Abstract
Purpose
The aim was to investigate the contribution of contrast-enhanced ultrasound (CEUS) to improve the results of US in the evaluation of recurrence in postsurgical Crohn’s disease (CD) and establish its role in the assessment of the severity.
Methods
Anastomotic site was assessed in 108 postsurgical CD patients with B-mode, color Doppler and CEUS. Bowel wall thickness (WT), transmural complications or stenosis, color Doppler grade, and bowel wall contrast enhancement (BWCE)—using time–intensity curves—were correlated with endoscopic Rutgeerts score. A receiver operating characteristic (ROC) curve was built to establish the best cutoff to predict recurrence and the severity. A US scoring system was elaborated in order to determine the grade of recurrence.
Results
Ileocolonoscopy detected recurrence in 90 (83.3%) subjects and severe recurrence in 62. WT ≥ 3 mm had an accuracy of 90.7% in the detection of endoscopic recurrence. The combination of parameters—WT ≥ 3 mm and BWCE (≥ 46%)—demonstrated similar accuracy (90.7%). A WT ≥ 5 mm showed the best specificity (100%) for the diagnosis of recurrence and a WT ≥ 6 mm the best specificity (95.7%) for the detection of severe recurrence. The combination of sonographic parameters—WT ≥ 6 mm or WT between 5 and 6 mm with BWCE ≥ 70%, or complications—obtained the best results grading the recurrence (sensitivity, specificity, and accuracy of 90.3%, 87%, and 88.9%, respectively).
Conclusions
US shows high sensitivity and specificity for the diagnosis of postsurgical recurrence. When combined with CEUS, it can improve the detection of severe recurrence.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recurrence of Crohn’s disease (CD) after an ileocolonic resection is one of the most important issues in the management of inflammatory bowel disease (IBD) [1]. Monitoring for postoperative recurrence is important to allow early intervention when CD recurs. Ileocolonoscopy has been considered to be the gold standard in the diagnosis and monitoring of postoperative recurrence. Endoscopic recurrence is defined and graded using the Rutgeerts score [1,2,3,4]. However, it is an invasive technique with recognized limitations, such as procedure-related discomfort, poor patient acceptance, and technically more difficult to complete in postoperative patients [5]. A range of clinical, serological, laboratory, radiological, and endoscopic parameters and procedures have been evaluated for assessing CD recurrence [6,7,8,9]. Several imaging techniques have been investigated for this purpose including intestinal ultrasonography (US), enterography/enteroclysis with computed tomography (CT) or magnetic resonance (MR), with promising results [10,11,12,13,14,15,16,17,18].
Bowell US is an interesting alternative noninvasive technique, safe and easy to perform repeatedly that allows the indirect visualization of the inflamed and thickened intestinal wall and the extraintestinal complications. Several studies have confirmed a very good accuracy of conventional US and small intestinal contrast ultrasound (SICUS) in the diagnosis of CD recurrence compared with endoscopy [10, 11, 18,19,20,21]. In a systematic review to assess the role of US in the management of CD described in the postoperative follow-up, the sensitivity of bowel US was 81.7% (95% CI, range 77–86.3%) and the specificity was 88.3% (95% CI, range 83.4–93.2%) [22].
Otherwise, the detection of severe recurrence in the management of postoperative CD represents an important issue in the treatment of the disease. Particularly, more severe lesions referring to i3 and i4 of the Rutgeerts score are associated with a greater likelihood of clinical relapse [1] and the therapeutic strategy must be modified [23]. Severity-based CT and MR scores have been correlated with the endoscopic Rutgeerts score, in order to differentiate between low- and high-grade lesions. For this purpose, the degree of active mucosal inflammation as measured by the layered pattern of enhancement, wall thickness (WT), and the comb sign (the vascular prominence) have been assessed [12, 15, 16, 24].
In order to increase the sensitivity of Doppler US in detecting vascularity of the bowel wall as a marker of inflammation, contrast-enhanced ultrasound (CEUS) has been used to provide a quantifiable measurement of the activity in the management of patients with CD. A recent meta-analysis has found that CEUS has high accuracy in the detection of active CD with a diagnostic accuracy exceeding 80% [25].
In a work published in 2013 by Paredes et al. [26], the contribution of the intravenous (iv) contrast agent in the detection of endoscopic recurrence in 60 CD patients with ileocolic resection was evaluated. In their preliminary results, they concluded that CEUS in combination with B-mode US (parietal thickness and presence of transmural complications) shows excellent results for the diagnosis of postoperative recurrence and allows better assessment of its severity. To date, as far as we know, there are no other studies that have investigated the value of CEUS in the specifically diagnosis of recurrence.
Our objective is to assess the contribution of CEUS to improve the results of B-mode and color Doppler US in the detection of postoperative recurrence and establish its role in the assessment of severity. In addition, we attempted to establish a new severity US scoring system to differentiate between low- and high-grade lesions, similar to the scales developed with MR or CT, which can help determine the most severe cases requiring a more aggressive attitude.
