Abstract
Background
Little is known about the natural history of perianal fistulas in Asian populations with Crohn’s disease (CD).
Aims
We investigated the incidence and outcomes of perianal CD (pCD) in Korean CD patients.
Methods
A nationwide population-based cohort of 6265 CD patients diagnosed in 2010–2014 was analyzed to investigate the incidence and outcomes of pCD. The results were validated in a hospital-based cohort of 2923 CD patients diagnosed in 1981–2015. Factors associated with pCD development were analyzed. The incidence and outcomes of pCD were compared between the prebiologic and biologic eras.
Results
pCD occurred in 39.2% of the population-based cohort and 56.1% of the hospital-based cohort during the median follow-up of 4.2 and 8.5 years, respectively. The cumulative incidence of pCD was 40.0% at 5 years after CD diagnosis in the population-based cohort and 62.5% at 20 years in the hospital-based cohort. In multivariate analysis, pCD development was positively associated with male sex, younger age and colonic involvement at diagnosis, early diagnosis, and CD diagnosis in the prebiologic era. The cumulative probability of proctectomy at 10, 20, and 30 years after pCD diagnosis was 2.9%, 12.2%, and 16.2%, respectively. The cumulative incidence of pCD occurring after CD diagnosis and the cumulative probability of proctectomy were significantly lower in the biologic era than in the prebiologic era (p < 0.001 and p = 0.03, respectively).
Conclusions
Compared with Western patients with CD, Korean patients show a high incidence of pCD but have a low probability of proctectomy, suggesting the favorable course of pCD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Perianal fistula is a common manifestation of Crohn’s disease (CD). Perianal fistulizing CD can lead to severe perianal pain, fecal incontinence, and anal stricture, eventually considerably impairing a patient’s quality of life [1, 2]. Also, the presence of perianal fistula has been reported to be associated with an increased risk of progression to complicated behavior, hospitalization, intestinal resection, postsurgical recurrence, and a disabling disease course in patients with CD [3,4,5,6]. Moreover, long-standing perianal fistulizing CD can be a risk factor for anorectal cancer [7, 8]. Therefore, perianal fistula is considered a predictor of poor prognosis in patients with CD.
Although the incidence of perianal fistula in patients with CD has been reported to be 13.9‒28.1% in Western population-based studies [3, 9,10,11,12,13], a recent meta-analysis [14] has suggested that perianal involvement is more common in Asian patients with CD than in Caucasian patients. However, this finding was limited by the discrepancy in the source of data between the Caucasian population (mostly population-based studies) and the Asian population (mostly hospital-based studies). To date, only 1 territory-wide study on perianal fistula in the Asian CD patients has been reported [15]. In this study, Mak et al. reported that the cumulative probability of developing perianal CD (pCD) was 16.2% at 10 years and 24.8% at 20 years among patients with CD in Hong Kong, which is comparable to that reported in previous Western population-based studies. These results indicate that more studies are needed before it can be concluded that Asian patients with CD have a higher incidence of perianal fistula.
Therefore, we performed a nationwide population-based study using claims data to investigate the incidence and outcomes of pCD in Korean patients with CD and validated the results in a well-defined, large hospital-based cohort.
Materials and Methods
Nationwide Population-Based Cohort Study
Study Population
The data source used in this nationwide population-based cohort study has been described in our previous study [16]. Briefly, we used the 2007–2016 data from the Health Insurance Review and Assessment Service (HIRA) claims database. The HIRA database contains claims data for the entire Korean population, including demographics, diagnoses, procedures, prescriptions, and registration information on rare intractable diseases (RIDs). The RID system in Korea provides copayment reductions for patients with RIDs, including CD [16,17,18,19]. To be registered in the RID program, all patients should meet the uniform diagnostic criteria distributed by the National Health Insurance (NHI) [17], and this application is reviewed by the corresponding health-care institution and the NHI.
Our algorithm for the identification of patients with CD has been described in our previous study [16]. Briefly, after establishing a washout period of 3 years (2007‒2009), incident CD cases between 2010 and 2014 were identified as those satisfying all of the following criteria: (1) diagnostic codes for CD (K50.0‒50.9) in the principal or subsidiary diagnostic field, (2) RID registration code for CD (V130), and (3) a prescription for CD-related medications. Patients were followed up until the end of 2016.
