and perianal diseases), which are important determinants of clinical outcomes [1, 2]. Regarding immunopathogenesis, CD exhibits a predominance of Th1 cytokine polarization and elevated levels of IFN-γ and IL-12. In contrast, a predominant Th2 response is observed in UC, in association with elevated levels of IL-5 and IL-13 [4, 5]. Moreover, new paradigms on the pathogenesis of IBD have been suggested, such as a shift from Th1 to Th17 in CD and from Th2 to Th23 in UC [6].
The nonclassical class I histocompatible HLA-G molecule, an immunomodulatory agent, is encoded by a gene located at the short arm of chromosome 6 and presents low variability compared to classical class I molecules (HLA-A/B/C). HLA-G exhibits restricted tissue distribution under physiological conditions and may be expressed in its membrane-bound (HLA-G1 to G4) or soluble (HLA-G5 to G7) isoforms. Among these isoforms, HLA-G1 and HLA-G5 are the most expressed in healthy tissues, such as trophoblasts, the thymus, cornea, erythroid, and endothelial precursors, and are considered the most important forms from a functional point of view [7]. The HLA-G molecule exerts its immunomodulatory activity by binding to inhibitory immunoglobulin-like transcript (ILT)-2 receptors, expressed by lymphoid and myeloid cells, and to ILT-4 receptors, present on myeloid cells. In addition, the killer immunoglobulin-like receptor KIR2DL4, present only in NK cells, has also been considered an HLA-G receptor [8]. The interaction of HLA-G with these receptors may produce several effects, such as the inhibition of CD8+ T and NK cytotoxic activity, decreased CD4+ T cell proliferation, the inhibition of antigen-presenting cells, and the impaired differentiation of B cells. In addition, HLA-G may shift Th1/Th2 polarization and may induce the proliferation of regulatory T cells [9, 10].
Previous studies evaluating the association between HLA-G and IBD have focused only on (1) intestinal specimens [11, 12], (2) HLA-G polymorphisms [13], and (3) HLA-G polymorphisms in association with soluble HLA-G (sHLA-G) [14, 15]. In the present study, we evaluated for the first time the association between plasma sHLA-G and tissue HLA-G expression in patients with IBD stratified according to disease localization and clinical behavior (for CD patients) and the extension of colonic involvement (for UC patients).
Methods
Patients
This study comprised 178 IBD patients (97 CD and 81 UC; 96 women; mean age at diagnosis 32.9 ± 13.5 years) diagnosed at the University Hospital, Ribeirão Preto Medical School, University of São Paulo (HCFMRP-USP), Brazil, between 2001 and 2016. The diagnosis of CD or UC was established on the basis of clinical, endoscopic, histopathological, and radiological aspects, and patients were classified according to the Montreal criteria [16]. A total of 120 unrelated healthy blood donors (62 women; mean age 34 ± 11 years; range: 18–64 years) from the same geographic region were also studied.
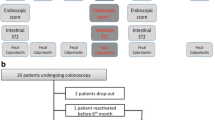
Plasma samples for sHLA-G levels were obtained from 178 untreated patients (97 CD and 81 UC) and from 120 healthy controls. Biopsies from the colon and the terminal ileum were obtained from 151 (90 CD; 61 UC) of the 178 patients included in the study. All tissue samples were obtained by the time the diagnosis was established and before the implementation of a specific treatment and evaluated for HLA-G expression. Thus, all patients had active disease, as evidenced by endoscopic and histologic analyses (moderate or severe inflammation in almost all samples). In addition, tissue samples from 28 patients with CD and 12 patients with UC who did not exhibit a response to clinical treatment were obtained by surgical resection. In this group, HLA-G expression was assessed at two levels: the lesion area, which corresponds to the resected segment, and the normal mucosa, in areas without lesions (surgical borders). In parallel, control specimens from 24 previously healthy individuals were obtained after surgical resection due to perforation during colonoscopy, impaction, and intestinal trauma (Fig. 1).
