Abstract
Objectives
Transarterial chemoembolization (TACE) improves the survival of patients with hepatocellular carcinoma (HCC); however, TACE treatment outcomes of patients with treatment-naïve HCC (TN-HCC) and those with recurrent HCC after curative resection (R-HCC) have not yet been compared.
Methods
We recruited 448 patients with TN-HCC, and 275 patients with R-HCC treated with TACE as first-line anti-cancer treatment.
Results
At first TACE, patients with TN-HCC showed a significantly lower proportion of male gender (74.9% vs. 84.3%), higher proportion of liver cirrhosis (61.9% vs. 49.3%), higher aspartate aminotransferase (median 48 vs. 31 IU/L), alanine aminotransferase (median 38 vs. 26 IU/L), alpha-fetoprotein (AFP) (median 96.6 vs. 7.7 ng/mL), and total bilirubin (mean 1.0 vs. 0.8 mg/dL) levels, longer prothrombin time (median 1.05 vs. 1.01 international normalized ratio), higher tumor number (mean 2.1 vs. 1.7), larger tumor size (median 3.1 vs. 1.6 cm), and lower proportion of Barcelona Clinic Liver Cancer stage 0-A (55.6% vs. 71.9%) than patients with R-HCC (all P < 0.05). Multivariate analysis showed that TACE for TN-HCC (vs. R-HCC) was an independent predictor of mortality (hazard ratio, 1.328; P = 0.024) with AFP level and tumor number (all P < 0.05). However, treatment outcomes between TN-HCC and R-HCC became statistically similar after propensity score-matched (PSM) analysis using liver cirrhosis, tumor size, and multiple tumors (P < 0.05).
Conclusions
Based on the similar TACE treatment outcomes observed with the PSM analysis, the current TACE treatment guideline for patients with TN-HCC might similarly be applied for patients with R-HCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is one of the most prevalent cancers worldwide, with over 22 million cases predicted over the next two decades [1]. Furthermore, it is the third most common cause of cancer-related death [2]. Although many advances in treatment and prevention have been made, > 20,000 deaths were estimated to occur due to HCC [3]. If HCC is detected in its early stage, curative treatments such as resection, transplantation, and local ablation can be applied [4]. In contrast, only palliative treatment such as transarterial chemoembolization (TACE) or sorafenib can be used for intermediate or advanced HCC [5,6,7].
Resection is a form of curative treatment for early HCC or a small proportion of intermediate HCC cases [4]. The long-term outcomes of curative resection for intermediate-stage HCC are significantly better (5-year survival, 37–50%) than palliative treatment modalities such as TACE (5-year survival, 12–16.5%) [8,9,10]. However, curative resection is applicable to only a small proportion of patients with early- or intermediate-stage HCC among all patients with HCC due to poor liver function, combined comorbidities, or old age [5]. In addition, because of the high rate of post-resection recurrence (2-year recurrence, 50–51%), [11, 12] long-term survival cannot be guaranteed for all patients with HCC treated with curative resection [13].
Among palliative treatments, based on the results of a recent meta-analysis in which 7 TACE trials showed higher 2-year survival rates than control supportive care, [14] most international guidelines recommend TACE as the standard treatment modality for intermediate-stage (Barcelona Clinic Liver Cancer [BCLC] stage B) multinodular HCC [15]. Recently, due to the heterogeneity in the outcomes of patients with BCLC B stage HCC treated with TACE, several subclassifications of patients with BCLC B stage HCC according to the Bolondi or Kinki criteria have been also proposed for determining TACE eligibility [16].
In addition, TACE has been frequently used to treat recurrent HCC after curative resection [17, 18]. Although the liver function of patients with recurrent HCC was originally sufficient to endure liver resection and recurrent HCC frequently presents in the early stages due to an intensive follow-up strategy using dynamic imaging studies after initial HCC resection, several factors such as relatively inadequate remaining liver volume for re-resection, postoperative adhesions, and multifocal recurrent tumors can make re-resection or ablation of the recurrent HCC difficult. However, most TACE studies focused on patients with HCC who did not undergo surgical resection and the applicability and treatment outcomes of TACE for patients with recurrent HCC has not been well documented.