Evaluation of pCD
pCD was defined as perianal fistula or perianal abscess that occurred in patients with CD. pCD cases were identified according to the diagnostic codes for perianal fistula (International Classification of Diseases, 10th edition [ICD 10] codes: K60.3, K60.4, K60.5) or perianal abscess (ICD 10 codes: K61, K61.0, K61.1, K61.2, K61.3, K61.4) in the principal or subsidiary diagnostic field. In patients with multiple diagnostic codes for pCD, the date of pCD diagnosis was defined as the first date on which the code for pCD was identified.
Patients with pCD who underwent proctectomy were identified according to the surgical procedure codes for abdominoperineal resection (claim codes: QA923, Q2923) and for total proctocolectomy with ileostomy (claim codes: QA925, Q2925). In addition, patients with pCD who underwent minor surgery for pCD (incision and drainage, seton operation, fistulostomy, and fistulectomy) were identified according to the surgical procedure codes for perianal fistula (claim codes: Q2910, Q2974, Q2975, Q2976, Q2977, Q2978, Q2979) and perianal abscess (claim codes: Q2881, Q2882, Q2883).
Hospital-Based Cohort Study
Study Population
CD patients who were seen at Asan Medical Center, a tertiary university hospital in Seoul, between June 1989 and December 2015 were enrolled in the hospital-based cohort study. All patients were diagnosed as having CD between 1981 and 2015 and were followed up until May 2018. The patients’ demographic and clinical information was retrieved from the Asan inflammatory bowel disease (IBD) registry, which has been prospectively maintained since 1997 [20]. CD diagnosis was based on conventional clinical, radiologic, endoscopic, and histopathologic criteria [20, 21].
Evaluation of pCD
A diagnosis of pCD was made if there was clinical, radiographic, endoscopic, or surgical evidence suggesting pCD [11]. pCD before CD diagnosis was defined as pCD that occurred at least 90 days before the time of CD diagnosis. pCD at CD diagnosis was defined as pCD that occurred within ± 90 days from the time of CD diagnosis. pCD after CD diagnosis was defined as pCD that occurred at least 90 days after the time of CD diagnosis. For the purpose of data analysis, patients who had pCD before CD diagnosis were assumed to have developed pCD on the day of CD diagnosis. The outcome of pCD was measured by the need for proctectomy.
Statistical Analysis
Categorical variables were presented as numbers with percentages, and continuous variables were presented as medians with interquartile ranges (IQRs). The Chi-square test was used to compare categorical variables, and Student’s t test was used for continuous variables. The cumulative incidence of pCD from the time of CD diagnosis and the cumulative probability of proctectomy from the time of pCD development were calculated using the Kaplan–Meier method. Further, to evaluate temporal changes, the results were stratified into 2 subgroups according to the year of CD diagnosis or pCD diagnosis (before 2005 vs. 2005 or later) and compared using the log-rank test. The year 2005 was chosen because reimbursement of anti-tumor necrosis factor (anti-TNF) agents for patients with CD started in 2005 in Korea. A logistic regression model was used to identify factors associated with pCD development and to calculate the odds ratios (ORs) and 95% confidence intervals (CIs). Variables with a p value < 0.1 in the univariate analysis were included in the multivariate analysis. All statistical analyses were performed using SAS Enterprise Guide software version 6.1 (SAS Institute Inc., Cary, NC) and SPSS version 21 (IBM Corp., Armonk, NY, USA), and p < 0.05 was considered to indicate statistical significance. The ethics committee of Asan Medical Center approved the study protocol.
Results
Nationwide Population-Based Cohort Study
Patient Characteristics
Among 6265 patients with CD newly diagnosed between 2010 and 2014, 4533 patients (72.4%) were men, and the median age at CD diagnosis was 22.5 years (IQR 17.3‒33.7 years).
