Abstract
Background
The activation of hepatic stellate cells (HSCs) is involved in hepatic fibrogenesis and is regulated by the decreased expression of peroxisome proliferator-activated receptor γ (PPARγ). Rosiglitazone (RGZ) is a highly potent agonist of PPARγ.
Aims
To clarify molecular regulatory mechanism of RGZ in the activation of HSCs in hepatic fibrosis.
Methods
A mouse model of hepatic fibrosis was established by carbon tetrachloride with or without RGZ intervention. A vector carrying pcDNA-HOTAIR was constructed and injected into a mouse model. HSCs were isolated from liver tissue and activated by transforming growth factor-β. The expression of miR-124-3p, HOTAIR, Col1A1, α-SMA, and PPARγ mRNAs was measured by quantitative real-time PCR. The level of PPARγ was measured by Western blotting. The interaction between HOTAIR and PPARγ was assessed by RNA immunoprecipitation (RIP) and RNA pull-down. The target gene of miR-124-3p was determined by luciferase reporter assay and RNA interference approaches.
Results
The expression of Col1A1 and α-SMA was reduced after RGZ intervention. Different expressions of HOTAIR and miR-124-3p were observed in liver tissue and HSCs. The luciferase reporter assay and RNA interference approaches indicated that miR-124-3p negatively regulated HOTAIR expression. RIP and RNA pull-down results revealed that PPARγ was interacted by HOTAIR. The therapeutic effect of RGZ on hepatic fibrosis was reversed by overexpression of HOTAIR.
Conclusions
RGZ inhibits the activation of HSCs by up-regulating miR-124-3p. The silencing of HOTAIR by miR-124-3p in HSC activation provided the foundation to understand interactions of ncRNAs and potential treatment target in hepatic fibrosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatic fibrosis is caused by chronic liver injury and wound healing reaction [1]. Specialized cells, such as myofibroblasts and hepatic stellate cells (HSCs), are major effectors of hepatic fibrogenesis [2]. The activation of HSCs is critical in the early phase of hepatic fibrosis, which is accompanied by increased mRNA expressions of α-smooth muscle actin (α-SMA) and α1(I) collagen (Col1A1) [3]. Peroxisome proliferator-activated receptor γ (PPARγ) is a kind of nuclear transcription factor belonging to the type II nuclear receptor superfamily and plays an important role in the proliferation, differentiation, and lipid metabolism of cells [4]. A previous study found that the expression of PPARγ was significantly decreased in activated HSCs [5]. In addition, the concentrations of ligands for PPARγ (15dPGJ2 and BRL49653) are characteristically changed in activated HSCs, suggesting the regulatory role of PPARγ in HSC activation [6]. As a highly potent agonist of PPARγ, rosiglitazone (RGZ) is widely used in the treatment of type 2 diabetes [7]. In recent studies, RGZ has been proved to have an antifibrotic effect in multiple organs [8,9,10]. Therefore, we assumed that RGZ may inhibit the activation of HSCs by increasing PPARγ expression, the mechanism behind which remains unclear.
Micro-RNA (miRNA) is a noncoding RNA of about 20 nucleotides in length that functions as a critical regulator in the pathogenesis of many diseases [11]. Recent studies have shown that miR-124 participates in the fibrogenesis of renal, pulmonary, and cartilage tissues [12,13,14]. In addition to the detection of miR-124 expression in cirrhosis liver cells [15], we suppose that miR-124 may be associated with hepatic fibrosis like other miRNAs—down-regulated miR-29a, 29b, 15a, 195, 200a, and 378a and up-regulated miR-34a, 34b, 15b, 16, 200c, and 199a—during the process of hepatic fibrosis [16]. However, the role miRNAs play in hepatic fibrosis is still unknown. Some miRNAs, such as the miR-29 family and the miR-34 family, are believed to induce cell apoptosis by modulating specific signaling pathways, and some miRNAs, such as the miR-199 family and the miR-200 family, are believed to be responsible for extracellular matrix deposition and the release of pro-fibrotic cytokines. A recent study also found that the expression of miR-124-3p could be regulated by RGZ in human alveolar macrophages [17]. For these reasons, miR-124-3p was chosen to be the potential miRNA target of RGZ in the activation of HSCs.
Long noncoding RNA (lncRNA) is a noncoding RNA of greater than 200 nucleotides that is involved in the regulation of various diseases [18]. Recent studies have found that lncRNAs are related to hepatic fibrogenesis [19]. Of the lncRNAs, HOTAIR has been confirmed to induce the activation of HSCs [20]. HOTAIR, a 2.2-kb-long lncRNA transcribed from the antisense strand of the HOXC gene [21], is believed to be suppressed by an miRNA (miR-141) in human cancer cells [22]. Meanwhile, we found that HOTAIR is one of the target lncRNAs of the miR-124-3p gene in the target gene prediction database (LncBase Predicted v.2). These data suggest that HOTAIR may be regulated by miR-124-3p in the activation of HSCs.
