Abstract
Background and Aims
Liver cirrhosis is a substantial health burden in the USA, but population-based data regarding the trend and medical expenditure are limited and outdated. We investigated the trends of inpatient admissions, costs, and inpatient mortality from 2005 to 2015 among cirrhotic patients.
Methods
A retrospective analysis was conducted using the National Inpatient Sample database. We adjusted the costs to 2015 US dollars using a 3% inflation rate. National estimates of admissions were determined using discharge weights.
Results
We identified 1,627,348 admissions in cirrhotic patients between 2005 and 2015. From 2005 to 2015, the number of weighted admissions in cirrhotic patients almost doubled (from 505,032 to 961,650) and the total annual hospitalization cost in this population increased three times (from 5.8 to 16.3 billion US dollars). Notably, admission rates varied by liver disease etiology, decreasing from 2005 to 2015 among patients with hepatitis C virus (HCV)-related cirrhosis while increasing (almost tripled) among patients with nonalcoholic fatty liver disease (NAFLD)-related cirrhosis. The annual inpatient mortality rate per 1000 admissions overall decreased from 63.8 to 58.2 between 2005 and 2015 except for NAFLD (27.2 to 35.8) (P < 0.001).
Conclusions
Rates and costs of admissions in cirrhotic patients have increased substantially between 2005 and 2015 in the USA, but varied by liver disease etiology, with decreasing rate for HCV-associated cirrhosis and for HBV-associated cirrhosis but increasing for NAFLD-associated cirrhosis. Inpatient mortality also increased by one-third for NAFLD, while it decreased for other diseases. Cost also varied by etiology and lower for HCV-associated cirrhosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Liver cirrhosis is the result of long-term progressive fibrosis from chronic liver diseases [1]. Patients with cirrhosis can develop liver synthetic dysfunction and portal hypertension, which may result in ascites, spontaneous bacterial peritonitis, encephalopathy, hydrothorax, hepatopulmonary syndrome, pulmonary hypertension, hepatorenal syndrome, gastroesophageal variceal bleeding [1], and hepatocellular carcinoma (HCC) [2]: each of which may require inpatient hospital care and cause premature death. In 2013, cirrhosis accounted for 1.95% of deaths worldwide [3]. In the USA, liver cirrhosis was the eighth leading cause of mortality, and responsible for approximately 49,500 deaths in 2010 [4]. In 2004, the total direct cost of medical care for cirrhosis and chronic liver diseases in the USA was estimated to be approximately $2.5 billion, while the indirect cost (due to time lost from work and leisure) was an estimated $10.6 billion [5].
According to recent data, the annual cirrhosis-related mortality in the USA has continued to increase [6, 7]. Patients with advanced liver disease such as decompensated cirrhosis and/or hepatocellular carcinoma (HCC) incurred a significant increase in hospitalizations, transplant listing, and mortality in the recent decade [8,9,10]. However, no study to date has quantified the inpatient burden of cirrhosis to include costs and inpatient mortality. Therefore, the purposes of this study were to quantify the annual inpatient admission rates, inpatient mortality rates, and hospital-associated costs for hospitalized patients with liver cirrhosis and HCC from 2005 to 2015. We also aimed to report the distribution of disease burden by race/ethnicity, age, and liver disease etiology to provide data for future research to include healthcare resource utilization analysis.
Methods
Data Source
Data were extracted from the National Inpatient Sample (NIS). The NIS database was developed for the Healthcare Cost and Utilization Project (HCUP) by the Agency for Healthcare Research and Quality [11]. The NIS database is the largest all-payer inpatient care database in the USA, which samples 20% of all discharges from US hospitals (excluding rehabilitation and long-term acute care hospitals, as well as federally funded facilities) [8].