Materials and Methods
Patient Characteristics
We prospectively evaluated 118 patients with CD established [27] who had been treated with intestinal resection with ileocolic anastomosis and were followed up in the Department of Gastroenterology. They were consecutively included in the study between January 2011 and July 2016, when a colonoscopy was required for any reason (evaluation for endoscopic recurrence, screening for colon cancer, changes in the clinical condition or for reviewing the treatment). Exclusion criteria included pregnancy, age younger than 18 years and contraindication to intravenous administration of US agent contrast. A maximum time interval of 90 days was chosen between colonoscopy and bowel US. To minimize the influence of pharmacological therapy, the medication for CD was stable between both studies in all patients.
Clinical activity was assessed at the time of endoscopy according to the Crohn’s disease activity index (CDAI) [28], and serological value of C-reactive protein (CRP) was registered.
This observational study was approved by the local ethics committee, and informed consent was obtained.
Examination Technique
Endoscopic Protocol
Colonoscopy examinations were performed under sedoanalgesia monitored by an anesthetist after the use of a polyethylene glycol electrolyte solution and a low-residue diet before the examination. They were conducted by an expert operator who was unaware of the results of the other examinations. A Pentax EC-380 LKP 4.2 colonoscope was used as a standardized routine procedure. For endoscopic evaluation, the Rutgeerts scoring was used [1]. Recurrence was considered when any aphthous lesion was detected in the anastomosis or neoterminal ileum (≥ i1). Severe endoscopic recurrence was defined as grade 3 or grade 4 of the Rutgeerts scoring (i3, i4).
Ultrasound Study
US examinations were performed by two expert operators (T.R. and MJ.M.) without oral contrast solution and with fasting for 4 h. The US examinations were performed (Toshiba, Aplio XG 80, Tokyo, Japan) with a 3.5 MHz convex and 6 MHz curved transducer and linear-array 5–10 MHz probe. Color Doppler parameters were optimized for maximal sensitivity with a preset designed for the detection of low-velocity flow in the intestinal wall, gain 1 dB, lower wall filter of 2 and a scale of 6 cm/s. A semiquantitative scale previously described was employed and graded as absent (grade 0), barely visible vascularity (grade 1), moderate vascularity (grade 2), and marked vascularity (grade 3) [29].
Wall thickness (WT) of the ileocolonic anastomosis or the neoterminal ileum was measured, and the vascularity pattern of the color Doppler flow was obtained. Extraintestinal perforating complications such abscesses, phlegmons, or fistulas were evaluated, and the presence of strictures was described in accordance with the existing literature [22, 30].
For the CEUS study, we used a 3.5–6 MHz convex probe in wideband contrast harmonic mode (pulse inversion, Toshiba Aplio) at low mechanical index (MI < 0.10), dynamic range 80, and the focal zone beneath the bowel wall. Sulfur hexafluoride microbubbles (SonoVue®, Bracco Imaging, Milan, Italy) a bolus of 2.4 mL through a three-way 20-gauge catheter into an antecubital vein, followed by 10 mL saline (0.9% NaCl) flush. To assess the vascularization of the involved bowel loop, the contrast uptake over quantitative analysis of the brightness intensity was measured over a period of 40 s, in regions of interest (ROI) located manually in the intestinal wall with at least 2 cm2. We used a dedicated software installed into the US equipment, and a time-intensity curve was automatically acquired. The quantitative measurement of the contrast uptake was obtained as the difference between the maximum enhancement value and the baseline value before the arrival of contrast. We calculated the bowel wall contrast enhancement (BWCE) by using the formula previously described [26, 31, 32]: brightness postcontrast (Bpost) − brightness precontrast (Bpre) × 100/brightness precontrast (Bpre). The cine clip was transferred to the picture archiving and communication system (PACS) of our radiology department.
Postsurgical CD recurrence was defined as an increased WT ≥ 3 mm as previously reported [11, 33]. Color Doppler flow was considered present when there is any signal color Doppler flow in the wall (grade ≥ 1). We used the BWCE of 46% as the reference for the diagnosis of recurrence based in previous studies [26, 34]. The threshold in detecting the severity was chosen according to the cutoff value of the receiver operating characteristic (ROC) curve. The anastomotic area was also assessed by a sonographic score developed to evaluate the severity of the recurrence. This score was empirically elaborated and includes transmural inflammation (WT, color Doppler grade, and mural enhancement), extramural complications, and stenosis.
Statistical Analysis
Results of numerical data are presented as mean, standard deviation (SD), 95% confidence interval (95% CI), median, and range. The diagnostic value of sonographic findings of B-mode, color Doppler US, and CEUS (parietal thickness, grade at color Doppler US, mural enhancement) for detecting recurrent CD in the neoterminal ileum and the severity of the recurrence was determined by comparing with the ileocolonoscopy. Sensitivity, specificity, the positive predictive value (PPV), negative predictive value (NPV), accuracy, and odds ratio (OR) were evaluated, with 95% CI, by means of the best cutoff value of the increase in wall brightness.
Receiver operating characteristic (ROC) analysis was helpful for determination of the optimal cutoff point for each quantitative parameter to identify subjects with postsurgical endoscopic recurrence and the severe forms of recurrence. To compare the different parameters, the areas under the ROC curved were obtained. The nonparametric Spearman’s sign rank correlation coefficient was used to assess the strength of relationships between the quantitative parameters and the Rutgeerts score.