Thus, here we investigated whether TACE survival outcomes differ between patients with recurrent HCC after curative resection and treatment-naïve patients with HCC treated with TACE, based on a propensity score-matched (PSM) analysis.
Methods
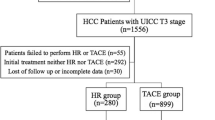
Patients in the database of Severance Hospital, Yonsei University College of Medicine, Seoul, South Korea, treated with TACE between 2005 and 2015 for treatment-naïve HCC (TN-HCC) and between 2003 and 2015 for recurrent HCC after curative resection (R-HCC) were considered eligible for this retrospective study. HCC was defined by histological or radiological evaluation with reference to European Association for the Study of the Liver guidelines [19]. Exclusion criteria included (1) anti-cancer treatments other than TACE; (2) combined use of other anti-cancer treatments and TACE; (3) serious medical comorbidity; (4) BCLC stage D HCC; (5) extrahepatic metastasis; and (6) insufficient clinical information (Fig. 1).
Flow of study population selection process. A total of 327 patients with TN-HCC treated with TACE between 2005 and 2015 and 539 patients with R-HCC treated with TACE between 2003 and 2015 were considered eligible. After the exclusion of 52 and 91 patients from the TN-HCC and R-HCC cohorts, respectively, according to our exclusion criteria, 275 patients with TN-HCC and 448 patients with R-HCC were ultimately included in the statistical analysis. TN-HCC, treatment-naïve hepatocellular carcinoma; TACE, transarterial chemoembolization; R-HCC, recurrent hepatocellular carcinoma
The study methodology was in accordance with the ethical guidelines of the 1975 Declaration of Helsinki and approved by the institutional review board of Severance Hospital. Informed consent was waived due to the retrospective nature of the study.
Surveillance Before the Diagnosis of TN-HCC or R-HCC
For patients with TN-HCC, 3- or 6-month interval ultrasound-based surveillance was usually used until the diagnosis of HCC [20]. Patients with R-HCC had a previous history of curative resection. At the time of resection, these patients were examined with CT, magnetic resonance imaging (MRI), hepatic angiography, and/or positron emission tomography to confirm tumor number, size, location, and extent as well as the existence of distant metastases. In addition to preoperative routine laboratory examinations and physical examination for Child–Pugh classification, the indocyanine green retention test was performed to determine liver reserve function. Indications for liver resection and the types of operative procedures were mainly determined based on the Makuuchi criteria, [21] and followed the anatomic definitions of segments and lobes of Couinaud [22]. The decision to resect was also made by preoperatively measuring liver stiffness using transient elastography [23]. Patients routinely underwent intraoperative ultrasonography to determine tumor location and extent and exclude the presence of additional lesions in the residual liver [24, 25]. After curative resection, patients were usually followed up to detect R-HCC using dynamic imaging including CT or MRI. The median time between surgery and HCC recurrence was 17.0 months (IQR 6.9–36.7 months).
TACE Procedure
Angiography of the superior mesenteric and hepatic arteries was performed before the start of TACE to determine factors such as tumor vascularity and portal vein patency. A combination of 5 mL of iodized oil contrast medium, 30–50 mg of adriamycin, and lipiodol was infused selectively to a sub-segmental branch or, if impossible, a segmental branch of the feeding artery. Afterward, embolization was performed using gelatin sponge particles. A dynamic liver computed tomography (CT) scan was used to detect residual viable tumors, and sequential TACE procedures were scheduled at 6- to 8-week intervals if the patient’s laboratory and clinical data suggested such and there was no evidence of critical portal vein invasion or extrahepatic spread [26].