Cohort Selection
This study included hospitalizations that occurred in adults (≥ 18 years) who had liver cirrhosis between 2005 and 2015 in the NIS database. We selected adults with cirrhosis using the cirrhosis ICD-9 and 10 codes found as either the primary diagnosis (DX1) or one of the secondary diagnoses (DX2-15 for years 2005–2009; DX2-DX25 for years 2010–2013; and Dx2-DX30 for years 2014–2015). In addition, to further identify our cohort of patients, we used the diagnostic codes associated with the presence of portal hypertension (thrombocytopenia, splenomegaly, presence of varices) and/or signs of clinical hepatic decompensation (ascites, hepatic encephalopathy, jaundice, variceal hemorrhage, spontaneous bacterial peritonitis, hepatic hydrothorax, and hepatorenal syndrome). These representative ICD-9 and ICD-10 codes are also listed in Supplemental Table 1 (under decompensated cirrhosis).
Liver-related admissions referred to hospitalizations with a primary diagnosis (DX1) of hepatitis C virus (HCV), hepatitis B virus (HBV), alcoholic liver disease (ALD), nonalcoholic fatty liver disease (NAFLD), cryptogenic cirrhosis, other liver disease, compensated cirrhosis, decompensated cirrhosis, and HCC. We defined cryptogenic cirrhosis using ICD-9 code 5715 and ICD-10 code K7460, K7469, and K740. Patients with “cirrhosis of unknown etiology” refer to patients with cirrhosis who did not have any of the listed etiologies including codes for cryptogenic. To define NAFLD, we not only used the codes for NAFLD but also defined patients with cryptogenic conditioned on obesity or diabetes as NAFLD. We excluded hospitalizations in patients with human immunodeficiency virus (HIV), HBV/HCV dual infection, those who had undergone liver transplantation, those with cirrhosis of unknown etiology or miscellaneous liver disease. We excluded admissions related to patients with dually infected viral hepatitis so that we could try to isolate the effect of specific liver disease etiologies on the study outcomes.
Comorbidities were identified via ICD-9 and ICD-10 codes (Supplemental Table 2). The comorbidities included cardiovascular disease, diabetes mellitus, hypertension, non-HCC cancers, hyperlipidemia, chronic obstructive pulmonary disease (COPD), chronic kidney disease, alcohol use, illicit drug use, and mental illness. Severe comorbidities defined for the purpose of this study included renal failure, cardiac failure, major neurologic events, severe hematologic conditions, multi-organ failure, sepsis, hepatic failure, and respiratory failure.
Outcomes and Definition
Trends for hospitalizations, inpatient deaths, and costs per hospitalization for adults with cirrhosis were the primary outcomes. Our secondary outcomes were to provide trend data for liver cirrhosis among adults by liver disease etiology/severity, age, and race/ethnicity. All costs were inflation adjusted (3%) to 2015 US dollar value.
Statistical Analysis
Discharge weights and strata provided by HCUP were applied to our data in order to provide estimates of the total number of admissions among cirrhotic patients, their inpatient mortality, and the associated costs. It is important to note that HCUP redesigned the NIS in 2012 to improve national estimates. As a result, HCUP suggested that all analyses for trends prior to and after 2012 utilize the new discharge trend weights so trend rates can be compared over time which we did [12].
Chi-square statistics were used to compare admission characteristics by time (2005–2008, 2009–2012, and 2013–2015), and inpatient mortality rates among the subgroups.
We used linear regression to perform trend analyses. To accurately reflect the trend of admissions by etiology considering the changes in all admissions over the years, we presented the trend of the proportion of admissions by etiology instead of the admission number of each etiology.
We used GLM with a gamma distribution and log-link function to estimate hospital costs per admission and per hospital day as well as the factors associated with cost which included age, sex, race/ethnicity, patient location, income quartile, insurance type, severity of cirrhosis to include HCC, disease etiology of cirrhosis, and comorbidities. In addition, to consider the changes in the number of diagnosis codes used by NIS over the years, we performed a sensitivity analysis on the main trend analyses using the original 15 diagnoses (DX1-DX15) over all years of the study. We used multivariable logistic regression to estimate the adjusted odds ratio (aOR) and confidence interval (CI) for the independent predictors of inpatient mortality. The covariates in the model were age, sex, race/ethnicity, patient location, income quartile, insurance type, severity of cirrhosis to include HCC, disease etiology of cirrhosis, and comorbidities.