This study has two aims: The first is to clarify whether RGZ is involved in the activation of HSCs, and the second is to determine the molecular mechanisms between miR-124-3p and HOTAIR in the activation of HSCs.
Methods
Mouse Model of Hepatic Fibrosis
Hepatic fibrosis was induced in 30 mice, as previously described [23]. Briefly, 40-week-old male Balb/c mice obtained from the Shanghai Experimental Animal Center (Chinese Academy of Sciences, Shanghai, China) and weighing 25–30 g were used in the study. The mice were maintained in a 12-h light/dark cycle at 22–25 °C with free access to food and water. Twenty-four of the animals were given intraperitoneal injections of diluted carbon tetrachloride (CCl4) (1:7 in sunflower oil) at 1 mL/kg body weight twice a week for 6 weeks to induce hepatic fibrosis. Six mice comprising the sham group (nonfibrotic) received only oil. After 2 weeks of CCl4 injections, 6 of the 24 CCl4-injected mice were randomly assigned to receive an injection of a lentivirus vector carrying pcDNA-HOTAIR (1 × 109 pfu only once [24]) in the tail vein. The control group received a pcDNA-lentivirus injection. For the final 2 weeks, 18 CCl4-injected mice (including pcDNA- and pcDNA-HOTAIR-injected mice) were randomly assigned to receive RGZ (4 mg/kg per day) by oral gavage. There were 6 mice in each group. After 6 weeks of CCl4 injections, the mice were killed and the liver tissue harvested.
Histopathology
Liver tissues were fixed in 10% formaldehyde of NaCl/Pi buffer with a pH of 7.4. After being dehydrated in alcohol, they were embedded in paraffin. Paraffin blocks were sliced in 4-µm pieces and stained with hematoxylin–eosin (HE) and Masson’s trichrome stain. This procedure was performed independently by two board-certified pathologists.
Isolation and Treatment of Primary HSCs
HSCs are normally in a quiet state but can become activated by the binding of bioactive TGF-β1 to TGF-β1 receptors on HSCs [25, 26]. Primary mouse HSCs were isolated by pronase/collagenase perfusion digestion, followed by subsequent density gradient centrifugation, as previously described [24]. Briefly, liver tissues were initially in situ digested with 0.05% pronase E (Roche, Shanghai, China) and 0.03% collagenase type IV (Sigma-Aldrich, Shanghai, China) and then further digested with a collagenase type IV, pronase E, and DNase I (Roche) solution at 37 °C in a shaking bath for 20 min. Subsequently, HSCs were isolated from nonparenchymal cells using 8.2, 12, and 18% Nycodenz solution (Sigma-Aldrich) at 1.45 kg and 4 °C without stop for 22 min due to the massive amount of vitamin A-storing lipid droplets in them. Primary HSCs were cultured in high-glucose Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin and were maintained in a humidified incubator with 5% CO2 at 37 °C. Primary HSCs were treated with 10 μM RGZ and TGF-β1 (10 ng/ml) for 24 h.
Quantitative Real-Time PCR (qRT-PCR) Analysis
Total RNA was extracted from mice liver tissue or cells using an isolation kit according to the manufacturer’s protocol. The cDNA was generated from miRNA, lncRNA, and mRNA using a cDNA Reverse Transcription Kit (Applied Biosystems, MA, USA). The expressions of miRNA, lncRNA, and mRNA were analyzed by qRT-PCR using Power SYBR Green PCR Master Mix (Applied Biosystems) with U6 or GAPDH as endogenous controls. Relative expression levels of all genes were calculated as 2−ΔΔCt.
Western Blotting
Total protein was extracted from mice liver tissue using RIPA lysis buffer. The concentration of total protein was quantified using a BCA kit (Thermo Fisher Scientific, MA, USA). The protein samples (20 μg/sample) were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to poly-vinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA, USA). After blocking with 5% fat-free milk in 1 × Tris-buffered saline containing 0.1% Tween 20 (TBST) for 1 h, the proteins were immunoblotted with primary antibodies at 4 °C overnight. After washing with 1 × TBST three times, the proteins were incubated with horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. After washing with 1 × TBST three times, protein signals were determined using a substrate chemiluminescence detection system (Thermo Fisher Scientific) and Image Lab Software (Bio-Rad, CA, USA).