Baseline demographic and clinical characteristics of 178 patients with inflammatory bowel disease (IBD) and of 120 healthy controls. CD: Crohn’s disease; UC: ulcerative colitis; IBD: inflammatory bowel disease; the Montreal classification of IBD: age at diagnosis: A1: < 16 years, A2: 16–40 years, A3: > 40 years; localization of CD: L1: ileal, L2: colonic, L3: ileocolonic, L4: upper GI tract; behavior of CD: B1: inflammatory, B2: stenosing, B3: fistulizing, P: perianal disease; SD: standard deviation
Soluble HLA-G Quantification
Soluble HLA-G was measured using the monoclonal MEM-G/9 [17, 18] antibody (shed sHLA-G1 and sHLA-G5 isoforms) as a capture antibody and the anti-human β2-microglobulin antibody as a detection antibody [19]. All incubation steps were performed at room temperature, followed by four washes using washing buffer (H2O, 1× PBS, 0.1% Tween 20). The plates were incubated for 30 min with the substrate (tetramethyl benzidine, Sigma-Aldrich, Saint Louis, MO), and absorbance was measured at 450 nm after adding 1 N HCl. Total sHLA-G levels were determined from a five-point standard curve (12.5–200 ng/mL) using dilutions of calibrated HLA-G5 purified from M8-HLA-G5 cell line culture supernatant. The results are expressed as ng/mL, restricting the limit of detection to ~ 1 ng/mL [18].
Immunohistochemistry for HLA-G
Formalin-fixed and paraffin-embedded intestinal specimens were placed onto polylysine-pretreated slides for the HLA-G immunohistochemical assay using the primary monoclonal antibody anti-HLA-G MEM-G/2 (EXBIO, Vestec, Czech Republic), diluted at 1:50, which recognizes all soluble and membrane-bound HLA-G molecules. The assay was carried out using a biotin-free immunoenzymatic antigen detection system (REVEAL Polyvalent HRP-DAB, Spring Bioscience, Pleasanton, CA) according to the manufacturer’s instructions. Slides were subsequently counterstained with hematoxylin [20].
An experienced pathologist blindly assessed intestinal HLA-G expression in a semiquantitative manner, stratifying immunoreactivity into four categories: (1) no staining (0); (2) mild expression, tissue specimens exhibiting < 25% cell staining (+); (3) moderate expression, tissue specimens exhibiting 25–50% cell staining (++); and (4) diffuse and intense expression, tissue specimens exhibiting > 50% cell staining (+++).
Statistical Analysis
Statistical analyses were performed using SPSS software (16.0). Comparisons of clinical features and sHLA-G levels between patients and controls were performed using the Kruskal–Wallis test, followed by Dunn’s approximation or using the Mann–Whitney U-test. The Chi-square and Fisher exact tests were used to compare proportions between the HLA-G-positive and HLA-G-negative groups. Logistic regression analysis was also used to determine the adjusted significance odds ratio (OR) and 95% confidence interval (95% CI) using SPSS 17.0 software (SPSS Inc., Chicago, IL). P values < 0.05 were considered significant.
Results
IBD Patients Exhibit Increased Plasma sHLA-G Levels
Compared to healthy controls (mean 6.00, SEM = 0.79 ng/mL), plasma sHLA-G levels from CD (mean 23.65, SEM = 2.47 ng/mL) and UC (mean 24.62, SEM = 5.33 ng/mL) patients were significantly increased (P < 0.0001 for each comparison). No significant difference was observed when CD patients were compared to UC patients (P > 0.9999). When sHLA-G plasma levels in CD patients were stratified according to disease location (ileal, colonic, and ileocolonic), sHLA-G plasma levels were higher in the ileocolonic location than in the colonic location (P = 0.026). Regarding CD behavior (fistulizing, stenosing, and inflammatory), no significant differences were observed (P > 0.05 for all comparisons). When UC patients were stratified according to disease extension (proctitis, left, or pancolitis), no significant associations were observed (P > 0.05 for all comparisons). These results are illustrated in Fig. 2.
HLA-G Expression Is Increased in the Tissue Inflammatory Cells of IBD Patients
HLA-G staining was universally observed in epithelial cells of the ileum and colon in specimens obtained from CD and UC patients, as well as in those obtained from healthy controls. However, HLA-G was differentially stained in the inflammatory cells of IBD patients.