Incidence of pCD
During the median follow-up period of 4.2 years (IQR 3.0‒5.4 years), pCD occurred at least once in 2456 (39.2%) of 6265 CD patients. Of them, 2038 patients (83.0%) were men, resulting in a male-to-female ratio of 4.9:1, which was higher than the ratio of 1.9:1 in patients without pCD (p < 0.001). The median age at CD diagnosis in patients with pCD was 19.0 years (IQR 16.2‒25.6 years), which was younger than the median age of 26.5 years (IQR 18.4‒40.3 years) in patients without pCD (p < 0.001).
Among 2456 patients with pCD, 1742 (70.9%) developed pCD before CD diagnosis, 344 (14.0%) developed pCD at CD diagnosis, and 370 (15.1%) developed pCD after CD diagnosis. Among patients who developed pCD after CD diagnosis, the median time from CD diagnosis to the first pCD episode was 1.6 years (IQR 0.8‒3.1 years). The cumulative incidence of pCD was 35.2% at 1 year, 37.9% at 3 years, and 40.0% at 5 years after CD diagnosis (Fig. 1).
Outcomes of pCD
Among 2456 patients with pCD, 1898 (77.3%) underwent minor surgery for pCD and 6 (0.2%) underwent proctectomy. Minor surgery included incision and drainage in 1175 patients (47.8%), fistulectomy and fistulotomy in 1015 patients (41.3%), and seton operation in 562 patients (22.9%). The remaining 552 patients (22.5%) were medically treated.
Hospital-Based Cohort Study
Patient Characteristics
Among a total of 2923 patients with CD, 2121 (72.6%) were men, and the median age at CD diagnosis was 22.8 years (IQR 18.8‒29.4 years) (Table 1). The disease location at CD diagnosis was ileal (L1) in 724 patients (24.8%), colonic (L2) in 193 patients (6.6%), ileocolonic (L3) in 1995 patients (68.3%), and unknown in 11 patients (0.4%). The disease behavior at CD diagnosis was inflammatory (B1) in 2296 patients (78.5%), structuring (B2) in 290 patients (9.9%), penetrating (B3) in 335 patients (11.5%), and unknown in 2 patients (0.1%).
Incidence of pCD
During the median follow-up period of 8.5 years (IQR 5.1‒13.0 years), pCD occurred at least once in 1639 (56.1%) of 2923 patients. Of them, 1276 patients (77.9%) were men, resulting in a male-to-female ratio of 4.5:1, which was significantly higher than the ratio of 1.9:1 in patients without pCD (p < 0.001, Table 1). The median age at CD diagnosis in patients with pCD was 21.8 years (IQR 18.4‒27.3 years), which was significantly younger than the median age of 24.2 years (IQR 19.5‒32.2 years) in patients without pCD (p < 0.001, Table 1). pCD developed in 317 (43.8%) of 724 patients with ileal (L1) disease, 121 (62.7%) of 193 patients with colonic (L2) disease, and 1196 (59.9%) of 1995 patients with ileocolonic (L3) disease. Among 1639 patients with pCD, 872 (53.2%) developed pCD before CD diagnosis, 470 (28.7%) developed pCD at CD diagnosis, and 297 (18.1%) developed pCD after CD diagnosis. In patients who developed pCD after CD diagnosis, the median time from CD diagnosis to the first pCD episode was 2.9 years (IQR 1.2‒6.2 years). Of all 2923 patients with CD, pCD was one of the initial manifestations of CD in 812 patients (27.8%). Moreover, 117 patients (4.0%) did not have any symptoms of CD other than pCD until the time of CD diagnosis.
The cumulative incidence of pCD was 47.7% at 1 year, 53.3% at 5 years, 57.1% at 10 years, 62.5% at 20 years, and 63.3% at 30 years after CD diagnosis (Fig. 1). The cumulative incidence of pCD that occurred after CD diagnosis was significantly lower in 2084 patients who were diagnosed as having and were treated for CD in 2005 or after than in 839 patients who were diagnosed as having and were treated for CD before 2005 (p < 0.001) (Fig. 2). Multivariate analysis revealed that male sex, younger age at CD diagnosis, colonic involvement at CD diagnosis, interval between gastrointestinal symptom onset and diagnosis ≤ 6 months, and CD diagnosis before 2005 were independently associated with the development of pCD (Table 2).