RNA Immunoprecipitation (RIP)
RNA immunoprecipitation (RIP) experiments were performed using the Magna RIP RNA-Binding Protein Immunoprecipitation Kit (Millipore) according to the manufacturer’s instructions. Briefly, the single-cell suspensions isolated from mouse liver or the HSCs at ~ 90% confluency in culture dishes (15 cm) were sequentially washed twice with ice-cold PBS, harvested into 15 ml conical tubes with 10 ml ice-cold PBS, and collected by centrifugation at 1500 rpm for 5 min at 4 °C. Next, cell pellets were resuspended in an equal pellet volume of complete RIP lysis buffer. The lysate was then incubated on ice for 5 min and stored at − 80 °C. Next, the RIP lysate was thawed quickly and centrifuged at 14,000 rpm for 10 min at 4 °C. Subsequently, 10 μl of the supernatant was transferred to new tubes as input, another 100 μl of the supernatant was added to the beads–antibody complex, and 900 μl of the RIP immunoprecipitation buffer was added to each RIP. The antibodies used for RIP were AGO2 and TLR4. All the tubes were incubated by rotating overnight at 4 °C. The immunoprecipitation tubes were centrifuged briefly, and the supernatant was discarded using a magnetic separator. The beads were then washed and the RNA purified. The precipitated RNA was detected by qRT-PCR or Western blotting.
Transfection
The cells (2 × 104 cells/well) were cultured in 24-well plates overnight and transiently transfected using transfection reagent lipofectamine 2000 (Invitrogen, MA, USA). Si-HOTAIR and the negative control (si-control) were designed and synthesized by GeneCopoeia (Guangzhou, China). The miR-124-3p mimic, negative control mimics (pre-NC), miR-124-3p inhibitor, and a negative control (NC) inhibitor were purchased from RiboBio Co., Ltd. (Guangzhou, China).
Luciferase Reporter Assay
The miR-124-3p binding sites in HOTAIR were mutated using the QuickChange site-directed mutagenesis system (Stratagene, La Jolla, CA, USA). For experimental validation of selected HOTAIR targets, cells were cultured in 24-well plates overnight and co-transfected with constructs containing wild-type (WT) or mutant (Mut) HOTAIR and miR-124-3p mimic or miR-124-3p inhibitor. After 48 h of transfection, luciferase activity was detected using a luciferase reporter assay system (Promega, WI, USA) according to the manufacturer’s protocol and was normalized to renilla luciferase activity.
RNA Pull-Down Assay
RNA pull-down assay was performed using a Thermo Scientific Pierce Magnetic RNA–Protein Pull-Down Kit according to the manufacturer’s instructions. Biotin was used as an affinity tag. RNA probes were biotinylated, complexed from HSC lysate, and then purified using magnetic beads. The RNA was detected using qRT-PCR analysis, and the proteins were detected by Western blotting.
Fibrotic Area Calculation
Liver tissues were initially embedded in paraffin, sliced into 5-μm serial sections, and placed on Colorfrost plus–plus slides. After de-paraffinization and rehydration, liver sections were stained with Masson’s trichrome to visualize fibrosis (blue area). The fibrosis area was measured using computerized planimetry and totaled for all sections. The area was determined by evaluating the total blue area per mm2 with NIH software. The size of the fibrotic area (%) was measured in 2–3 sections (five images captured per section and calculated data averaged per section) from each group.
Statistical Analysis
Statistical analysis was performed using SPSS version 18.0 (SPSS Inc, Chicago, IL, USA) with a Student’s t test or analysis of variance. The data were expressed as the mean ± standard deviation (SD). P < 0.05 was considered statistically significant. All experiments were performed in triplicate.
Results
Expressions of Col1A1, α-SMA, and HOTAIR in RGZ-Treated Liver Tissues
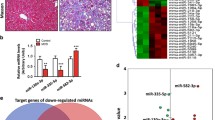
We determined the expression of Col1A1 and α-SMA, both molecular markers of HSC activation, in the liver tissues of the mouse model. The levels of Col1A1 and α-SMA mRNA and the levels of Col1A1 and α-SMA protein were increased in the CCl4 group. These levels were decreased after RGZ intervention compared to the sham group (Fig. 1a). In screening lncRNAs, we chose LFAR1, MEG3, PVT1, APTR, GAS5, and HOTAIR as candidate lncRNAs. The qRT-PCR results showed the expression of LFAR1, HOTAIR, PVT1, and APTR was increased in liver tissues and that MEG3 and GAS5 were decreased in the CCl4 group. After the RGZ treatment, only the expression of HOTAIR was reversed (Fig. 1b). Therefore, HOTAIR was revealed to have a characteristic change with RGZ intervention.
Expression of Col1A1, α-SMA, and lncRNAs in mouse model liver tissue. a The mRNA and protein levels of Col1A1 and α-SMA in the sham (n = 6), CCl4 (n = 6), and CCl4 + RGZ (n = 6) groups. b The expression of different types of lncRNAs in the sham (n = 6), CCl4 (n = 6), and CCl4 + RGZ (n = 6) groups was determined by qRT-PCR. *P < 0.05 versus sham. #P < 0.05 versus CCl4
Expression of miR-124-3p, HOTAIR, and PPARγ in Activated HSCs
To explore the expression of miR-124-3p, HOTAIR, and PPARγ in activated HSCs, we isolated HSCs from liver tissues and treated them with TGF-β1 to induce HSC activation. The results of qRT-PCR and Western blotting analysis showed that the mRNA and protein levels of Col1A1 and α-SMA were both increased after TGF-β1 induction compared to the control group. After RGZ intervention, the expressions of Col1A1 and α-SMA mRNA and protein levels were significantly reduced (Fig. 2a). Regarding the expression of miR-124-3p, HOTAIR, and PPARγ, the qRT-PCR results showed that the expression of miR-124-3p was significantly decreased after TGF-β1 induction but was increased after RGZ intervention (Fig. 2b), which was totally reversed in the expression of HOTAIR (Fig. 2c). The levels of PPARγ mRNA and protein were changed along with miR-124-3p according to qRT-PCR and Western blotting results (Fig. 2d).