Multiple linear regression analysis was employed to represent the relationship between the endoscopy grading of the neoterminal ileum, (dependent variable) [35] and the kinetic parameters (independent variables), to calculate the regression coefficients (β).
The Statistical Package for the Social Sciences (SPSS) version 20.0 (SPSS Inc, Chicago, USA) and STATA 14.0 (StataCorp LP) were used to describe and analyze the data, and a p value < 0.05 was considered to indicate a statistically significant difference.
Results
Initially, we included 118, of which ten (8.5% of the total) were excluded for incomplete colonoscopy, eight of them for impossibility to reach the anastomosis due to stenosis. The remaining 108 subjects constituted our study group. Main demographic, surgical, and clinical characteristics of the study population are reported in Table 1. A complete colonoscopy and US/CEUS were performed in the 108 subjects, and none experienced adverse event concerning the use of intravenous contrast. The median time between the colonoscopy and US was 1 month and 28 days (0–86 days), with 45 cases performed in less than one month. A time interval between the operation and examinations (colonoscopy or US) for recurrent CD was between 3 months to 30 years (mean 6 years).
Colonoscopy detected recurrence in 90/108 (83.3%) subjects. The endoscopy Rutgeerts scores were: grade 0 in 18 subjects (16.7%); grade 1 in 15 (14.8%); grade 2 in 12 (11.1%); grade 3 in 23 (21.3%); and grade 4 in 39 (36.1%). The mean age of the 90 studies in which endoscopic recurrence was detected was 42 years (range 18–74), 42 females and 48 males. Recurrence was detected in 81% of smokers and in 79% of nonsmokers without significant differences (p = 0.123). There were no significant differences between endoscopic recurrence and number of previous resections, type of anastomosis, nor previous treatment received; however, 72% of the cases with ileocecal resection and 96% with ileocolonic resection had endoscopic recurrence, showing significant differences (p < 0.008). Among the 108 subjects included in the study, 36 (33%) had a CDAI higher than 150 points while in 24 patients (22.2%) the CRP showed a value > 10 mg/L. The sensitivity, specificity, positive predictive value and negative predictive value for CDAI and CRP for the detection of recurrence were 37.8%, 88.9%, 94.4%, 22.2% and 25.6%, 98.9%, 95.8% and 57.3%, respectively.
Analysis of the US and CEUS Parameters and the Rutgeerts Score
Comparison of Rutgeerts score and ultrasound parameters (WT or BWCE) is shown in Fig. 1a, b. A good correlation was obtained for thickness and endoscopic recurrence. WT did not significantly differ among subjects with score i2, i3, or i4 (Fig. 1a).
Representation of the box diagrams of wall thickness (a), and wall contrast enhancement (b) according to different Rutgeerts scores. The lower and upper edges of the boxes represent the values of 25th percentile to the 75th, respectively, and the central point of the median values. The extremes mark the upper and lower values and outside the outliers
Assessment of Recurrence Diagnosis
Table 2 shows the results of the different sonographic parameters analyzed in the diagnosis of recurrence. Thickness ≥ 3 mm displayed the best accuracy for the diagnosis of recurrence (90.7%). With this parameter, we correctly diagnose 85/90 recurrences and 13/18 without recurrence. In the five subjects with a false positive (FP) result, the thickness of the wall was between 3 and 5 mm, while among the five subjects with false negative (FN) results, the endoscopic grade was i1 in three patients and i2 in two subjects.
We analyzed the ROC curve to assess the capacity of the different variables to the diagnosis of recurrence. The area under the curve (AUC) obtained for the WT was 0.92 (95% CI 0.86–0.98). The optimal cutoff value for the prediction of recurrence was 3.5 mm (sensitivity 90%, specificity 83%). The value of 5 mm showed a specificity of 100%. The AUC for the BWCE was 0.90 (95% CI 0.81–0.99), and the optimal cutoff value for predicting recurrence was > 46% (sensitivity 89%, specificity 83%).
The logistic regression analysis showed that both parameters, WT (p = 0.006) and BWCE (p = 0.01), were independent predictors of endoscopic recurrence. In the comparative analysis of the different areas under the ROC curves of the US parameters for the diagnosis of recurrence, no statistically significant differences were found between WT and contrast enhancement (p = 0.061).
To analyze which combination of ultrasound variables offered the greatest capacity for the detection of recurrence, we used the values described in previous studies and the variables that in our logistic regression analysis were independent predictors. The combination of thickness ≥ 3 mm and enhancement value ≥ 46% improved the specificity of these two variables individually, with the same accuracy and slight decrease of sensitivity (Table 2). With this combination, we correctly diagnose 82/90 recurrences and 16/18 of patients without recurrence with only two false positive (Fig. 2). The AUC for this combination was 0.90 (CI 95%, 0.81–0.99).