Statistical Analysis
Continuous variables are expressed as median with interquartile range (IQR), whereas categorical variables are expressed as percentages. Baseline characteristic comparisons were performed using Student’s t test for continuous variables and the Chi square test for categorical values. Survival rates were calculated using the Kaplan–Meier method and compared using the log-rank test. Independent risk factors for survival were estimated using a multivariate Cox proportional hazard regression analysis.
To adjust for the differences in clinical characteristics between TN-HCC and R-HCC patients, a balanced cohort was assembled using a PSM analysis with a 1:1 ratio. Two PSM models were used: PSM-I adjusted for liver cirrhosis and PSM-II adjusted for liver cirrhosis, tumor number, and maximal tumor size, which were significantly imbalanced between the groups.
Only variables with a P value < 0.05 in univariate analysis were subjected to multivariate analysis unless the variables overlapped and interfered with multivariate analysis accuracy. Statistical analyses were performed using SPSS software 24.0 for Windows (SPSS Inc., Chicago, IL, USA), and two-tailed P values < 0.05 were considered statistically significant.
Results
Baseline Characteristics
After excluding 91 and 52 patients from the R-HCC and TN-HCC cohorts, respectively, according to our exclusion criteria, 448 TN-HCC patients and 275 patients with R-HCC (total, 723; 584 men, 139 women) were finally included in the statistical analysis (Fig. 1). Baseline demographics and characteristics of the entire study populations are shown in Table 1. Median patient age was 59.8 years. Liver cirrhosis was found in 379 (52.7%) patients. Multiple tumors were found in 342 (47.3%) patients, and the average maximal tumor diameter was 2.0 (IQR, 1.4–3.2) cm. Of the study population, 539 patients (74.4%) had hepatitis B virus-related HCC.
Comparison Between TN-HCC and R-HCC Patients
TN-HCC patients (n = 275, 38.0%) had a significantly higher percentage of liver cirrhosis (61.9% vs. 49.3%), higher aspartate aminotransferase (AST; median 48 vs. 31 IU/L), higher alanine aminotransferase (ALT; median 38 vs. 26 IU/L), alpha-fetoprotein (AFP; median 96.6 vs. 7.7 ng/mL), higher total bilirubin (mean 0.97 vs. 0.84 mg/dL), longer international normalized ratio of prothrombin time (median 1.05 vs. 1.01), higher number of tumors (mean 2.1 vs. 1.7), larger maximal tumor diameter (median 3.1 vs. 1.6 cm), and lower proportion of BCLC stage 0-A (55.6% vs. 71.8%) (all P < 0.05), whereas R-HCC patients (n = 448, 62.0%) included a significantly higher percentage of men (84.3% vs. 74.3%) (P < 0.05) (Table 1). Except for the lower proportion of male gender, TN-HCC patients had more unfavorable characteristics than R-HCC patients.
Follow-Up and Survival
The median follow-up period of the entire study population was 32.4 (IQR, 17.3–61.4) months. The cumulative survival rates at 2, 4, 6, 8, and 10 years were 64.7, 33.2, 15.5, 4.4, and 1.4%, respectively (Supplementary Fig. 1). The median follow-up periods of the TN-HCC and R-HCC patients were statistically similar (35.0 [IQR, 12.5–65.1] vs. 31.8 [IQR, 18.7–59.2], P = 0.813). The cumulative survival rate for TN-HCC and R-HCC patients was 61.3% and 51.1% at 2 years, 36.8% and 31.0% at 4 years, 13.1% and 17.0% at 6 years, 3.3% and 5.1% at 8 years, and 1.1% and 1.6% at 10 years, respectively (Supplementary Fig. 1). Additional TACE was mostly performed for the recurred HCC in two groups (173 [62.9%] patients in TN-HCC group and 296 [65.6%] patients in R-HCC group, respectively).