All statistical analyses were two-tailed, with a significance level of 0.05. We performed all analyses using STATA version 14 (Stata Corp, College Station, TX).
Ethics Statement with Data Use Agreement
This study was determined to be exempt by the Stanford University Institutional Review Board, Stanford, California. A data use agreement was obtained from the Agency for Healthcare Research and Quality for use of the NIS data.
Results
Study Population Characteristics
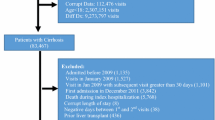
A total of 1,627,348 hospitalizations were included in the main analysis and 1,361,943 in the analysis involving liver disease etiologies (Supplementary Fig. 1). The national total hospitalizations were 7,882,801.
Table 1 shows the baseline characteristics of the study sample (characteristics of the total national hospitalization cohort provided by Supplementary Table 3). Male patients (~ 60%) and 45–64-year-olds (~ 60%) represented the largest groups of hospitalized cirrhotic patients across the study periods. By race/ethnicity, the largest proportion of admissions were in White (51.3–64.0%), followed by Hispanic (13.53–14.34%), Black (8.7–11.6%), and then Asian populations (1.8–2.0%). The majority of admissions were associated with decompensated cirrhosis (52.1–59.7%). Medicare was the primary insurance for approximately 40% of the admissions and was the most frequent payer. By trend analysis, the proportions of patients who were 65 and older, female, White, recipients of public insurance (Medicare or Medicaid) or who had HCC, NAFLD, or “other” liver diseases increased significantly over time (P < 0.001).
Number of Annual Admissions
Time Trends: Overall and by Liver Disease Etiology
Overall, the total estimated number of annual admissions in patients with cirrhosis rose between 2005 and 2015 (P < 0.001) (Fig. 1). Specifically, annual admissions rose from 505,032 in 2005 to 961,650 in 2015 which equals a 90.4% increase. A sensitivity analysis restricted to the original 15 diagnoses (DX1-DX15) used by the NIS throughout the whole study showed the same increasing trend of admission numbers (P < 0.001; Supplementary Fig. 2).
The time trend of total hospital cost and number of hospital admissions among patients with liver cirrhosis from 2005 to 2015. The x axis represents the time period from 2005 to 2015. The y axis on the left shows total hospital cost in billion US dollars, while the y axis on the right shows number of admissions in patients with cirrhosis
In terms of the etiology of liver disease, ALD, HCV, and cryptogenic liver disease accounted for the majority of admissions throughout 2005–2015 (Fig. 2). However, the proportion of admissions associated with these three etiologies decreased from 2005 to 2015, while the proportion of admissions associated with NAFLD almost tripled (from 0.03 to 0.11, P < 0.001). Although the proportion of HBV-related admissions witnessed only a small decline over time (12% decrease from 2005 to 2015), the decrease was significant (P < 0.001).
The time trend of proportion of hospital admission in patients with cirrhosis by etiology from 2005 to 2015 (P for trend < 0.001 for all etiologies). The x axis represents the time period from 2005 to 2015. The y axis shows the proportion of hospital admissions in patients with cirrhosis by etiology. HBV: hepatitis B virus, HCV: hepatitis C virus, ALD: alcoholic liver disease, NAFLD: nonalcoholic fatty liver disease, other liver disease: autoimmune hepatitis, alpha-1-antitrypsin deficiency, hemochromatosis, disorders of copper metabolism
Time Trend: By Liver Disease Severity, Age, and Race/Ethnicity
The distribution and severity of admissions in patients with cirrhosis varied among the racial/ethnic groups. Among White, Black, and Hispanic individuals, most hospitalizations occurred in the 45–64 age group, while the greatest proportion of admissions among Asian individuals occurred in the oldest age group (≥ 65 years). Within each racial/ethnic group, except for the Asian group, hospital admissions in patients with liver cirrhosis seemed to rise over time for patients 65 years or older, while decreasing in the younger groups (Supplementary Fig. 3).