We performed five comparisons regarding HLA-G expression: (1) epithelial cells in patients and controls, (2) inflammatory cells in patients and controls, irrespective of the lesion location and disease behavior, (3) inflammatory cells in CD patients stratified according to disease behavior, (4) inflammatory cells in CD patients stratified according to disease location and (5) inflammatory cells in UC patients stratified according to disease extension.
HLA-G was expressed primarily in the epithelial cells of healthy controls (83.3%) and in specimens from both UC (88.5%) and CD (85.6%, P = 0.21) patients.
The expression of HLA-G in inflammatory cells (plasma cells and lymphocytes) was observed in 88.5% of UC specimens, 62.2% of CD specimens and only 13.1% of healthy control specimens (P < 0.001). Regarding the magnitude of tissue HLA-G expression in the inflammatory cells of CD patients, we observed that 37.8% (n = 34) of the specimens did not express HLA-G, 45.6% (n = 41) presented mild expression, and 16.6% (n = 15) exhibited moderate/strong expression. In the intestinal specimens of UC patients, there was no expression of HLA-G in 11.5% (n = 7), mild expression in 60.7% (n = 37), and moderate/strong expression in 27.9% (n = 17). Only 3 of 23 healthy control specimens exhibited mild HLA-G expression in inflammatory cells, while the other 20 specimens did not exhibit any HLA-G expression. Compared to controls, both UC and CD specimens exhibited an increased magnitude of HLA-G expression in inflammatory cells (P < 0.0001 and P = 0.0002, respectively). A comparison between patients revealed that HLA-G expression in inflammatory cells was greater in UC than in CD samples (P = 0.0011). Table 1 illustrates these results.
The multivariate analysis, adjusted for sex, age, and skin color, showed that the magnitude of HLA-G expression in inflammatory cells was increased in both UC and CD specimens compared to that in control specimens [P = 0.001; OR = 6.10; 95% CI = 2.14–17.41].
HLA-G Expression Is Increased in Tissue from Surgical Resection Samples
In samples from IBD patients (28 CD and 12 UC), HLA-G expression in epithelial cell borders (mucosa exhibiting no lesion) was similar to that in lesion areas, which was closely similar to that found in healthy control samples (24 specimens). HLA-G expression in inflammatory cells in UC lesion areas (83.3%) was significantly increased compared to that in areas exhibiting no lesion (16.7%, P = 0.002). A similar result was observed for CD specimens (border = 17.9% and lesion = 82.1%, P < 0.001). A comparison between UC and CD specimens regarding HLA-G expression in borders or lesions revealed no significant differences; however, the expression of HLA-G in the lesion areas of UC specimens was significantly greater than that in CD specimens (P = 0.02). Similarly, HLA-G expression in inflamed areas in both CD and UC specimens was significantly greater than that in noninflamed areas (P < 0.001 for both comparisons). The patterns of HLA-G expression in these samples are shown in Fig. 3.
a Normal mucosa exhibiting positive labeling for HLA-G in almost all epithelial cells (black arrow) and few inflammatory cells (white arrow). b Ulcerative colitis lesion biopsy showing staining for HLA-G in epithelial cells (black arrow) and numerous inflammatory cells (white arrow). c Ulcerative colitis excision (pancolitis) showing staining for HLA-G in epithelial cells (black arrow) and absence of labeling in inflammatory cells—the yellow arrow points a cryptic abscess. d Crohn’s lesion biopsy showing staining for HLA-G in epithelial cells (black arrow), which partially covers an inflammatory pseudopolyp with frequently stained inflammatory cells (white arrow). e Crohn’s lesion biopsy exhibiting absence of staining for HLA-G—the green arrow points a multinucleated giant cell (sclerosing Crohn’s disease). (HLA-G immunohistochemistry, ×20 magnification). Different HLA-G expression patterns: A, B and C- intense, D-moderate, E-no staining
Relationships Between HLA-G Expression and Crohn’s Disease Location and Phenotype and Ulcerative Colitis Disease Extent
Among CD patients with the inflammatory phenotype, 17.6% (n = 3) did not express HLA-G in plasma cells and lymphocytes; 35.3% (n = 6) presented mild expression, and 47.1% (n = 8) presented moderate/strong expression. In fistulizing CD, 47.2% (n = 17) of the patients did not express HLA-G; 44.4% (n = 16) presented mild expression, and 8.4% (n = 3) showed moderate/strong expression. In stenosing CD samples, 38.9% (n = 14) did not express HLA-G; 50% (n = 18) presented mild expression, and 11.1% (n = 4) exhibited moderate/strong expression. Comparisons between these behaviors revealed that the inflammatory phenotype exhibited greater HLA-G expression than the fistulizing and stenosing phenotypes (P = 0.0407) (Table 1).