Expression of miR-124-3p, HOTAIR, and PPARγ in activated HSCs before and after RGZ intervention. a HSCs were isolated from liver tissue and activated by TGF-β1. The mRNA and protein levels of Col1A1 and α-SMA were detected by qRT-PCR and Western blotting. b The expression of miR-124-3p in activated HSCs before and after RGZ intervention. c The expression of HOTAIR in activated HSCs before and after RGZ intervention. d The mRNA and protein levels of PPARγ in activated HSCs before and after RGZ intervention. *P < 0.05 versus control. #P < 0.05 versus TGF-β1
Target Site of miR-124-3p on HOTAIR
The software predicted that miR-124-3p could bind to the 3′UTR region of HOTAIR (Fig. 3a). To elucidate the target relationship between them, we introduced an miR-124-3p inhibitor and mimic into HSCs. We found that the interference of miR-124-3p could up-regulate the activity of 3′UTR in HOTAIR compared to the NC group and could promote the expression of HOTAIR (Fig. 3b). A luciferase reporter assay showed that overexpressed miR-124-3p could inhibit the activity of 3′UTR in HOTAIR and could inhibit HOTAIR expression (Fig. 3c). RIP revealed that miR-124-3p was associated with the AGO2 protein and the expression of HOTAIR. The expression of miR-124-3p in HSCs was reduced when transfected with miR-124-3p inhibitor, but miR-124-3p mimic transfection could reverse this reduction (Fig. 3d).
Target relationship between miR-124-3p and HOTAIR. a Complementary sequence information of miR-124-3p and HOTAIR. b The luciferase activity of WT or Mut HOTAIR and the HOTAIR mRNA level after down-regulating miR-124-3p. c The luciferase activity of WT or Mut HOTAIR and the HOTAIR mRNA level after up-regulating miR-124-3p. d The fold enrichment of miR-124-3p and HOTAIR of HSCs after down-regulating and up-regulating miR-124-3p using RIP and qRT-PCR. *P < 0.05 versus NC. #P < 0.05 versus pre-NC
RGZ Down-Regulated HOTAIR and Up-Regulated miR-124-3p
To demonstrate the regulation between RGZ and miR-124-3p/HOTAIR in activated HSCs, we first used TGF-β1 to activate mouse HSCs. The qRT-PCR results showed that the expression of miR-124-3p was decreased in activated HSCs, whereas HOTAIR expression was enhanced. After RGZ intervention, miR-124-3p expression was increased. HSCs transfected with miR-124-3p inhibitor could reverse this increase (Fig. 4a). On the contrary, HOTAIR expression was reversed after RGZ intervention (Fig. 4b). These data revealed the interaction between the two ncRNAs in HSC activation.
RGZ down-regulated HOTAIR and up-regulated miR-124-3p. HSCs were activated by TGF-β1, treated with RGZ, and transfected with miR-124-3p inhibitor. The expression of miR-124-3p and HOTAIR is shown in a, b, respectively, based on qRT-PCR. *P < 0.05 versus control. #P < 0.05 versus TGF-β1. &P < 0.05 versus NC
PPARγ Level Was Affected by HOTAIR in HSCs
We used qRT-PCR and RIP procedures to determine the interaction between HOTAIR and PPARγ. First, we confirmed that PPARγ was an RNA pull-down compound of HOTAIR (Fig. 5a) and that HOTAIR accumulated in protein samples precipitated by TLR4 (Fig. 5b). In mouse HSCs, the protein level of PPARγ was increased when HOTAIR was knocked out. Overexpressed HOTAIR in mouse HSCs decreased the protein level of PPARγ (Fig. 5c). However, the PPARγ mRNA level remained unchanged whether HOTAIR was knocked out or overexpressed (Fig. 5d). The overexpression of HOTAIR promoted PPARγ degradation under cycloheximide (CHX) treatment in mouse HSCs (Fig. 5e). Taken together, the PPARγ level was confirmed to be affected by HOTAIR expression.