Among Asians, a larger proportion of admissions occurred in patients with HCC (19.4% vs. 3.8% in White, 6.2% in Black, and 5.4% in Hispanic patients, P < 0.001). In fact, admissions with HCC were more frequent in the Asian cirrhotic population across all age groups: 28.7% of admissions in the ≥ 65 age group, 26.7% in the 45–64 age group, and 17.0% in the 18–44 age group (all P < 0.001) (Supplementary Fig. 4).
There were also significant differences in the distribution of liver disease etiology and age among racial/ethnic groups. Admissions in patients with HCV infection and ALD accounted for > 60% of admissions for White, Black, and Hispanic patients and were the most frequent etiologies in those younger than 65 (Supplementary Figs. 5 and 6). On the other hand, approximately 30% of admissions for Asian patients with cirrhosis were related to HBV (vs. < 5% in the other major racial/ethnic groups). (Supplementary Fig. 6).
Inpatient Mortality
In total, there were 469,808 inpatient all-cause deaths during 2005–2015, averaging 42,710 deaths annually. HBV had the highest inpatient mortality rate (77.9 per 1000 admissions, 95% CI 75.1–80.8, P < 0.001) (Fig. 3).
In-hospital mortality rate in patients with liver cirrhosis by etiology during 2005–2015. The x axis represents different etiology groups. The y axis shows mortality rate per 1000 hospitalizations. The error bar refers to 95% confidence interval of each etiology group. The p values on the left side show whether the difference of the mortality rate within subgroups is significant. HBV: hepatitis B virus, HCV: hepatitis C virus, ALD: alcoholic liver disease, NAFLD: nonalcoholic fatty liver disease, other liver disease: autoimmune hepatitis, alpha-1-antitrypsin deficiency, hemochromatosis, disorders of copper metabolism
Between 2005 and 2015, the total number of yearly all-cause deaths increased from 32,234 to 56,010 (a 73.8% increase). However, the inpatient mortality rate actually decreased from 63.8 to 58.2 per 1000 hospital admissions between 2005 and 2015. This trend was consistent across race/ethnicities, age groups and liver disease etiologies except for NAFLD, whose rate increased from 27.2 to 35.8 per 1000 hospital admissions (Supplemental Fig. 7).
In a multivariable model that controlled for age, sex, race/ethnicity, insurance type, income quartile, patient location, severity of cirrhosis, cause of cirrhosis, and comorbidities, older age (aOR = 2.39, P < 0.001 for those 65 or older) being Asian (aOR = 1.06, P < 0.001), having HCC (aOR = 3.36, P < 0.001), being self-pay (aHR: 1.28, P < 0.001) or having HBV (aOR = 1.05, P < 0.001), cryptogenic cirrhosis (aOR = 1.64, P < 0.001), or ALD (aOR = 1.32, P < 0.001) compared to HCV were all associated with a higher rate of in-hospital deaths. (Table 2).
Hospital Costs
The total cost of hospital admissions among patients with cirrhosis almost tripled from $5.8 billion in 2005 to $16.3 billion in 2015 (P < 0.001) (Fig. 1), with similar findings in a sensitivity analysis restricted to the original 15 diagnoses (DX1-DX15) (Supplementary Fig. 2).
After adjusting for age, sex, race/ethnicity, insurance type, income quartile, hospital location, severity of cirrhosis, etiology of cirrhosis, and comorbidities, we found that the hospital cost per admission was higher among the racial/ethnic minorities, as were admissions related to HBV and cryptogenic cirrhosis (Supplementary Table 4).