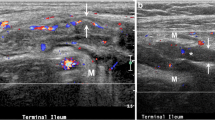
A 33-year-old woman with Crohn’s disease with ileocolic anastomosis. a Transverse ultrasound of the neoterminal ileum showing slight WT of 3.9 mm (between cursors). b Contrast-enhanced ultrasound shows a mild enhancement in the same section of the neoterminal ileum. The time–intensity curve shows a BWCE of 23% (baseline value, 95; maximum value, 117). With the criterion of thickness, it would be considered as a sign of recurrence in the perianastomotic area. In this patient, the value of contrast enhancement modifies the result and we classify it without signs of recurrence. The colonoscopy (not shown) displayed no lesions in the anastomosis and neoterminal ileum
Assessment of Recurrence Severity
Endoscopy detected 62 subjects with severe recurrence (57.4%). Statistically significant differences were found between WT in not severe cases (mean 3.5 mm ± SD 1.4, range 2–7 mm) compared to severe forms (mean 6 mm ± SD 1.7, range 3-14 mm) (p < 0.0001). The AUC for the WT in the detection of severe recurrence was 0.87 (95% CI 0.81–0.92). The best cutoff point for this curve was thickness of 5 mm (sensitivity 74%, specificity 82%) but the value of 6 mm showed the best specificity (95.7%).
Regarding enhancement parameters and endoscopic severity, significant differences were found between BWCE of not severe cases (mean 54.3% ± SD 30.7%) and severe cases (mean 79.9% ± SD 24%, p < 0.0001). The AUC for the BWCE was 0.73 (95% CI 0.63–0.84), and the best cutoff point was 70%, with sensitivity of 61% and specificity of 70%.
All cases with severe color Doppler flow (grade 3) had endoscopic criteria of severity (10/10). Regarding the group with moderate Doppler flow, 34 of 43 cases also had endoscopic criteria of severe recurrence. Combining the moderate and severe color Doppler groups, the accuracy for the diagnosis of endoscopic severity was 75% (sensitivity 71%, specificity 80%).
Table 3 shows the results for the best US parameters described previously in the diagnosis of severe endoscopy recurrence. The WT ≥ 5 mm provided the best results, although the accuracy only reached 77.8%.
In the present series, 14 of the 108 subjects showed transmural complications or stenosis, (17 complications: six fistulas, two abscesses and nine stenoses). All of them showed endoscopic signs of recurrence (sensitivity 43.8%, specificity 100%, accuracy 83.3), and 13/14 patients showed severe endoscopic recurrence (Rutgeerts grade i3 and i4). Only there was one case with US stenosis with mild endoscopic recurrence (grade i1), displaying a thickness of 4.2 mm and BWCE of 31%, suggestive of fibrotic stenosis in the US examination. None of the fistulas or abscesses was described in the endoscopy.
In order to obtain a more efficient diagnosis of severe recurrence, we searched for combinations of the different sonographic parameters. The combination with the best results was the presence of at least one of the following features: WT ≥ 6 mm, or WT between 5 and 6 mm with BWCE ≥ 70%, or presence of complications (fistulas, abscesses, or stenosis) (Table 3). These parameters were chosen on the basis of our results and the analysis of the literature: Thickness of 5 mm is the reference of the literature; 6 mm and the presence of complications were highly specific in our series; and 70% of enhancement was the ROC value with the best results. Combining these parameters as signs of severity, the accuracy was 88.9% and OR 62.22, getting a good concordance with endoscopy (k = 0.773, p < 0.0001) (Fig. 3). By using this combination, only six out of 62 subjects (9.7%) with nonsevere endoscopic recurrence had US parameters of severity.
A 47-year-old man with ileocolic resection for Crohn’s disease, with severe recurrence of the neoterminal ileum in the ileocolonoscopy (Rutgeerts score: grade 3). a Ultrasound image of the neoterminal ileum shows increased WT (5.6 mm) indicated between cursors. b Contrast-enhanced ultrasound illustrates the marked enhancement of the wall. The region of interest (ROI) is placed in the thickened wall. The time–intensity curve shows an enhanced contrast of 74% (baseline value, 75; maximum value, 131) indicating severe recurrence
The logistic regression analysis showed that WT (β = 1.411, p = 0.0001), color Doppler grade (β = 0.989, p = 0.015) and BWCE (β = 1.223, p = 0.007) were independent predictors of severe endoscopic recurrence. The Hosmer–Lemeshow goodness-of-fit test was p = 0.729 for the logistic regression model.
Ultrasound Scoring System of Recurrence Severity
In order to investigate a scoring system that would allow us to classify the recurrence in high or low grade, we constructed an US scale. We included the parameters that were independent predictors of severity in our logistic regression analysis: parietal thickness, color Doppler grade and wall contrast enhancement of the anastomosis. Extraintestinal complications were also included in the scale, since this feature was highly specific to the diagnosis of severe endoscopic recurrence. In this scoring scale, we use the cutoff values with the best results in the diagnosis of recurrence and in the detection of endoscopic severity. Table 4 shows the score of each of the US parameters assigned, with a possible score between 0 and 8. Later, subjects were dichotomized into two categories of severity: nonsevere (score 0–3) and severe (score 4–8). US severity score showed values of sensitivity, specificity, and accuracy of 90.3%, 73.9%, and 83.3%, respectively (Table 3). With this US scale, we obtained a good concordance (k = 0.635, p < 0.0001) and a high NPV (85%), with 56 of 62 cases correctly diagnosed as severe and six cases with severe endoscopy with a low US score, incorrectly diagnosed as low risk.