Considering CD location, no significant differences were observed regarding HLA-G expression in inflammatory cells observed in ileum, ileocolonic, and colonic specimens (P = 0.1622, Table 1).
The multivariate analysis, adjusted for sex, age, phenotype, location, and perianal disease, for CD patients showed that the magnitude of HLA-G expression in inflammatory cells was increased in samples with the inflammatory CD phenotype compared to that in samples with the fistulizing CD phenotype [P = 0.017; OR = 0.11; 95% CI = 0.02–0.67].
Regarding UC disease extent, no significant differences were observed regarding HLA-G expression in inflammatory cells between specimens from patients with pancolitis, left colitis, or proctitis (P = 0.1212).
Discussion
In this study, we assessed sHLA-G levels and tissue HLA-G expression in patients with IBD and evaluated, for the first time, the association between different expression profiles and IBD clinical features, including CD location and behavior and UC disease extent.
HLA-G tissue expression was restricted to the apical surface of the intestinal epithelial cells in healthy controls as well as in CD and UC specimens. No significant differences were observed regarding HLA-G expression in intestinal biopsy samples, considering inflamed and noninflamed areas in specimens from either biopsy or surgical resection. Although few tissues constitutively express HLA-G, this and other studies [12, 21] have demonstrated that epithelial cells of the terminal ileum and colon obtained from healthy donors as well as from CD and UC patients exhibit similar HLA-G expression. A contrasting result was obtained by Torres et al. [11], who reported the presence of HLA-G expression restricted to the apical surface of intestinal epithelial cells only in UC samples.
In contrast to epithelial HLA-G expression, we observed an abnormally increased number of plasma cells and lymphocytes that stained positive for HLA-G in the lamina propria of CD and UC specimens. Comparisons between subgroups of IBD patients revealed the following interesting results: (1) Irrespective of disease extent, UC specimens exhibited an increased number of infiltrating cells that stained positive for HLA-G compared to CD specimens, (2) CD specimens from patients with the inflammatory phenotype presented an increased number of plasma cells and lymphocytes expressing HLA-G compared to specimens from patients with either the stenosing or fistulizing phenotype, suggesting that HLA-G expression may protect against the development of severe lesions, and (3) in specimens from CD and UC patients who underwent surgical resection (usually performed for more severe disease), HLA-G expression was higher in the lesion areas (inflammatory cells) than in the margins (normal mucosa). A similar study conducted by our group in a different population from Northeastern Brazil (3000 km away from the patients described in this study) revealed similar results regarding plasma cell/lymphocyte HLA-G expression in the lamina propria of CD and UC patients; however, in the previous study, HLA-G expression in infiltrating cells was not studied in correlation to the clinical features of IBD [12].
Several hypotheses may explain the increased plasma cell/lymphocyte HLA-G expression in IBD specimens. (1) Since plasma cell/lymphocyte HLA-G expression is scarcely observed in healthy samples, the inflammatory milieu may account for the increased HLA-G expression in IBD samples, with a possible role of common mediators (TNF and IL-10) that increase HLA-G expression [22]. Indeed, a positive correlation between TNF and IL-10 levels is observed depending on the type of underlying IBD [12]. (2) Plasma cell/lymphocyte HLA-G expression may also represent an attempt of immune cells to suppress chronic inflammation.