Interaction between HOTAIR and PPARγ by RNA pull-down and RIP. a The protein of the HOTAIR pull-down compound compared with the NC group. b The level of HOTAIR in the protein sample of TLR4. *P < 0.05 versus NC or IgG. c The level of PPARγ when HOTAIR was knocked out or overexpressed in mouse HSCs. d The level of PPARγ mRNA after knocking out or overexpressing HOTAIR. e Relative PPARγ level in HSCs co-treated with HOTAIR and CHX, an inhibitor for protein synthesis. *P < 0.05 versus control
RGZ Regulated PPARγ Expression Through miR-124-3p/HOTAIR
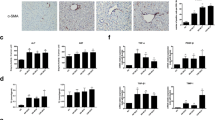
Western blotting and qRT-PCR were performed to explain the mechanism by which RGZ regulates the expression of PPARγ. The results showed that the expression of miR-124-3p was decreased after HSC activation, but after RGZ intervention the miR-124-3p expression was increased; this increase could be reversed after transfection with the miR-124-3p inhibitor. However, the change in miR-124-3p expression had no statistical difference after knocking out HOTAIR (Fig. 6a). The activation of HSCs caused an increase in HOTAIR expression. After RGZ intervention, HOTAIR expression was decreased, but the down-regulation of miR-124-3p caused increased HOTAIR expression. Knocking out HOTAIR could reverse this increase (Fig. 6b). The mRNA and protein levels of PPARγ were significantly decreased in activated HSCs. After RGZ intervention, both levels were increased but were reduced after the down-regulation of miR-124-3p. This decrease could be reversed by knocking out HOTAIR (Fig. 6c).
RGZ regulated PPARγ expression through miR-124-3p/HOTAIR. HSCs were activated by TGF-β1 and treated with RGZ. They were then transfected with miR-124-3p inhibitor, si-control, or si-HOTAIR. The expression of miR-124-3p, HOTAIR, and PPARγ is shown in a, b, and c, respectively. #P < 0.05 versus TGF-β1. &P < 0.05 versus NC. $P < 0.05 versus si-control
RGZ Inhibited the Activation and Proliferation of HSCs by miR-124-3p/HOTAIR
To specify the effect of RGZ in HSCs, we detected the levels of Col1A1 and α-SMA mRNA and their proteins and the cell proliferation of HSCs using qRT-PCR and Western blotting. The results showed that the mRNA and protein expressions of Col1A1 and α-SMA were significantly increased under the stimulation of TGF-β1 but were decreased after RGZ intervention. The down-regulation of miR-124-3p increased mRNA and protein expressions of Col1A1 and α-SMA, but this increase could be reversed by knocking out HOTAIR (Fig. 7a). The activation of HSCs promoted cell proliferation, while RGZ inhibited cell proliferation. The down-regulation of miR-124-3p increased cell proliferation as well, but knocking out HOTAIR reversed this cell proliferation (Fig. 7b).
Proliferation of TGF-β1-induced mouse HSCs. HSCs were activated by TGF-β1 and treated with RGZ. They were then transfected with miR-124-3p inhibitor, si-control, or si-HOTAIR. a The mRNA and protein levels of Col1A1 and α-SMA were determined by qRT-PCR and Western blotting. b The cell proliferation of mouse HSCs. *P < 0.05 versus control. #P < 0.05 versus TGF-β1. &P < 0.05 versus NC. $P < 0.05 versus si-control
Effect of HOTAIR in Hepatic Fibrosis
To further determine whether RGZ reverses liver fibrosis via HOTAIR, a lentivirus vector carrying pcDNA-HOTAIR was injected into the tail veins of mice. After 4 weeks of infection, mouse liver tissues were obtained; the staining specimens are shown in Fig. 8a. Histopathology revealed few necrotic cells and little fibrosis in the sham group. After CCl4 treatment, fatty degeneration and necrosis were clear, and Masson’s staining revealed nodular fibrosis with collagen deposition. After RGZ intervention, fatty degeneration and necrosis were significantly reversed. However, hepatic fibrosis increased when infected with pcDNA-HOTAIR. The fibrotic area was significantly reduced after RGZ intervention and was increased after pcDNA-HOTAIR injection (Fig. 8a). Similarly, the expression of HOTAIR was decreased after RGZ intervention but was reversed after pcDNA-HOTAIR injection, whereas Western blotting revealed that PPARγ expression increased after RGZ intervention. The expression of miR-124-3p was increased after RGZ intervention, although the change with pcDNA-HOTAIR injection was not statistically different (Fig. 8b). Based on these results, we confirmed that RGZ could alleviate hepatic fibrosis by up-regulating miR-124-3p and that the expression of PPARγ was regulated by miR-124-3p/HOTAIR pathway.