The AUC obtained for sonographic score was 0.813 (95% CI 0.72–0.90, p = 0.005) and for the combination of US parameters was 0.886 (95% CI 0.81–0.95, p = 0.001) in the evaluation of severe endoscopic recurrence.
Discussion
Several studies have already demonstrated that US is an adequate technique for the detection of recurrence of postsurgical CD in the anastomotic segment and for the estimation of severity with sensitivity ranging from 77 to 98% and specificity from 60 to 96% [10, 11, 18,19,20,21, 36,37,38]. However, except in the study of Paredes et al. [26] with preliminary results, the use of CEUS has not been evaluated for the study of the recurrence diagnosis. In our study, only parietal thickness and BWCE were independent predictors of recurrence. WT ≥ 3 mm discriminated with high accuracy the presence of endoscopic recurrence, showing the best results (AUC = 0.92, and PPV = 94.4%). The combination of WT ≥ 3 mm and BWCE (≥ 46%) demonstrated similar results (AUC = 0.90, and PPV = 97.6%).
Both, in works that use B-mode ultrasound, and those that use oral contrast (SICUS), WT of the anastomosis and/or preanastomotic segment is considered the best parameter in the assessment of recurrence [10, 11, 18, 21, 26, 36]. In our study, performed without oral contrast, mural thickness was also the parameter that showed the best area under ROC curve for the diagnosis of recurrence with a value of 0.92, better than the AUC of BWCE (0.90). Using the reference value of thickness ≥ 3 mm, we obtained a high sensitivity, specificity, and accuracy for the diagnosis of recurrence (94.4%, 72.2%, and 90.7%, respectively). On the other hand, thickness of 5 mm showed a specificity of 100%, so this thickness guarantees great security for the diagnosis of recurrence.
Similar to MR and CT studies, sonographic intravenous contrast is used in CD patients mainly to assess the inflammatory activity and to characterize the stenosis [39,40,41,42]. This contrast agent is able to evaluate the mural microvascularization, and it is submitted to less interobserver and intraobserver variability than color Doppler US. To quantify the contrast mural enhancement, we chose time–intensity curves because they allow more objective interpretation of the results than the visual analysis [43, 44]. In our evaluation, contrast agent injection did not significantly increase the accuracy of mural thickness in the diagnosis of anastomotic recurrence. When we considered as a sign of recurrence the combination of a thickness ≥ 3 mm and an enhancement ≥ 46%, the specificity increased (Table 2) identifying cases without endoscopic recurrence with a wall thickness ≥ 3 mm and in which the contrast showed wall contrast enhancement < 46%. Paredes et al. [26], using the combination of WT > 3 mm and/or contrast enhancement > 46%, were able to identify some cases of thickness < 3 mm with endoscopic recurrence, which allowed detecting cases of early inflammatory activity.
On the other hand, only few studies have investigated the contribution of imaging techniques in the classification of severity of recurrence compared with endoscopy (Rutgeerts index) [10,11,12, 15, 18, 37]. In our study, we were able to verify the usefulness of the US in the estimation of endoscopic severity. Parietal thickness was also one of the isolated parameters that best predicted severe recurrence; however, the combination of parietal thickness with other ultrasound parameters, including contrast enhancement and presence of complications, improved the ability of US for discriminating the severe cases.
A WT > 5 mm has been previously associated with the diagnosis of moderate–severe recurrence [11, 18, 45]. In our hands, a thickness ≥ 5 mm showed a sensitivity of 74% and specificity of 82%, with moderate agreement with the endoscopy (k = 0.556). Otherwise, the value of 6 mm showed the higher specificity (95.7%).
We have found that the presence of transmural complications (fistulas, abscesses, or stenosis) is very specific for the diagnosis of recurrence and highly related to severe recurrence (PPV 93%). Moreover, these features cannot be assessed with endoscopy, as it was demonstrated in the eight cases of penetrating disease visualized in US, and their identification may alter management plans including the initiation of antibiotics therapy, the use of immunosuppressive or biological agent, or even the surgical decision.
Previous studies have analyzed the usefulness of CEUS in the assessment of endoscopic severity in CD [34, 42, 43, 46], but only one in the setting of postoperative patient [26], where the authors demonstrated the utility of B-mode US combined with CEUS to detect almost all cases with severe recurrence, with a sensitivity of 94% and specificity of 73.1%. In the present study, we have found that the combination of several parameters (WT ≥ 6 mm or WT between 5 and 6 mm with contrast enhancement ≥ 70% or presence of complications) or a US scoring system showed results similar to the study of Paredes et al., with a sensitivity of 90.3% and a specificity between 74 and 87% and a high NPV (87% and 85%, respectively).