Though the cost per admission appeared higher for liver-related admission for certain etiologies such as cryptogenic cirrhosis, the hospital costs per day were fairly similar for the different liver disease etiologies and for both all-cause and liver-related admissions (Supplementary Figs. 8a and 8b).
Discussion
In this study, we provided updated 2015 national estimates and trends for hospital admission, mortality and costs for patients with cirrhosis overall and by race/ethnicity, age, and liver disease etiology-specific subgroups. We found that overall the number of hospitalizations among patients with cirrhosis increased by ~ 90% over this study period totaling 16.3 billion USD in 2015, a dramatic rise from the last reported figure of $9.8 billion in 2011 [13]. Notably, the number of NAFLD-associated admissions tripled and its inpatient mortality increased by 32%.
The proportion of HBV-associated cirrhotic admissions decreased over time, but the decrease was modest (12% over a 10-year period), despite the implementation of universal vaccination since 1991 and the availability of oral anti-HBV therapy since 1998 and ample data showing its effectiveness in decreasing HCC incidence and liver-related complications and mortality in treated patients [14, 15]. One possible explanation for this may be the suboptimal connection to care that has been well reported for many patients with chronic hepatitis B [16,17,18]. However, these hypotheses will require further investigation.
Our study found that patients aged 65 and over, female, White, recipients of public insurance (Medicare or Medicaid), and those having HCC, NAFLD, or other liver diseases experienced a significant increase in hospitalizations. Some of these associations were intuitive (e.g., age ≥ 65, HCC), while others were less apparent, though the association with NAFLD was likely related to metabolic and cardiovascular comorbidities being prevalent within these patient populations [19]. An unexpected finding was that the costs associated with those on Medicare or self-pay were significantly less than those with other insurance. One possible explanation for this result is that patients who were self-pay and/or were 65 years and older were 1.3 and 2.4 times (respectively) more likely to die while in the hospital, which in turn can shorten their length of stay and thus costs. Another possible explanation may be that Medicare may be discharged sooner from the hospital than others, as reported by a brief by the Agency for Healthcare Quality for patients with Medicare Advantage plans [20]. However, further exploration is needed to better understand this dynamic.
From 2005 to 2015, the rate of inpatient mortality per 1000 hospitalizations actually decreased by 8.7%, though the total number of inpatient deaths per year for patients with cirrhosis increased by 73.8% (from 32,234 to 56,010). The decreasing overall inpatient mortality rate may also be due to the increasing use of palliative care/hospice care. A recent study by the Veterans Administration reported a decrease in inpatient mortality rate, but an actual increase in mortality rate following discharge [21].
However, inpatient mortality did not decrease but actually increased by one-third for NAFLD-associated cirrhosis between 2005 and 2015. This, coupled with a three-times rising trend in hospital admissions in patients with NAFLD-associated cirrhosis during the same time period, highlights the importance of NAFLD as a growing and major contributor to liver disease morbidity and mortality in the USA. We should note that part of the observed increase in NAFLD-associated disease burden might be due to the increasing awareness and reporting for NAFLD in the more recent years rather than reporting NAFLD as cryptogenic cirrhosis which has often been cited as “burned out nonalcoholic steatosis.” In fact, cryptogenic cirrhosis is the most frequent cause of cirrhosis all-cause and liver-related hospitalization and a major contributor to mortality in this study. As such, this phenomenon must be followed as our understanding and ability to diagnose NAFLD increases such that NAFLD may carry a greater burden than what we are presently able to report given the limitations of national databases [22, 23].
Regarding alcoholic cirrhosis, we did not find a rising trend as recently reported by a claims database study that grouped patients with other concurrent liver disease (e.g., HCV) together with patients with only a diagnosis code for alcoholic cirrhosis, yielding a higher disease burden for ALD [24]. In the current study, patients were classified as ALD if they did not have another chronic liver disease. However, it is important to point out that ALD is the second most frequent reason for liver cirrhosis in the 45–64-year-old age group and does carry a substantial financial burden. Thus, we suggest that there must be continued focus on interventions for ALD, since it may overtake HCV as the leading liver disease as efforts to curb the impact of viral hepatitis are taking effect.