Outcomes of pCD
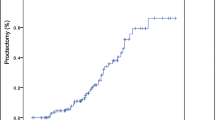
Among 1639 patients with pCD, 1329 (81.1%) underwent minor surgery for pCD and 103 (6.3%) underwent major surgery, including proctectomy and fecal diversion. The remaining 207 patients (12.6%) were only medically treated. The natural course of pCD in 103 patients who underwent major surgery is presented in Fig. 3. Proctectomy was performed in 57 (3.5%) of 1639 pCD patients during a median follow-up period of 125.7 months (IQR 71.6–180.9 months) after pCD diagnosis. The cumulative probability of proctectomy was 2.9% at 10 years, 12.2% at 20 years, and 16.2% at 30 years after pCD diagnosis (Fig. 4). The cumulative probability of proctectomy was significantly lower in 1118 patients who were diagnosed as having CD and pCD in 2005 or after than in 443 patients who were diagnosed as having CD and pCD before 2005 (p = 0.030) (Fig. 5).
Discussion
In the present study, we evaluated the incidence of pCD in Korean patients with CD by using both population-based and hospital-based cohorts. To the best of our knowledge, this study is the first to evaluate the incidence of pCD in East Asian CD patients in a nationwide population-based cohort. In this nationwide population-based cohort study, 33.3% of the patients developed pCD before or at CD diagnosis, and an additional 5.9% developed pCD during a median follow-up period of 4.2 years after CD diagnosis. These rates are much higher than those reported in Western population-based studies [9,10,11,12, 22, 23], in which only 8.1–16.7% of patients developed pCD before or at CD diagnosis, and, even though cases of pCD that occurred during the median follow-up period of 8.4–16.2 years after CD diagnosis were added, only 13.9–28.1% of patients developed pCD [3, 9,10,11,12,13].
The incidence of pCD in our population-based study should be interpreted with caution. Although the pCD incidence in our population-based study was higher than that in Western population-based studies, it was much lower than the pCD incidence in our hospital-based study. However, the impact of referral bias in our hospital-based study may be lower than that in Western hospital-based studies because most CD patients are managed at university hospitals in Korea. Therefore, the incidence of pCD in our population-based study might have been underestimated because of the following reasons. First, there is a strong possibility that the code for pCD was missed in some patients with pCD, because pCD is a subsidiary diagnosis. Second, considering that the washout period for excluding patients with preexisting CD was only 3 years in our study and that pCD may precede the onset of CD by many years, there is a possibility that some pCD episodes were not included. Third, the median follow-up duration after CD diagnosis was only 4.2 years in our population-based study, which contributed to lowering the proportion of patients with pCD.
The cumulative probability of proctectomy in our hospital-based cohort study was 2.9% at 10 years and 12.2% at 20 years after pCD diagnosis, which is lower than the reported probability of 20% and 22%, respectively, in a population-based study from Olmsted County [11]. However, the proctectomy rate in our study is comparable to that of a territory-wide study from Hong Kong, in which 1.7% of patients with pCD underwent proctectomy during a median follow-up period of 8.8 years [15]. These results suggest that the clinical course of pCD in East Asian CD patients may be better than that in Western patients. However, to conclude that the prognosis of pCD in East Asian CD patients is better than that in Western patients, factors that may affect the proctectomy rate need to be analyzed. First, a lower proctectomy rate may be attributed to milder disease severity, a lower proportion of complex fistula, or better response to treatment. However, it is impossible to verify these issues because of a lack of comparative data. Second, the development of new therapeutic modalities such as anti-TNF agents may have led to a lower proctectomy rate in our study. The study from Olmsted County demonstrated that the cumulative proctectomy rate was significantly lower in the biologic era than in the prebiologic era [11]. This finding was validated in our present study. Moreover, 71.3% of our patients were diagnosed as having CD in the biologic era. Therefore, the impact of anti-TNF agents in lowering the overall proctectomy rate may be greater in our study than in previous Western studies [12, 24]. However, the 10-year cumulative proctectomy rate in our study is lower than that in the study from Olmsted County [11], not only in the whole study period (2.9% vs. 20%) but also in the biologic era (2.3% vs. > 10%). Thus, the higher proportion of patients diagnosed as having CD in the biologic era cannot totally explain the lower proctectomy rate in our study. Third, the lower acceptance of proctectomy by patients and/or physicians may have contributed to the low proctectomy rate in Korea, especially in the early period after pCD diagnosis. This assumption is supported by the finding that the gap in cumulative proctectomy rate between the Olmsted County study [11] and our study decreased as the duration of pCD increased: 17.1% at 10 years, 9.8% at 20 years, and 5.8% at 30 years. Longer-term studies are needed to clarify this issue.