Independent Risk Factors for Mortality
The univariate analysis indicated that liver cirrhosis, higher AST, higher AFP, higher tumor number, larger maximal tumor diameter, and TACE for TN-HCC (vs. R-HCC) were significant risk factors for mortality (all P < 0.05). On the multivariate analysis, together with a higher AFP and higher tumor numbers (all P < 0.05), TACE for TN-HCC (vs. R-HCC) was independently associated with a higher risk of mortality (hazard ratio [HR], 1.328; 95% confidence interval [CI], 1.038–1.700; P = 0.024) (Table 2). Antiviral therapy for hepatitis B virus did not influence the risk of mortality and recurrence (P = 0.920 and 0.381, respectively).
The cumulative survival rate of patients with a high AFP (> 400 ng/mL) was significantly lower than that of patients with a low AFP (≤ 400 ng/mL) (P < 0.001, log-rank test). Similarly, the cumulative survival rate of patients with multiple tumors and TACE for TN-HCC was significantly lower than that of patients with a single tumor and TACE for R-HCC (all P < 0.001, log-rank test) (Fig. 2).
Cumulative overall survival according to AFP level (a), tumor number (b), and treatment group (TACE for TN-HCC vs. R-HCC) (c). The cumulative survival rate of patients with a high AFP level (> 400 ng/mL) was significantly lower than that of patients with a low AFP level (≤ 400 ng/mL) (P < 0.001, log-rank test). Similarly, the cumulative survival rate of patients with multiple tumors and TACE for TN-HCC was significantly lower than that of patients with a single tumor and TACE for R-HCC (all P < 0.001, log-rank test). AFP, alpha-fetoprotein; TACE, transarterial chemoembolization; TN-HCC, treatment-naïve hepatocellular carcinoma; R-HCC, recurrent hepatocellular carcinoma
When patients with BCLC stage B (n = 197) and C (n = 50) were selected for subgroup analysis (Supplementary Table 1), the risk of mortality was statistically similar between TACE for TN-HCC and R-HCC in univariate analysis (P = 0.498).
Comparison of Early and Late HCC Recurrence
Because early (≤ 2 years) and late recurrent (> 2 years) HCCs have shown different prognosis in previous studies, [12] our subpopulation of patients with R-HCC was divided into those with early and those with late recurrent HCC (Supplementary Table 2). Patients with early recurrent HCC (n = 242) had significantly higher ALT levels (median 26.0 vs. 23.0 IU/L), a higher tumor number (mean 4.2 vs. 2.0), a higher percentage of multiple tumors (55.2 vs. 33.3%), and a lower proportion of BCLC stage 0-A (66.7% vs. 78.5%) than patients with late recurrent HCC (n = 42) (all P < 0.05).
Independent Predictors of Mortality Considering Early and Late Recurrent HCCs
When patients with early recurrent HCC were selected from the R-HCC subgroup (Supplementary Table 3), higher AFP level, lower platelet count, and greater tumor number were independent predictors of mortality on the multivariate analysis (all P < 0.05), where the risk of mortality was similar between TACE for early recurrent HCC and TN-HCC, even on univariate analysis (P = 0.069). When patients with late recurrent HCC were selected from the R-HCC subgroup (Supplementary Table 3), in addition to having higher AFP levels and larger maximal tumor sizes, TACE for TN-HCC was the independent predictor of a higher risk of mortality (HR, 2.964; 95% CI, 1.546–5.719; P = 0.001) (all P < 0.05).
In the cohort of early recurrent HCC and TN-HCC, the cumulative survival rate of patients with a high AFP level (> 400 ng/mL) was significantly lower than that of those with a low AFP level (≤ 400 ng/mL) (P < 0.001, log-rank test). Similarly, the cumulative survival rate of patients with multiple tumors was significantly lower than that of those with a single tumor (P < 0.001, log-rank test). However, the cumulative survival rate of patients with TN-HCC was not significantly different from that of those with early recurrent HCC (P = 0.068, log-rank test) (Supplementary Fig. 2).
In the cohort of patients with late recurrent HCC and TN-HCC, the cumulative survival rate of patients with a high AFP level (> 400 ng/mL) was significantly lower than that of those with a low AFP (≤ 400 ng/mL) (P < 0.001, log-rank test). Similarly, the cumulative survival rate of TN-HCC patients was significantly lower than that of patients with late recurrent HCC in addition to those with multiple tumors (all P < 0.001, log-rank test) (Supplementary Fig. 3).