Regarding sHLA-G plasma levels, (1) both CD and UC patients exhibited increased sHLA-G plasma levels compared to controls, (2) increased sHLA-G levels were observed irrespective of the clinical features of CD (location: ileal, colonic, and ileocolonic; and behavior: inflammatory, stenotic, and penetrating phenotypes) and UC disease extent (proctitis, colitis left, and pancolitis), and (3) a comparison of sHLA-G plasma levels between CD and UC specimens revealed no significant differences. In concert, these results indicate that local and systemic shared mechanisms may be involved in the induction of HLA-G gene expression in IBD. In addition, the increased HLA-G expression in inflammatory cells at the intestinal level may contribute to the increased plasma sHLA-G levels in IBD patients, since total sHLA-G plasma levels are the sum of the soluble forms plus cellular exosomes.
Previous studies on the effects of treatment and therapeutic regimens on HLA-G expression in IBD have reported conflicting results. While one study found that treatment did not influence the production of sHLA-G [23], another study indicated that IBD treatment may differentially act on HLA-G production in IBD patients [24]. To avoid the influence of treatment, plasma sHLA-G levels in this series were evaluated only in IBD patients waiting for the institution of any specific therapy.
Some limitations of the present study should be addressed. First, regarding our findings in tissue samples, this was a retrospective study, with specimens originating from the routine medical follow-up of a single tertiary reference university hospital. Second, specimens used as healthy controls comprised a relatively small sample compared to those from IBD patients. Third, the semiquantitative method for HLA-G staining, despite having been carried out by a single independent pathologist with expertise in pathology of the digestive tract and “blinded” to clinical and endoscopy data, was not validated by a second pathologist. Furthermore, the present study, despite the significant and potentially relevant results, may not represent the general Brazilian population of IBD patients because the patients included had more severe disease manifestations, justifying follow-up in a tertiary reference university hospital. Finally, although our center admits patients from the whole country, the large majority of them originate from a specific geographical area in Southeastern Brazil.
Although significant differences were observed in terms of the immune response among different subsets of IBD patients, both CD and UC exhibit deregulated immune mechanisms characterized by distinct inflammatory profiles [4]. On the other hand, patients with CD and UC share common inflammatory pathways, such as the TNF pathway [25], associated with the induction of HLA-G production [16], which may explain, at least partially, the results observed in our study. Taken together, our results show findings similar to those in the literature regarding HLA-G expression in epithelial cells from IBD patients and healthy controls; however, in the lamina propria, plasma cell/lymphocyte HLA-G expression is frequently observed in IBD specimens, particularly in more severe cases. Increased lamina propria plasma cell/lymphocyte HLA-G expression along with increased plasma sHLA-G levels counterregulates immune system deregulation, an appanage of the immune checkpoint HLA-G molecule.
References
Baumgart D, Sandborn W. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;369:1641–1657.
Fakhoury M, Negrulj R, Mooranian A, Al-Salami H. Inflammatory bowel disease: clinical aspects and treatments. J Inflamm Res. 2014;7:113–120.
Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19:5A–36A.
Van Wijk F, Cheroutre H. Mucosal T cells in gut homeostasis and inflammation. Expert Rev Clin Immunol. 2010;6:559–566.
Ferguson LR, Gentschew L. Inflammatory bowel disease pathogenesis. In: Advanced Nutrition and Dietetics in Gastroenterology. 2014:169–179.
Di Sabatino A, Biancheri P, Rovedatti L, MacDonald TT, Corazza GR. New pathogenic paradigms in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:368–371.
Fainardi E, Castellazzi M, Stignani M, Morandi F, et al. Emerging topics and new perspectives on HLA-G. Cell Mol Life Sci. 2011;68:433–451.
Gonçalves MAG, Le Discorde M, Simões RT, et al. Classical and non-classical HLA molecules and p16INK4a expression in precursors lesions and invasive cervial cancer. Eur J Obstet Gynecol Reprod Biol. 2008;141:70–74.