Various MR and CT scores have been proposed for the assessment of recurrence severity using mural and extramural findings of the neoterminal ileum in correlation with Rutgeerts score. Sailer et al. [15] in 2008 classified 30 postsurgical CD patients into two groups: “low grade” (≤ i2) and “high grade” (i3/i4), achieving a concordance of 95% (k = 0.84). In the same way, other authors used CT enterography/enteroclisis to correlate with Rutgeerts score with excellent concordance (k = 0.87) [12, 47].
Finally, we constructed an US scoring system including US parameters, color Doppler, mural enhancement and extramural complications. The agreement between the ultrasound scale and the endoscopic score was good (k = 0.635). The dichotomization into two groups, score < 4 or ≥ 4, provided a high sensitivity (90.3%) and specificity (73.9%) in the severe recurrence detection. These results are slightly lower than those published with MR or CT scales [17, 47], but it is worth noting that our study group was much higher than in these studies and the predictive negative value was higher.
We want to highlight the usefulness of ultrasound contrast, which gives us information about the degree of vascularization of the intestinal segments in relation to the anastomosis, in a very specific way, contributing to determine the severity and the prognoses. One of the most important problems in the management of postsurgical recurrence is the follow-up strategy that includes the assessment of the effectiveness of the treatment [8, 48]. Colonoscopy is still the most accurate tool, but it can be technically impossible in up to 10% of the cases [5] (in our study, 8.5% of the total colonoscopies) and is poorly tolerated, especially in long-term follow-up. Thus, alternatives techniques better tolerated than colonoscopy such as fecal markers (calprotectin) or imaging tests are proposed, and in the case of radiological studies with the ability to study the stenoses located in anastomosis or the presence of extramural complications. Ultrasound is a cheap and comfortable technique, well tolerated and as demonstrated in this study with high diagnostic performance. Of special interest is the high correlation of wall thickness with the Rutgeerts endoscopic grades and the great capacity to detect patients with severe recurrence. As we have been able to demonstrate, the use of intravenous contrast improves the results, increasing the specificity to detect severe cases. The availability of the US with a scoring model similar to endoscopy that classifies recurrent patients with low or high degree of severity should encourage us to consider the use of this technique in the management and follow-up algorithm of this type of patients.
One of the limitations of the study is the time elapsed between US and colonoscopy in some patients. This may have contributed to a bias in the results, although all patients maintained the same treatment without modification between the two tests. In this sense, only those subjects who remained clinically stable were included. However, there could have been some cases in which the treatment would have been effective or, on the contrary, it would have worsened in the course of time between the two techniques, and this could have influenced in false positive or negative sonographic results. Another limitation is the interobserver or intraobserver variability of the ultrasound technique. The parietal thickness was the parameter that obtained the best results for the diagnosis of recurrence. In the only work that has evaluated the interobserver variability of sonographic signs, WT showed the best interobserver agreement (k = 0.72–1) [49]. For the estimation of the color Doppler flow, a semiquantitative measurement was used. Likewise, the analysis of the contrast enhancement by means of the time–intensity curves allows obtaining the increase in brightness in relation to the basal state, which results in more objective analysis. Finally, we performed all the examinations with the same US machine and quantitative measurements obtained with the software packages of different commercial US equipments are not interchangeable. The multitude of ultrasound systems and image analysis software provide inherent difficulty in comparing results of different hospitals; therefore, different ultrasound manufactures should obtain their optimal values.
Conclusions
In conclusion, intestinal US is a valuable and noninvasive tool for assessing postoperative endoscopic recurrence in patients with CD. Based on our results, the assessment of the anastomotic wall thickness can demonstrate with high precision the existence of signs of recurrence. The use of CEUS would be useful in the identification of patients with signs of severity in the anastomotic wall. We believe that the use of an US scoring model, similar to endoscopy that classifies recurrent patients in low or high degree of severity, is beneficial for management and can sometimes be decisive, especially in cases where colonoscopy is not possible or is incomplete.
References
Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. Predictability of the postoperative course of Crohn’s disease. Gastroenterology. 1990;99:956–963.
Olaison G, Smedh K, Sjodahl R. Natural course of Crohn’s disease after ileocolic resection: endoscopically visualised ileal ulcers preceding symptoms. Gut. 1992;33:331–335.
Rutgeerts P, Geboes K, Vantrappen G, Kerremans R, Coenegrachts JL, Coremans G. Natural history of recurrent Crohn’s disease at the ileocolonic anastomosis after curative surgery. Gut. 1984;25:665–672.
Van Assche G, Dignass A, Reinisch W, et al. The second European evidence-based consensus on the diagnosis and management of Crohn’s disease: Special situations. J Crohn’s Colitis [Internet]. 2010;4:63–101.
Shah HA, Paszat LF, Saskin R, Stukel TA, Rabeneck L. Factors associated with incomplete colonoscopy: A population-based study. Gastroenterology. 2007;132:2297–2303.
Biancone L, Onali S, Calabrese E, et al. Non-invasive techniques for assessing postoperative recurrence in Crohn’s disease. Dig Liver Dis. 2008;40:S265–S270.
De Cruz P, Kamm MA, Prideaux L, Allen PB, Desmond PV. Postoperative recurrent luminal Crohn’s disease: A systematic review. Inflamm Bowel Dis. 2012;18:758–777.