PSM Analysis
Because the baseline characteristics were significantly different between patients with TN-HCC and those with R-HCC, a PSM analysis was performed. When the proportion of liver cirrhosis was adjusted in the PSM analysis (PSM-I), 271 pairs were selected. When multiple tumor and maximal tumor size were additionally adjusted for in the PSM analysis (PSM-II), 159 pairs were selected (Table 3).
TACE for TN-HCC was independently associated with a high risk of mortality (HR, 1.335; 95% CI, 1.015–1.755; P = 0.038) together with AFP level and tumor number in the PSM-I cohort (all P < 0.05), whereas it did not significantly influence the risk of mortality in further adjusted models (PSM-II) (Table 4). The cumulative survival rate of patients with TN-HCC was significantly lower than that of those with R-HCC in the PSM-I cohort (P = 0.001, log-rank test), whereas it was statistically similar in the PSM-II cohort (P = 0.067, log-rank test) (Fig. 3).
Cumulative overall survival rates by treatment group (TACE for TN-HCC vs. R-HCC) in PSM-I (a), PSM-II (b). The cumulative survival rates of patients with TN-HCC was significantly lower than that of patients with R-HCC in PSM-I cohort (P = 0.001, log-rank test), but it was statistically similar in the PSM-II cohort (P = 0.067, log-rank test). TACE, transarterial chemoembolization; TN-HCC, treatment-naïve hepatocellular carcinoma; R-HCC, recurrent hepatocellular carcinoma; PSM, propensity score-matched
Discussion
In this study, patients with TN-HCC showed unfavorable clinical characteristics at the time of the first TACE compared to patients with R-HCC. Accordingly, TACE for TN-HCC was selected as one of the independent risk factors for mortality (HR, 1.328) together with higher AFP level and tumor number. When patients with R-HCC were divided into two groups (early HCC recurrence and late HCC recurrence), TACE for TN-HCC was independently associated with an increased risk of mortality (HR, 2.964) in patients with late recurrence but not in patients with early recurrence (P > 0.05). After PSM analysis to adjust for the imbalanced baseline characteristics at the time of TACE, the risks of mortality between TACE for TN-HCC and TACE for R-HCC were statistically similar (P > 0.05).
Our study has several strength and issues. Primarily, to the best of our knowledge, our study is the first to compare the survival outcomes of TACE for patients with TN-HCC and those with R-HCC. Based on the proven survival benefit of TACE for unresectable HCC patients compared to conservative care only, [27] a significant number of studies have shown the treatment efficacy of TACE on the survival of patients with TN-HCC, particularly 2-year survival [14, 28]. On the other hand, few studies have reported the outcomes of TACE for patients with R-HCC. Choi et al. evaluated the efficacy and safety of TACE for R-HCC after resection and found that time to recurrence (> 6 months) and favorable tumor-node-metastasis stage (I vs. III) of R-HCC at the time of TACE were independently associated with better survival outcomes [18]. In contrast, our study compared the treatment efficacy of TACE for patients with TN-HCC and those with R-HCC and found that its outcome was significantly better in patients in the R-HCC cohort but was statistically similar between the two cohorts after PSM adjustment. These findings support the idea that the use of dynamic imaging is important in detecting R-HCC as early as possible for a better prognosis. Thus, the current treatment guidelines for TACE might be applicable to patients with R-HCC based on the similar TACE outcomes after PSM adjustment shown here.