There were limitations to our study. First, we could only analyze the data by hospitalization events instead of individual patients, and we were not able to report whether patients had multiple admissions. Second, the database does not capture death outside of the hospital, so the total mortality rates in patients with cirrhosis may be higher than we reported. Third, we cannot perform state-level analysis, since the NIS database was sampled by hospital rather than state-level features limiting our ability to study regional differences [25]. Finally, diagnoses may not have always been coded correctly, which is a standard limitation across all studies utilizing administrative databases. However, the databases we utilized are well regarded to be relatively reliable and are widely used. We also worked to compensate for this by including all diagnosis codes that would be associated with cirrhosis.
Conclusion
In 2015, there were 961,650 hospital admissions associated with cirrhosis, responsible for 56,010 deaths (58.2 death per 1000 hospital admissions) and 16.3 billion USD in cost. Between 2005 and 2015, admission rates and inpatient mortality both increased for NAFLD-associated cirrhosis, while decreasing or remaining stable for other etiologies. Hospital cost per admission was higher among racial/ethnic minorities. These data should inform stakeholders to plan targeted interventions to prevent these costly admissions.
References
Nusrat S, Khan MS, Fazili J, Madhoun MF. Cirrhosis and its complications: evidence based treatment. World J Gastroenterol. 2014;20:5442–5460.
Asrani SK, Larson JJ, Yawn B, Therneau TM, Kim WR. Underestimation of liver-related mortality in the United States. Gastroenterology. 2013;145(375–382):e372.
Mokdad AA, Lopez AD, Shahraz S, et al. Liver cirrhosis mortality in 187 countries between 1980 and 2010: a systematic analysis. BMC Med. 2014;12:145.
Murray CJ, Atkinson C, Bhalla K, et al. The state of US health, 1990–2010: burden of diseases, injuries, and risk factors. Jama. 2013;310:591–608.
Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part I: overall and upper gastrointestinal diseases. Gastroenterology. 2009;136:376–386.
Kim D, Li AA, Perumpail BJ, et al. Changing trends in etiology- and ethnicity-based annual mortality rates of cirrhosis and hepatocellular carcinoma in the United States. Hepatology (Baltimore, Md.). 2018.
Tapper EB, Parikh ND. Mortality due to cirrhosis and liver cancer in the United States 1999–2016: observational study. BMJ (Clinical research ed.). 2018;362:k2817.
Kim D, Cholankeril G, Li AA, et al. Trends in hospitalizations for chronic liver disease-related liver failure in the United States, 2005–2014. Liver Int Off J Int Assoc Study Liver. 2019.
Kim D, Li AA, Perumpail BJ, et al. Changing trends in etiology-based and ethnicity-based annual mortality rates of cirrhosis and hepatocellular carcinoma in the United States. Hepatology (Baltimore, Md.). 2019;69:1064–1074.
Goldberg D, Ditah IC, Saeian K, et al. Changes in the prevalence of hepatitis C virus infection, nonalcoholic steatohepatitis, and alcoholic liver disease among patients with cirrhosis or liver failure on the waitlist for liver transplantation. Gastroenterology. 2017;152:e1091.
HCUP Nationwide Inpatient Sample (NIS). Healthcare cost and utilization project (HCUP). City 2011.
Overview of the National (Nationwide) Inpatient Sample (NIS). Available at: https://www.hcup-us.ahrq.gov/nisoverview.jsp (2015). Accessed 12.03.2018.
Allen AM, Kim WR, Moriarty JP, Shah ND, Larson JJ, Kamath PS. Time trends in the health care burden and mortality of acute on chronic liver failure in the United States. Hepatology (Baltimore, Md.). 2016;64:2165–2172.