Effect of HOTAIR in liver fibrosis. a The HE and Masson staining and fibrotic area in the sham, CCl4, CCl4 + RGZ, CCl4 + RGZ + pcDNA, and CCl4 + RGZ + pcDNA-HOTAIR groups. b The expression of miR-124-3p, HOTAIR, and PPARγ using qRT-PCR and Western blotting. *P < 0.05 versus sham. #P < 0.05 versus CCl4. &P < 0.05 versus NC
Discussion
Hepatic fibrogenesis is a complex and regulated process. Typical therapeutic strategies include reducing oxidative stress; improving insulin signaling; activating the farnesoid X receptor, fibrosis-targeted inhibitors of hedgehog signaling, and combined PPAR-α/δ agonists; and manipulating altered gut microbiota using probiotics or microbiota transfer [2]. In the present study, we focused on the PPARγ agonist and the underlying mechanism through which RGZ alleviates hepatic fibrosis. Clinically, RGZ is widely used in type 2 diabetes and is known as a highly potent agonist of PPARγ [7]. It has not been used in treating liver fibrosis. Despite this, RGZ has been shown to have a therapeutic function in hepatic fibrosis. For instance, Bennett et al. [23] confirmed that the combination of serelaxin and RGZ treatment for 2 weeks was effective in significantly reducing established hepatic fibrosis. However, they did not further explore the molecular mechanism. Our findings show that RGZ can alleviate hepatic fibrosis through a specific mechanism; the up-regulation of miR-124-3p expression resulted in the reduction in HOTAIR, thus significantly increasing PPARγ levels.
PPARs are ligand-activated transcription factors with three PPAR isoforms (α, β/δ, and γ) that differ in their distribution in tissues and in ligand specificity [27]. As we know, low levels of PPARγ are proved to activate HSCs [28, 29], which is a key step in the pathogenesis of liver fibrosis [30]. In our work, we detected the expression of PPARγ in TGF-β1-treated HSCs and found the low expression of PPARγ. A previous study isolated quiescent HSCs and hepatocytes from the liver tissue of mice and compared the difference in the expression of PPARγ in the two cell types. The study found that PPARγ expression in quiescent HSCs was significantly higher than in hepatocytes and found a marked reduction in PPARγ expression in activated HSCs [31]. The low expression of PPARγ in Kupffer cells in promoting hepatic fibrosis was also reported [32]. With these findings, a deficiency of PPARγ was demonstrated to promote hepatic fibrosis in both parenchymal liver cells (i.e., hepatocytes) and in nonparenchymal liver cells (i.e., Kupffer cells and HSCs). Our work indicates that the up-regulation of PPARγ by the PPARγ agonist RGZ in HSCs attenuated liver fibrogenesis, although the liver protective effect and the underlying mechanism via the up-regulation of PPARγ in other cell types deserve further investigation.
Recently, the number of molecules and pathways that are targets for antifibrotic therapy has increased [2, 33, 34], as many studies have shown that the dysregulation of miRNA affects the cell proliferation and differentiation involved in fibrogenesis [35]. For example, miR-29 [36] and miR-19b [37] have been identified as acting as fibrogenetic miRNAs. In our work, we found that miR-124 is a downstream molecule of RGZ in preventing hepatic fibrosis, which is in agreement with previous studies [12,13,14]. This indicates that miR-124 may participate in the fibrotic progress of renal, pulmonary, and cartilage tissues.
In addition to miRNA, the above mechanism requires the participation of another important RNA—lncRNA-HOTAIR. HOTAIR is a 2.2-kb-long lncRNA that acts like a protein coding gene [38]. Recent studies have shown that HOTAIR promotes malignancy [39, 40]. Both lncRNAs and miRNAs have been proved to participate in many critical biological processes, such as cell proliferation, apoptosis, and differentiation [41]. In particular, miRNAs have been found to inhibit the crucial process of target RNA transcription [42], and lncRNAs have been shown to have facilitative or suppressive effects on the gene regulatory network [43]. However, the relationship between miRNAs and lncRNAs remained unclear. In recent years, it has been found that miRNAs may mediate the degradation of lncRNA and prevent the binding of lncRNA to its targeting molecules [44, 45]. This phenomenon adds a significant new dimension to the miRNA-mediated regulation of gene expression in cells. Our results show that HOTAIR is the downstream target gene of miR-124-3p and that the expression of PPARγ significantly increased with the competing combination of miR-124-3p and HOTAIR.
In conclusion, we established the role of RGZ in determining the progression of hepatic fibrosis via the miR-124-3p/HOTAIR pathway. We also revealed that the low level of HOTAIR consistently maintained the high expression of PPARγ, thereby inhibiting the activation of HSCs. This study provided new insights about the mechanisms of RGZ in alleviating hepatic fibrosis and suggested RGZ as a potential medication for fibrosis treatment.
Change history
29 March 2023
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1007/s10620-023-07926-0
References
Trautwein C, Friedman SL, Schuppan D, Pinzani M. Hepatic fibrosis: concept to treatment. J Hepatol. 2015;62:S15–S24.
Mehal WZ, Schuppan D. Antifibrotic therapies in the liver. Semin Liver Dis. 2015;35:184–198.