Our study has several strengths. First, this is the largest study to evaluate the incidence and outcomes of pCD. Second, both nationwide population-based and hospital-based cohorts were used in this study. The population-based data enabled overcoming the referral bias of the hospital-based data, whereas the hospital-based data enabled a detailed analysis that would be impossible with administrative data. Third, because the Asan IBD registry has been prospectively maintained since 1997 [20], detailed information on pCD could be obtained without missing the history of pCD that occurred long before the diagnosis of CD. Despite these strengths, our study has a limitation in that potential contributing factors of pCD outcomes, such as types of pCD (simple vs. complex type) and disease activity at pCD diagnosis, were not considered in this study.
In conclusion, when compared with Western CD patients, Korean CD patients show a high incidence of pCD, but may have a low probability of proctectomy, suggesting the favorable course of pCD.
References
Vollebregt PF, van Bodegraven AA, Markus-de Kwaadsteniet TML, van der Horst D, Felt-Bersma RJF. Impacts of perianal disease and faecal incontinence on quality of life and employment in 1092 patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2018;47:1253–1260.
Mahadev S, Young JM, Selby W, Solomon MJ. Quality of life in perianal Crohn’s disease: what do patients consider important? Dis Colon Rectum. 2011;54:579–585.
Bernell O, Lapidus A, Hellers G. Risk factors for surgery and postoperative recurrence in Crohn’s disease. Ann Surg. 2000;231:38–45.
Beaugerie L, Seksik P, Nion-Larmurier I, Gendre JP, Cosnes J. Predictors of Crohn’s disease. Gastroenterology. 2006;130:650–656.
Lakatos PL, Czegledi Z, Szamosi T, et al. Perianal disease, small bowel disease, smoking, prior steroid or early azathioprine/biological therapy are predictors of disease behavior change in patients with Crohn’s disease. World J Gastroenterol. 2009;15:3504–3510.
Kobayashi T, Hisamatsu T, Suzuki Y, et al. Predicting outcomes to optimize disease management in inflammatory bowel disease in Japan: their differences and similarities to Western countries. Intest Res. 2018;16:168–177.
Beaugerie L, Carrat F, Nahon S, et al. High risk of anal and rectal cancer in patients with anal and/or perianal Crohn’s disease. Clin Gastroenterol Hepatol. 2018;16:892–899-e892.
Lee HS, Park SH, Yang SK, et al. The risk of colorectal cancer in inflammatory bowel disease: a hospital-based cohort study from Korea. Scand J Gastroenterol. 2015;50:188–196.
Schwartz DA, Loftus EV Jr, Tremaine WJ, et al. The natural history of fistulizing Crohn’s disease in Olmsted County, Minnesota. Gastroenterology. 2002;122:875–880.
Thia KT, Sandborn WJ, Harmsen WS, Zinsmeister AR, Loftus EV Jr. Risk factors associated with progression to intestinal complications of Crohn’s disease in a population-based cohort. Gastroenterology. 2010;139:1147–1155.
Park SH, Aniwan S, Scott Harmsen W, et al. Update on the natural course of fistulizing perianal Crohn’s disease in a population-based cohort. Inflamm Bowel Dis. 2019;25:1054–1060.
Hellers G, Bergstrand O, Ewerth S, Holmstrom B. Occurrence and outcome after primary treatment of anal fistulae in Crohn’s disease. Gut. 1980;21:525–527.
Gottgens KW, Jeuring SF, Sturkenboom R, et al. Time trends in the epidemiology and outcome of perianal fistulizing Crohn’s disease in a population-based cohort. Eur J Gastroenterol Hepatol. 2017;29:595–601.