Lok AS, McMahon BJ, Brown RS Jr, et al. Antiviral therapy for chronic hepatitis B viral infection in adults: a systematic review and meta-analysis. Hepatology (Baltimore, Md.). 2016;63:284–306.
Lin D, Yang HI, Nguyen N, et al. Reduction of chronic hepatitis B-related hepatocellular carcinoma with antiviral therapy, including low risk patients. Alimentary pharmacology & therapeutics. 2016;44:846–855.
Rosenberg ES, Rosenthal EM, Hall EW, et al. Prevalence of hepatitis C virus infection in US states and the district of Columbia, 2013 to 2016. Jama Netw Open. 2018;1:e186371.
Cohen C, Holmberg SD, McMahon BJ, et al. Is chronic hepatitis B being undertreated in the United States? J Viral Hepat.. 2011;18:377–383.
Spradling PR, Xing J, Rupp LB, et al. Infrequent clinical assessment of chronic hepatitis B patients in United States general healthcare settings. Clin Infect Dis Off Publ Infect Dis Soc Am. 2016;63:1205–1208.
Nguyen AL, Park H, Nguyen P, Sheen E, Kim YA, Nguyen MH. Rising inpatient encounters and economic burden for patients with nonalcoholic fatty liver disease in the USA. Dig Dis Sci. 2019;64:698–707. https://doi.org/10.1007/s10620-018-5326-7
Raetzman SO, Hines AL, Barrett ML, Karaca Z. Hospital stays in medicare advantage plans versus the traditional medicare fee-for-service program, 2013: statistical Brief#198. ed. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs, City; Agency for Healthcare Research and Quality (US);2006.
Kanwal F, Tansel A, Kramer JR, Feng H, Asch SM, El-Serag HB. Trends in 30-day and 1-year mortality among patients hospitalized with cirrhosis from 2004 to 2013. Am J Gastroenterol. 2017;112:1287–1297.
Golabi P, Bush H, Stepanova M, et al. Liver transplantation (LT) for cryptogenic cirrhosis (CC) and nonalcoholic steatohepatitis (NASH) cirrhosis: data from the scientific registry of transplant recipients (SRTR): 1994 to 2016. Medicine. 2018;97:e11518.
Thuluvath PJ, Kantsevoy S, Thuluvath AJ, Savva Y. Is cryptogenic cirrhosis different from NASH cirrhosis? J Hepatol. 2018;68:519–525.
Mellinger JL, Shedden K, Winder GS, et al. The high burden of alcoholic cirrhosis in privately insured persons in the United States. Hepatology (Baltimore, Md.). 2018;68:872–882.
Khera R, Angraal S, Couch T, et al. Adherence to methodological standards in research using the national inpatient sample. Jama. 2017;318:2011–2018.
Acknowledgments
This research used the NIS dataset provided by HCUP. We thank the HCUP Data Partners that contributed to HCUP. The state organizations were listed here: www.hcup-us.aORq.gov/hcupdatapartners.jsp.
Funding
None to disclose.
Author information
Authors and Affiliations
Contributions
MHN and BZ had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. MHN was involved in study concept, supervision, and guarantor of the article. BZ, YHY, EI, RC, and MHN were involved in study design. BZ, YHY, LH, RC, and MHN contributed to drafting of the manuscript. BZ, YHY, DJ, and MHN helped in data analysis. YHY, BZ, and MHN contributed to data collection. All the authors were involved in data interpretation, review, and revision of the manuscript, and all the authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zou, B., Yeo, Y.H., Jeong, D. et al. A Nationwide Study of Inpatient Admissions, Mortality, and Costs for Patients with Cirrhosis from 2005 to 2015 in the USA. Dig Dis Sci 65, 1520–1528 (2020). https://doi.org/10.1007/s10620-019-05869-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-019-05869-z