Hsu W-H, Lee BH, Hsu YW, Pan TM. Peroxisome proliferator-activated receptor-gamma activators monascin and rosiglitazone attenuate carboxymethyllysine-induced fibrosis in hepatic stellate cells through regulating the oxidative stress pathway but independent of the receptor for advanced glycation end products signaling. J Agric Food Chem. 2013;61:6873–6879.
Kersten S, Desvergne B, Wahli W. Roles of PPARs in health and disease. Nature. 2000;405:421.
Marra F, Efsen E, Romanelli RG, et al. Ligands of peroxisome proliferator-activated receptor gamma modulate profibrogenic and proinflammatory actions in hepatic stellate cells. Gastroenterology. 2000;119:466–478.
Miyahara T, Schrum L, Rippe R, et al. Peroxisome proliferator-activated receptors and hepatic stellate cell activation. J Biol Chem. 2000;275:35715–35722.
Lu Y, Ma D, Xu W, Shao S, Yu X. Effect and cardiovascular safety of adding rosiglitazone to insulin therapy in type 2 diabetes: a meta-analysis. J Diabetes Investig. 2015;6:78–86.
Zhao C, Chen W, Yang L, Chen L, Stimpson SA, Diehl AM. PPARgamma agonists prevent TGFbeta1/Smad3-signaling in human hepatic stellate cells. Biochem Biophys Res Commun. 2006;350:385–391.
Asano T, Yamazaki H, Kasahara C, et al. Identification, synthesis, and biological evaluation of 6-[(6R)-2-(4-fluorophenyl)-6-(hydroxymethyl)-4,5,6,7-tetrahydropyrazolo[1,5-a]pyr imidin-3-yl]-2-(2-methylphenyl)pyridazin-3(2H)-one (AS1940477), a potent p38 MAP kinase inhibitor. J Med Chem. 2012;55:7772–7785.
Yoshihara D, Kurahashi H, Morita M, et al. PPARgamma agonist ameliorates kidney and liver disease in an orthologous rat model of human autosomal recessive polycystic kidney disease. Am J Physiol Renal Physiol. 2011;300:F465–F474.
Cech TR, Steitz JA. The noncoding RNA revolution-trashing old rules to forge new ones. Cell. 2014;157:77–94.
Zell S, Schmitt R, Witting S, Kreipe HH, Hussein K, Becker JU. Hypoxia induces mesenchymal gene expression in renal tubular epithelial cells: an in vitro model of kidney transplant fibrosis. Nephron Extra. 2013;3:50–58.
Gong M, Liang T, Jin S, et al. Methylation-mediated silencing of miR-124 facilitates chondrogenesis by targeting NFATc1 under hypoxic conditions. Am J Transl Res. 2017;9:4111–4124.
Wang D, Zhang H, Li M, et al. MicroRNA-124 controls the proliferative, migratory, and inflammatory phenotype of pulmonary vascular fibroblasts. Circ Res. 2014;114:67–78.
Jiang J, Gusev Y, Aderca I, et al. Association of microRNA expression in hepatocellular carcinomas with hepatitis infection, cirrhosis, and patient survival. Clin Cancer Res. 2008;14:419–427.
Jiang X-P, Ai WB, Wan LY, Zhang YQ, Wu JF. The roles of microRNA families in hepatic fibrosis. Cell Biosci. 2017;7:34.
Wang Y, Hu M. Peroxisome proliferators activated receptor γ protects against acute lung injury alveolar macrophages inflammation by upregulating miR-124 expression. Chin J Lung Dis. 2015;8:160–165.
Carninci P, Kasukawa T, Katayama S, et al. The transcriptional landscape of the mammalian genome. SPJ. 2005;309:1559–1563.
Sun M, Kraus WL. From discovery to function: the expanding roles of long noncoding RNAs in physiology and disease. Endocr Rev. 2015;36:25–64.
Bian EB, Wang YY, Yang Y, et al. Hotair facilitates hepatic stellate cells activation and fibrogenesis in the liver. Biochimica et Biophysica Acta. 2017;1863:674–686.
Rinn JL, Kertesz M, Wang JK, et al. Functional demarcation of active and silent chromatin domains in human HOX loci by non-coding RNAs. Cell. 2007;129:1311–1323.
Chiyomaru T, Fukuhara S, Saini S, et al. Long non-coding RNA HOTAIR is targeted and regulated by miR-141 in human cancer cells. J Biol Chem. 2014;289:12550–12565.
Bennett RG, Simpson RL, Hamel FG. Serelaxin increases the antifibrotic action of rosiglitazone in a model of hepatic fibrosis. World J Gastroenterol. 2017;23:3999–4006.
Zhang K, Han X, Zhang Z, et al. The liver-enriched lnc-LFAR1 promotes liver fibrosis by activating TGFβ and Notch pathways. Nat Commun. 2017;8:144.
Ikeda K, Wakahara T, Wang YQ, Kadoya H, Kawada N, Kaneda K. In vitro migratory potential of rat quiescent hepatic stellate cells and its augmentation by cell activation. Hepatology. 1999;29:1760–1767.
Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;28:2247–2250.
Armoni M, Harel C, Karnieli E. PPARγ gene expression is autoregulated in primary adipocytes: ligand, sumoylation, and isoform specificity. Horm Metab Res. 2015;47:89–96.
Zhang Q, Xiang S, Liu Q, et al. PPARγ antagonizes hypoxia-induced activation of hepatic stellate cell through cross mediating PI3K/AKT and cGMP/PKG signaling. PPAR Res. 2018. https://doi.org/10.1155/2018/6970407.
Kweon S-M, Chi F, Higashiyama R, Lai K, Tsukamoto H. Wnt pathway stabilizes MeCP2 protein to repress PPAR-γ in activation of hepatic stellate cells. PLoS ONE. 2016;11:e0156111.
Troeger JS, Mederacke I, Gwak GY, et al. Deactivation of hepatic stellate cells during liver fibrosis resolution in mice. Gastroenterology. 2012;143:1073. https://doi.org/10.1053/j.gastro.2012.06.036.
Morán-Salvador E, Titos E, Rius B, et al. Cell-specific PPARγ deficiency establishes anti-inflammatory and anti-fibrogenic properties for this nuclear receptor in non-parenchymal liver cells. J Hepatol. 2013;59:1045–1053.
Odegaard JI, Ricardo-Gonzalez RR, Eagle AR, et al. Alternative M2 activation of Kupffer cells by PPARδ ameliorates obesity-induced insulin resistance. Cell Metab. 2008;7:496–507.
Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, nonalcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956–965.
Yang J-J, Tao H, Li J. Hedgehog signaling pathway as key player in liver fibrosis: new insights and perspectives. Expert Opin Therap Targets. 2014;18:1011–1021.
Noetel A, Kwiecinski M, Elfimova N, Huang J, Odenthal M. microRNA are central players in anti- and profibrotic gene regulation during liver fibrosis. Front Physiol. 2012;3:49.
Roderburg C, Urban GW, Bettermann K, et al. Micro-RNA profiling reveals a role for miR-29 in human and murine liver fibrosis. Hepatology. 2010;53:209–218.
Lakner AM, Steuerwald NM, Walling TL, et al. Inhibitory effects of microRNA 19b in hepatic stellate cell-mediated fibrogenesis. Hepatology. 2012;56:300–310.
Liang YG, Liu ZT, Guo SX. Ultrasound reverses adriamycin-resistance in non-small cell lung cancer via positive regulation of BRAF-activated non-coding RNA (BANCR) expression. Clin Surg Res Commun. 2017;1:18–23.
Zhang A, Zhao JC, Kim J, et al. LncRNA HOTAIR enhances the androgen-receptor-mediated transcriptional program and drives castration-resistant prostate cancer. Cell Rep. 2015;13:209–221.
Bhan A, Mandal SS. LncRNA HOTAIR: a master regulator of chromatin dynamics and cancer. Biochim Biophys Acta. 2015;1856:151–164.
Shenoy A, Blelloch RH. Regulation of microRNA function in somatic stem cell proliferation and differentiation. Nat Rev Mol Cell Biol. 2014;15:565–576.
Song R, Walentek P, Sponer N, et al. miR-34/449 miRNAs are required for motile ciliogenesis by repressing cp110. Nature. 2014;510:115–120.
Sun Q, Csorba T, Skourti-Stathaki K, Proudfoot NJ, Dean C. R-loop stabilization represses antisense transcription at the Arabidopsis FLC locus. Science. 2013;340:619–621.
Ghosal S, Das S, Sen R, Basak P, Chakrabarti J. Circ2Traits: a comprehensive database for circular RNA potentially associated with disease and traits. Front Genet. 2013;4:283.
Wang J, Liu X, Wu H, et al. CREB up-regulates long non-coding RNA, HULC expression through interaction with microRNA-372 in liver cancer. Nucl Acids Res. 2010;38:5366–5383.
Acknowledgments
This study was funded by Zhejiang Provincial Natural Science Foundation (Grant Number: LY16H030014) and Zhejiang Provincial Medical Technology Project (Grant Number: 2019KY104) and Zhejiang Provincial Science Technology Project (Grant Number: 2015C37101).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the Institutional Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University. All of these experiments in the current research were in compliance with the government policies and defined protocols.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
This article has been retracted. Please see the retraction notice for more detail:https://doi.org/10.1007/s10620-023-07926-0
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhi, Sc., Chen, Sz., Li, Yy. et al. RETRACTED ARTICLE: Rosiglitazone Inhibits Activation of Hepatic Stellate Cells via Up-Regulating Micro-RNA-124-3p to Alleviate Hepatic Fibrosis. Dig Dis Sci 64, 1560–1570 (2019). https://doi.org/10.1007/s10620-019-5462-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-019-5462-8