Donadi EA, Castelli EC, Arnaiz-Villena A, Roger M, Rey D, Moreau P. Implications of the polymorphism of HLA-G on its function, regulation, evolution and disease association. Cell Mol Life Sci. 2011;68:369–395.
Menier C, Rouas-Freiss N, Favier B, Lemaoult J, Moreau P, Carosella ED. Recent advaces on the non-classical major histocompatibility complex class I HLA-G molecule. Tissue Antigens. 2010;75:201–206.
Torres MI, Le Discorde M, Lorite P, et al. Expression of HLA-G in inflammatory bowel disease provides a potential way to distinguish between ulcerative colitis and Crohn’s disease. Int Immunol. 2004;16:579–583.
Gomes RG, de Brito CAA, Martinelli VF, et al. HLA-G is expressed in intestinal samples of ulcerative colitis and Crohn’s disease patients and HLA-G5 expression is differentially correlated with TNF and IL-10 cytokine expression. Hum Immunol. 2018;79:477–484.
Glas J, Töök HP, Tonenchi L, et al. The 14-bp deletion polymorphism in the HLA-G gene displays significant differences between ulcerative colitis and Crohn’s disease and is associated with ileocecal resection in Crohn’s disease. Int Immunol. 2007;19:621–626.
Zidi I, Ben Yahia H, Bortolotti D, et al. Association between sHLA-G and HLA-G 14-bp deletion/insertion polymorphism in Crohn’s disease. Int Immunol. 2015;27:289–296.
Rizzo R, Bortolotti D, Baricordi OR, Fainardi E. New insights into HLA-G and inflammatory diseases. Inflamm Allergy Drug Targets. 2012;11:448–463.
Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749–753.
Rebmann V, LeMaoult J, Rouas-Freiss N, Carosella ED, Grosse-Wilde H. Quantification and identification of soluble HLA-G isoforms. Tissue Antigens. 2007;69:143–149.
Rebmann V, LeMaoult J, Rouas-Freiss N, Carosella ED, Grosse-Wilde H. Report of the wet workshop for quantification of soluble HLA-G in Essen, 2004. Hum Immunol. 2005;66:853–863.
Menier C, Saez B, Horejsi V, et al. Characterization of monoclonal antibodies recognizing HLA-G or HLA-E: new tools to analyze the expression of nonclassical HLA class I molecules. Hum Immunol. 2003;64:315–326.
Crispim JCO, Mendes-Junior CT, Wastowski IJ, et al. Frequency of insertion/deletion polymorphism in exon 8 of HLA-G and kidney allograft outcome. Tissue Antigens. 2008;71:35–41.
Downs-Kelly E, Schade AE, Hansel DE. The role of HLA-G in gastrointestinal inflammatory disease and malignancy. Semin Cancer Biol. 2007;17:451–458.
Opal SM, DePalo VA. Anti-inflammatory cytokines. Chest. 2000;117:1162–1172.
Rizzo R, Melchiorri L, Simone L, et al. Different production of soluble HLA-G antigens by peripheral blood mononuclear cells in ulcerative colitis and Crohn’s disease: a noninvasive diagnostic tool? Inflamm Bowel Dis. 2008;14:100–105.
Zelante A, Borgoni R, Galuppi C, et al. Therapy modifies HLA-G secretion differently in Crohn’s disease and ulcerative colitis patients. Inflamm Bowel Dis. 2011;17:E94–E95.
De Souza HSP, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol. 2016;13:13–27.
Funding
This research was supported by the Brazilian Research Foundations CNPq (Grant #304931/2014-1) and CAPES (Finance code 001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have no conflicts of interest.
Ethics approval and consent to participate
This retrospective cross-sectional study was approved by the Research Ethics Committee of the University Hospital, Ribeirão Preto Medical School, University of São Paulo, Brazil (HCFMRP-USP, protocol # 17469/2015).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
da Costa Ferreira, S., Sadissou, I.A., Parra, R.S. et al. Increased HLA-G Expression in Tissue-Infiltrating Cells in Inflammatory Bowel Diseases. Dig Dis Sci 66, 2610–2618 (2021). https://doi.org/10.1007/s10620-020-06561-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-020-06561-3