Second, our study had a relatively large sample size (> 700 patients overall) and long-term follow-up (> 10 years), which enhanced its statistical power. Because of the large sample size, we could divide our cohort with R-HCC into early and late recurrence subgroups and analyze their TACE outcomes versus those of the TN-HCC. The risk of mortality was statistically similar among the patients with TN-HCC and those with early R-HCC, whereas the TACE outcomes of patients with TN-HCC were significantly poorer than those of patients with late R-HCC. The similarity between the early R-HCC and TN-HCC subgroups could most likely be explained by the fact that early R-HCC might be associated with unfavorable tumor factors such as microscopic vascular invasion or poor differentiation, [29] although the overall outcomes were favorable among patients with R-HCC compared to those of patients with TN-HCC. In contrast, patients with late R-HCC would most likely be those who underwent successful resection and had de novo HCC formation [29]. In addition, patients with R-HCC might have received frequent follow-up based on dynamic imaging studies such as CT scans [30]. All of these factors would ensure that tumors would be found earlier in TN-HCC patients, resulting in their favorable TACE outcomes. Additionally, when patients with BCLC B and C were selected, no significant difference in the risk of mortality was found. Furthermore, based on the large sample size, we could use the PSM analysis to ensure balance between various clinical characteristics at the time of TACE between the TN-HCC and R-HCC cohorts.
Third, considering the significantly different characteristics of the two cohorts at the time of TACE, we performed a PSM analysis. Surgical resection is generally reserved for patients with BCLC stage A HCC and preserved liver function to prevent liver failure after resection. Indeed, patients with R-HCC showed a significantly lower proportion of liver cirrhosis, lower AST and ALT levels, lower total bilirubin levels, and lower prothrombin times as well as favorable tumor characteristics. Accordingly, TACE outcomes were better in patients with R-HCC than in those with TN-HCC. However, the outcomes became similar after PSM.
We are also aware of several issues in our study that remain unresolved. First, despite the study’s large sample size and long-term follow-up, the baseline characteristics of the two cohorts at the time of TACE were significantly different. Although the PSM analysis was adopted to balance the populations, a risk of selection bias persisted. Specifically, patients with R-HCC were theoretically cured after curative resection, but factors related to surgical technique, tumor stage prior to surgery, and histological features of HCC likely affected our final results. Second, although we adjusted for several factors in the PSM analysis, the large decrease in sample size seen in the PSM-II cohort may weaken the conclusions of our study. Thus, additional studies with larger sample sizes are needed to validate our results. In addition, due to the skewed distribution of AFP level at the time of TACE between patients with TN-HCC and those with R-HCC, AFP level, which can affect clinical outcomes [31], it could not be analyzed in the PSM analysis. Third, because of the retrospective nature of our study, we were not able to control the decision to resect and perform TACE rather than using other anti-cancer treatment modalities. Therefore, the possibility of selection bias in terms of treatment modality might have affected our final results.
In conclusion, the treatment outcomes of TACE for patients with TN-HCC and R-HCC were similar in the PSM analysis. Accordingly, the current treatment guidelines for TACE in patients with TN-HCC might be similarly applied for patients with R-HCC after curative resection.
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- TACE:
-
Transarterial chemoembolization
- BCLC:
-
Barcelona Clinic Liver Cancer
- PSM:
-
Propensity score-matched
- TN-HCC:
-
Treatment-naïve HCC
- R-HCC:
-
Recurrent HCC after curative resection
- MRI:
-
Magnetic resonance imaging
- CT:
-
Computed tomography
- IQR:
-
Interquartile range
- AST:
-
Aspartate aminotransferase
- ALT:
-
Alanine aminotransferase
- AFP:
-
Alpha-fetoprotein
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
References
Ghouri YA, Mian I, Rowe JH. Review of hepatocellular carcinoma: Epidemiology, etiology, and carcinogenesis. J Carcinog. 2017;16:1.
El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142(1264–1273):e1261.
Balogh J, Victor D, Asham EH, et al. Hepatocellular carcinoma: a review. J Hepatocell Carcinoma. 2016;3:41–53.
Daher S, Massarwa M, Benson AA, et al. Current and future treatment of hepatocellular carcinoma: an updated comprehensive review. J Clin Transl Hepatol. 2018;6:69–78.
Song MJ, Bae SH. Newer treatments for advanced hepatocellular carcinoma. Korean J Intern Med. 2014;29:149–155.