Yamamoto T. Diagnosis and monitoring of postoperative recurrence in Crohn’s disease. Expert Rev Gastroenterol Hepatol. 2014;9:55–66.
Marteau P, Laharie D, Colombel J-F, et al. Inter-observer variation study of the Rutgeerts score to assess endoscopic recurrence after surgery for Crohn’s disease. J Crohns Colitis. 2016;10:1001–1005.
Paredes JM, Ripollés T, Cortés X, et al. Non-invasive diagnosis and grading of postsurgical endoscopic recurrence in Crohn’s disease: usefulness of abdominal ultrasonography and 99mTc-hexamethylpropylene amineoxime-labelled leucocyte scintigraphy. J Crohn’s Colitis [Internet]. 2010;4:537–545.
Rispo A, Bucci L, Pesce G, et al. Bowel onography for the diagnosis and grading of postsurgical recurrence of Crohn’s disease. Inflamm Bowel Dis. 2006;12:486–490.
Minordi LM, Vecchioli A, Poloni G, Guidi L, De Vitis I, Bonomo L. Enteroclysis CT and PEG-CT in patients with previous small-bowel surgical resection for Crohn’s disease: CT findings and correlation with endoscopy. Eur Radiol. 2009;19:2432–2440.
Soyer P, Boudiaf M, Sirol M, et al. Suspected anastomotic recurrence of crohn disease after ileocolic resection: Evaluation with CT enteroclysis. Radiology. 2010;254:755–764.
Choi IY, Park SH, Park SH, et al. CT Enterography for surveillance of anastomotic recurrence within 12 months of bowel resection in Patients with Crohn’ s disease: an observational study using an 8-year registry. Gastrointest Imaging. 2017;18:906–914.
Sailer J, Peloschek P, Reinisch W, Vogelsang H, Turetschek K, Schima W. Anastomotic recurrence of Crohn’s disease after ileocolic resection: Comparison of MR enteroclysis with endoscopy. Eur Radiol. 2008;18:2512–2521.
Koilakou S, Sailer J, Peloschek P, et al. Endoscopy and MR enteroclysis: Equivalent tools in predicting clinical recurrence in patients with Crohn’s disease after ileocolic resection. Inflamm Bowel Dis. 2010;16:198–203.
Gallego Ojea JC, Echarri Piudo AI, Porta Vila A. Enfermedad de Crohn: Utilidad de la RM-enterografía en la detección de recurrencias posquirúrgicas. Radiologia. 2011;53:552–559.
Castiglione F, Bucci L, Pesce G, et al. Oral contrast-enhanced sonography for the diagnosis and grading of postsurgical recurrence of Crohn’s disease. Inflamm Bowel Dis. 2008;14:1240–1245.
Calabrese E, Petruzziello C, Onali S, et al. Severity of postoperative recurrence in Crohn’s disease: Correlation between endoscopic and sonographic findings. Inflamm Bowel Dis. 2009;15:1635–1642.
Pallotta N, Giovannone M, Pezzotti P, et al. Ultrasonographic detection and assessment of the severity of Crohn’s disease recurrence after ileal resection. BMC Gastroenterol. 2010;10:69.
Onali S, Calabrese E, Petruzziello C, et al. Endoscopic vs ultrasonographic findings related to Crohn’s disease recurrence: A prospective longitudinal study at 3 years. J Crohn’s Colitis. 2010;4:319–328.
Calabrese E, Maaser C, Zorzi F, Kannengiesser K, Hanauer SB, Bruining DH et al. Bowel ultrasonography in the management of Crohn’s disease. A review with recommendations of an international panel of experts. Inflamm Bowel Dis. 2016;22:1168–1183.
Domènech E, López-Sanromán A, Nos P, et al. Recomendaciones del Grupo Español de Trabajo en Enfermedad de Crohn y Colitis Ulcerosa (GETECCU) sobre la monitorización, prevención y tratamiento de la recurrencia posquirúrgica en la enfermedad de Crohn. Gastroenterol Hepatol. 2017;40:472–483.
Gallego JC, Echarri AI, Porta A, Ollero V. Ileal Crohn’s disease: MRI with endoscopic correlation. Eur J Radiol. 2011;80:8–12.
Ma X, Li Y, Jia H, et al. Contrast-enhanced ultrasound in the diagnosis of patients suspected of having active Crohn’s disease: meta-analysis. Ultrasound Clin. 2015;41:659–668.
Paredes J, Ripollés T, Cortés X, et al. Contrast-enhanced ultrasonography: usefulness in the assessment of postoperative recurrence of Crohn’s disease. J Crohn’s Colitis. 2013;7:192–201.
Lennard-Jones JE. Classification of inflammatory bowel disease. Scand J Gastroenterol Suppl. 1989;170:2–9.
Best WR, Becktel JM, Singleton JW, Kern F. Development of a Crohn’s disease activity index. National cooperative Crohn’s disease study. Gastroenterology. 1976;70:439–444.
Spalinger J, Patriquin H, Miron M-C, et al. Doppler US in patients with Crohn disease: Vessel density in the diseased bowel reflects disease activity. Radiology. 2000;217:787–791.