Shi HY, Levy AN, Trivedi HD, Chan FKL, Ng SC, Ananthakrishnan AN. Ethnicity Influences phenotype and outcomes in inflammatory bowel disease: a systematic review and meta-analysis of population-based studies. Clin Gastroenterol Hepatol. 2018;16:190–197-e111.
Mak WY, Mak OS, Lee CK, et al. Significant medical and surgical morbidity in perianal Crohn’s disease: results from a territory-wide study. J Crohns Colitis. 2018;12(12):1392–1398.
Chang K, Lee HS, Kim YJ, et al. Increased risk of herpes zoster infection in patients with inflammatory bowel diseases in Korea. Clin Gastroenterol Hepatol. 2018;16:1928–1936-e1922.
Kim HJ, Hann HJ, Hong SN, et al. Incidence and natural course of inflammatory bowel disease in Korea, 2006–2012: a nationwide population-based study. Inflamm Bowel Dis. 2015;21:623–630.
Wei SC. Differences in the public medical insurance systems for inflammatory bowel disease treatment in Asian countries. Intest Res. 2016;14:218–223.
Kim JA, Yoon S, Kim LY, Kim DS. Towards actualizing the value potential of Korea Health Insurance Review and Assessment (HIRA) data as a resource for health research: strengths, limitations, applications, and strategies for optimal use of HIRA data. J Korean Med Sci. 2017;32:718–728.
Park SH, Yang SK, Park SK, et al. Long-term prognosis of Crohn’s disease and its temporal change between 1981 and 2012: a hospital-based cohort study from Korea. Inflamm Bowel Dis. 2014;20:488–494.
Ye BD, Yang SK, Cho YK, et al. Clinical features and long-term prognosis of Crohn’s disease in Korea. Scand J Gastroenterol. 2010;45:1178–1185.
Sjoberg D, Holmstrom T, Larsson M, et al. Incidence and clinical course of Crohn’s disease during the first year—results from the IBD Cohort of the Uppsala Region (ICURE) of Sweden 2005–2009. J Crohns Colitis. 2014;8:215–222.
van den Heuvel TRA, Jeuring SFG, Zeegers MP, et al. A 20-year temporal change analysis in incidence, presenting phenotype and mortality, in the Dutch IBDSL cohort-can diagnostic factors explain the increase in IBD incidence? J Crohns Colitis. 2017;11:1169–1179.
Bell SJ, Williams AB, Wiesel P, Wilkinson K, Cohen RC, Kamm MA. The clinical course of fistulating Crohn’s disease. Aliment Pharmacol Ther. 2003;17:1145–1151.
Acknowledgments
We thank the Korean Health Insurance Review and Assessment Service and the National Health Insurance Service for providing the insurance claims data.
Funding
This work was supported by a Korean Health Technology R&D Project Grant from the Korea Health Industry Development Institute to Suk-Kyun Yang (A120176), funded by the Ministry of Health and Welfare. Ho-Su Lee was supported by the National Research Foundation of Korea (NRF) MRC Grant funded by the Korean government (MSIT) (2018R1A5A2020732).
Author information
Authors and Affiliations
Contributions
EMS, HSL, and SKY were involved in planning and conducting the study. EMS, HSL, YJK, EHO, NSH, JK, SWH, SHP, DHY, BDY, JSB, SJM, YSY, CSY, and SKY contributed to collecting data. EMS, HSL, YJK, and SKY helped in statistical analysis and interpretation of data. EMS and HSL contributed to drafting the manuscript. SKY was involved in study supervision and critical revision of the manuscript for important intellectual content.
Corresponding author
Ethics declarations
Conflict of interest
Suk-Kyun Yang received a research grant from Janssen Korea Ltd.; however, this funding was not related to the topic of this study. The remaining authors have no conflict of interest or financial ties to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Song, E.M., Lee, HS., Kim, YJ. et al. Incidence and Outcomes of Perianal Disease in an Asian Population with Crohn’s Disease: A Nationwide Population-Based Study. Dig Dis Sci 65, 1189–1196 (2020). https://doi.org/10.1007/s10620-019-05819-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-019-05819-9