Lee EW, Khan S. Recent advances in transarterial embolotherapies in the treatment of hepatocellular carcinoma. Clin Mol Hepatol. 2017;23:265–272.
Dika IE, Abou-Alfa GK. Treatment options after sorafenib failure in patients with hepatocellular carcinoma. Clin Mol Hepatol. 2017;23:273–279.
Zhao YN, Zhang YQ, Ye JZ, et al. Hepatic resection versus transarterial chemoembolization for patients with barcelona clinic liver cancer intermediate stage child-pugh a hepatocellular carcinoma. Exp Ther Med. 2016;12:3813–3819.
Wang JH, Changchien CS, Hu TH, et al. The efficacy of treatment schedules according to barcelona clinic liver cancer staging for hepatocellular carcinoma—survival analysis of 3892 patients. Eur J Cancer. 2008;44:1000–1006.
Yin X, Zhang L, Wang YH, et al. Transcatheter arterial chemoembolization combined with radiofrequency ablation delays tumor progression and prolongs overall survival in patients with intermediate (BCLC B) hepatocellular carcinoma. BMC Cancer. 2014;14:849.
Lacaze L, Scotté M. Surgical treatment of intra hepatic recurrence of hepatocellular carcinoma. World J Hepatol. 2015;7:1755–1760.
Portolani N, Coniglio A, Ghidoni S, et al. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg. 2006;243:229–235.
Xu XS, Liu C, Qu K, et al. Liver transplantation versus liver resection for hepatocellular carcinoma: a meta-analysis. Hepatobiliary Pancreat Dis Int. 2014;13:234–241.
Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37:429–442.
Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022.
Kudo M, Arizumi T, Ueshima K, et al. Subclassification of BCLC B stage hepatocellular carcinoma and treatment strategies: proposal of modified Bolondi’s subclassification (Kinki criteria). Dig Dis. 2015;33:751–758.
Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg. 2000;232:10–24.
Choi JW, Park JY, Ahn SH, et al. Efficacy and safety of transarterial chemoembolization in recurrent hepatocellular carcinoma after curative surgical resection. Am J Clin Oncol. 2009;32:564–569.
Anonymous. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943.
Jung KS, Kim SU, Ahn SH, et al. Risk assessment of hepatitis B virus-related hepatocellular carcinoma development using liver stiffness measurement (FibroScan). Hepatology. 2011;53:885–894.
Makuuchi M, Kosuge T, Takayama T, et al. Surgery for small liver cancers. Semin Surg Oncol. 1993;9:298–304.
Couinaud C. Le foie; études anatomiques et chirurgicales. Paris: Masson; 1957.
Kim SU, Kim BK, Han KH. Clinical application of liver stiffness measurement using transient elastography: a surgical perspective. Digestion. 2013;88:258–265.
Kim SU, Ahn SH, Park JY, et al. Prediction of postoperative hepatic insufficiency by liver stiffness measurement (FibroScan(®)) before curative resection of hepatocellular carcinoma: a pilot study. Hepatol Int. 2008;2:471–477.
Lee SH, Kim SU, Jang JW, et al. Use of transient elastography to predict de novo recurrence after radiofrequency ablation for hepatocellular carcinoma. Onco Targets Ther. 2015;8:347–356.
Kim BK, Shim JH, Kim SU, et al. Risk prediction for patients with hepatocellular carcinoma undergoing chemoembolization: development of a prediction model. Liver Int. 2016;36:92–99.
Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–1171.
Camma C, Schepis F, Orlando A, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology. 2002;224:47–54.
Colecchia A, Schiumerini R, Cucchetti A, et al. Prognostic factors for hepatocellular carcinoma recurrence. World J Gastroenterol. 2014;20:5935–5950.
Yu SJ. A concise review of updated guidelines regarding the management of hepatocellular carcinoma around the world: 2010–2016. Clin Mol Hepatol. 2016;22:7–17.