Maconi G, Carsana L, Fociani P, et al. Small bowel stenosis in Crohn’s disease: Clinical, biochemical and ultrasonographic evaluation of histological features. Aliment Pharmacol Ther. 2003;18:749–756.
Ripollés T, Rausell N, Paredes JM, Grau E, Martínez MJ, Vizuete J. Effectiveness of contrast-enhanced ultrasound for characterisation of intestinal inflammation in Crohn’s disease: A comparison with surgical histopathology analysis. J Crohn’s Colitis. 2013;7:120–128.
Ripollés T, Paredes JM, Martínez-Pérez MJ, et al. Ultrasonographic changes at 12 weeks of anti-TNF drugs Predict 1-year sonographic response and clinical outcome in Crohn’s disease: A multicenter study. Inflamm Bowel Dis. 2016;22:2465–2473.
Paredes JM, Ripollés T, Cortés X, et al. Abdominal sonographic changes after antibody to tumor necrosis factor (Anti-TNF) alpha therapy in crohn’s disease. Dig Dis Sci. 2010;55:404–410.
Ripollés T, Martínez MJ, Paredes JM, Blanc E, Flors L, Delgado F. Crohn disease: Correlation of findings at contrast-enhanced US with severity at endoscopy. Radiology [Internet]. 2009;253:241–248.
Gareen IF, Gatsonis C. Primer on multiple regression models for diagnostic imaging research. Radiology. 2003;229:305–310.
Andreoli A, Cerro P, Falasco G, Giglio LA, Prantera C. Role of ultrasonography in the diagnosis of postsurgical recurrence of Crohn’s disease. Am J Gastroenterol. 1998;93:1117–1121.
Biancone L, Calabrese E, Petruzziello C, et al. Wireless capsule endoscopy and small intestine contrast ultrasonography in recurrence of Crohn’s disease. Inflamm Bowel Dis. 2007;13:1256–1265.
Orlando A, Mocciaro F, Renna S, et al. Early post-operative endoscopic recurrence in Crohn’s disease patients: Data from an Italian Group for the study of inflammatory bowel disease (IG-IBD) study on a large prospective multicenter cohort. J Crohn’s Colitis. 2014;8:1217–1221.
Pauls S, Gabelmann A, Schmidt SA, et al. Evaluating bowel wall vascularity in Crohn’s disease: A comparison of dynamic MRI and wideband harmonic imaging contrast-enhanced low MI ultrasound. Eur Radiol. 2006;16:2410–2417.
Robotti D, Cammarota T, Debani P, Sarno A, Astegiano M. Activity of Crohn disease: Value of color-power-Doppler and contrast-enhanced ultrasonography. Abdom Imaging. 2004;29:648–652.
Quaia E. Contrast-enhanced ultrasound of the small bowel in Crohn’s disease. Abdom Imaging. 2013;38:1005–1013.
Piscaglia F, Nolsoe C, Dietrich CF, et al. The EFSUMB guidelines and recommendations on the clinical practice of contrast enhanced ultrasound (CEUS): Update 2011 on non-hepatic applications. Ultraschall Der Med. 2012;33:33–59.
De Franco A, Di Veronica A, Armuzzi A, Roberto I, Marzo M, De Pascalis B et al. Ileal Crohn disease: mural microvascularity quantified with contrast-enhanced US correlates with disease activity. Radiology. 2012;262:680–688.
Ripollés T, Martínez-Pérez MJ, Blanc E, et al. Contrast-enhanced ultrasound (CEUS) in Crohn’s disease: technique, image interpretation and clinical applications. Insights Imaging. 2011;2:639–652.
Rispo A, Imperatore N, Tesla A, et al. Diagnostic accuracy of ultrasonography in the detection of postsurgical recurrence in Crohn’s disease: A systematic review with meta-analysis. Inflamm Bowel Dis. 2018;24:977–988.
Moreno N, Ripollés T, Paredes JM, et al. Usefulness of abdominal ultrasonography in the analysis of endoscopic activity in patients with Crohn’s disease: Changes following treatment with immunomodulators and/or anti-TNF antibodies. J Crohn’s Colitis. 2014;8:1079–1087.
Mao R, Gao X, Zhu Z, et al. CT enterography in evaluating postoperative recurrence of Crohn’s disease after ileocolic resection: complementary role to endoscopy. Inflamm Bowel Dis. 2013;19:977–982.
De Cruz P, Kamm MA, Hamilton AL, et al. Crohn’s disease management after intestinal resection: A randomised trial. Lancet. 2015;385:1406–1417.
Fraquelli M, Sarno A, Girelli C, et al. Reproducibility of bowel ultrasonography in the evaluation of Crohn’s disease. Dig Liver Dis. 2008;40:860–866.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Martínez, M.J., Ripollés, T., Paredes, J.M. et al. Intravenous Contrast-Enhanced Ultrasound for Assessing and Grading Postoperative Recurrence of Crohn’s Disease. Dig Dis Sci 64, 1640–1650 (2019). https://doi.org/10.1007/s10620-018-5432-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-018-5432-6