Kim BK, Ahn SH, Seong JS, et al. Early alpha-fetoprotein response as a predictor for clinical outcome after localized concurrent chemoradiotherapy for advanced hepatocellular carcinoma. Liver Int. 2011;31:369–376.
Acknowledgments
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT & Future Planning (2016R1A1A1A05005138). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
DS Kim and SU Kim designed this study; DS Kim and SU Kim carried out the data analysis and wrote the manuscript; TS Lim, MY Jeon, BK Kim, JY Park, DY Kim, SH Ahn, KH Han, O Baatarkhuu, and SU Kim contributed to inclusion of patients, acquisition and analysis of data; all authors contributed to the interpretation of results, critical revision of the manuscript, and approved the final manuscript; SU Kim is the guarantor.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10620_2019_5701_MOESM2_ESM.eps
Supplementary figure 1. Cumulative overall survival rates of the entire study population (A), TN-HCC subgroup (B), and R-HCC subgroup (C). The median follow-up period of the entire study population was 32.4 (interquartile range [IQR], 17.3–61.4) months. The cumulative survival rates at 2, 4, 6, 8, and 10 years were 64.7%, 33.2%, 15.5%, 4.4%, and 1.4%, respectively. The median follow-up periods of the TN-HCC and R-HCC patients were statistically similar (35.0 [IQR, 12.5–65.1] vs. 31.8 [IQR, 18.7–59.2], P = 0.813). The cumulative survival rates of the TN-HCC and R-HCC patients were 61.3% and 51.1% at 2 years, 36.8% and 31.0% at 4 years, 13.1% and 17.0% at 6 years, 3.3% and 5.1% at 8 years, and 1.1% and 1.6% at 10 years, respectively. TN-HCC, treatment-naïve hepatocellular carcinoma; R-HCC, recurrent hepatocellular carcinoma; IQR, interquartile range (EPS 2419 kb)
10620_2019_5701_MOESM3_ESM.eps
Supplementary figure 2. Cumulative overall survival rates by AFP (A) and treatment group (TACE for TN-HCC vs. TACE for early recurrent HCC) (B). In the early recurrent HCC and TN-HCC cohorts, the cumulative survival rate of patients with a high AFP level (>400 ng/mL) was significantly lower than that of patients with a low AFP level (≤400 ng/mL) (P < 0.001, log-rank test). Similarly, the cumulative survival rate of patients with multiple tumors was significantly lower than that of patients with a single tumor (P < 0.001, log-rank test). However, the cumulative survival rate of patients with TN-HCC was not significantly different from that of patients with R-HCC (P = 0.068, log-rank test). AFP, alpha-fetoprotein; TACE, transarterial chemoembolization; TN-HCC, treatment-naïve hepatocellular carcinoma; R-HCC, recurrent hepatocellular carcinoma (EPS 2495 kb)
10620_2019_5701_MOESM4_ESM.eps
Supplementary figure 3. Cumulative overall survival rates by AFP level (A), tumor number (B), and treatment group (TACE for TN-HCC vs. TACE for late recurrent HCC) (C). In the late recurrent HCC and TN-HCC cohorts, the cumulative survival rate of patients with a high AFP level (>400 ng/mL) was significantly lower than that of patients with a low AFP level (≤400 ng/mL) (P < 0.001, log-rank test). Similarly, the cumulative survival rate of patients with TN-HCC was significantly lower than that of patients with late recurrent HCC in addition to multiple tumors (all P < 0.001, log-rank test). AFP, alpha-fetoprotein; TACE, transarterial chemoembolization; TN-HCC, treatment-naïve hepatocellular carcinoma; R-HCC, recurrent hepatocellular carcinoma (EPS 1884 kb)
Rights and permissions
About this article
Cite this article
Kim, D.S., Lim, T.S., Jeon, M.Y. et al. Transarterial Chemoembolization in Treatment-Naïve and Recurrent Hepatocellular Carcinoma: A Propensity-Matched Outcome Analysis. Dig Dis Sci 64, 3660–3668 (2019). https://doi.org/10.1007/s10620-019-05701-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-019-05701-8