Abstract
Background and Objective
Optimization strategies with infliximab (IFX) are increasingly used as rescue therapy for steroid refractory acute severe ulcerative colitis (ASUC). We aim to determine if intensified IFX induction improves colectomy rate and identifies outcome predictors.
Methods
Hospitalized adult patients who received IFX for ASUC between 2010 and 2016 were identified. We compared standard inductions (5 mg/kg) vs high-dose induction (10 mg/kg) with 3-month colectomy rate as primary outcome.
Results
Seventy-two patients (62.5% male, median age 38.5) were identified. Thirty-seven patients (51.3%) received 5 mg/kg IFX and 35 received 10 mg/kg. Baseline clinical, biochemical and endoscopic parameters were well matched between these two groups. 10 mg/kg was more likely to be used by clinicians from 2014 onwards (p < 0.001). Three-month colectomy rate was 9.7%; which was not significantly different between the standard (5.4%) and high-dose (14.3%) IFX induction (p = 0.205). CRP ≥ 60 (OR 10.9 [95% CI 1.23–96.50], p = 0.032), hemoglobin ≤ 90 g/L (OR 15.6 [95% CI 2.61–92.66], p = 0.036) and albumin < 30 g/L (OR 9.4 [95% CI 1.06–83.13], p = 0.044) were associated with increased risk of colectomy at 3 months in univariate regression analysis.
Conclusion
Use of high-dose infliximab rescue therapy did not improve 3-month colectomy-free survival in this cohort. Tailored use in high-risk patients may be beneficial although further validation is required.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute severe ulcerative colitis (ASUC) constitutes a medical emergency. Intravenous corticosteroids have been the mainstay of management for many years [1]. However, 30–40% of acute severe UC patients will be refractory to steroids and subject to urgent colectomy for disease control [2, 3]. Colectomy reduces mortality rate in UC patients, but it is often associated with a poorer quality of life and considerable morbidity, including infertility, pouchitis, and pouch failure, rendering it an unattractive alternative to most patients [4,5,6]. The use of infliximab (IFX) rescue therapy in ASUC has become standard practice since the publication of successful rescue therapy in severe hospitalized ASUC with a single 5-mg/kg dose of IFX [7]. Colectomy was avoided in 61% of cases after a median follow-up of 538 days [6].
ASUC is associated with high circulating levels of TNF, representing an increased inflammatory burden and more rapid drug clearance [8, 9]. Accelerated drug clearance is seen in states with higher CRP and hypoalbuminemia, with systemic inflammation increasing monoclonal antibody catabolism resulting in decreased clinical response. Furthermore, IFX is lost into stools of patients with ASUC. High fecal concentrations of IFX in the first days after therapy begins are associated with primary nonresponse [10]. This highlights that these patients potentially require higher modified dosing strategies, such as more frequent IFX administration or higher drug dosages to maintain therapeutic drug levels during the induction period. The optimal induction regimen for patients with ASUC is not known.
There exists enormous variation in practice among inflammatory bowel disease specialists regarding IFX induction regimens [11]. Identifying predictors of response to IFX is important as it might help in carefully selecting patients who will benefit from anti-TNF therapy. Furthermore, there is sparse data and follow-up time on outcomes in acute IFX rescue therapy in the literature. Few retrospective studies have since been published on intensified therapy with conflicting results [12,13,14].
The primary objective of this study was to investigate if high-dose IFX induction regimen was associated with improved 3-month colectomy-free outcomes. The secondary objectives are to identify predictors of favorable clinical outcomes and evaluate the safety of different induction regimens.
Materials and Methods
Study Population and Design
We performed a retrospective cohort study including patients who were hospitalized and received at least one intravenous infusion of IFX as rescue therapy for ASUC between July 2010 and July 2016 at either of the three tertiary teaching hospitals at McGill University, in Montreal, Canada. In the standard-dose (5 mg/kg) and high-dose (10 mg/kg) group, IFX induction regimen was at 0, 2, 6 weeks and maintenance at every 8 weeks. All hospitalized patients that received IFX were identified through the hospital centralized pharmacy electronic database. The clinical data were extracted from the patient’s electronic medical records and inpatient pharmacy dispensing record. We included patients with established diagnosis of ulcerative colitis, Mayo score of ≥ 6, Mayo endoscopic score of 2 and above, and meeting Oxford criteria for requiring medical rescue therapy for ASUC: C-reactive protein (CRP) > 45 mg/mL and a stool frequency of 3–8 stools per day, or stool frequency greater than 8 stools per day on day 3 after the initiation of treatment with intravenous glucocorticoids [2]. All patients who received a diagnosis of UC limited to the rectum, indeterminate colitis, Crohn’s disease, or patients who had a previous surgical resection or positive Clostridium difficile infection prior to IFX induction were excluded from the study. The choice of IFX dosing regimen was at the discretion of the treating clinician.
For all eligible patients, the following demographic and clinical variables were collected and analyzed: age, sex, weight, height, date of diagnosis and duration of UC prior to IFX, and extent of disease (as defined by the Montreal Classification) [15], previous and concomitant medical therapy, dose of IFX, dose intervals, escalations, number of infusions, need for hospitalization, and colectomy at 30 days, 90 days and 2 years. Additionally, serological variables including hemoglobin concentration (Hb), albumin, and C-reactive protein (CRP) were also collected both at presentation prior to IV steroid and just prior to IFX therapy.
Ethics Considerations
The study was approved by the McGill University Health Centre and Jewish General Hospital institutional review board and ethics committee (REB: 15-630-MUHC) on June 30, 2015. The research protocol conforms to ethical guidelines of the 1975 Declaration of Helsinki and local regulations.
Statistical Analysis
Statistical analyses were performed using Statistical Package for the Social Sciences (SPSS 24; IBM, New York, USA). All continuous data were expressed as median and interquartile range (IQR). For univariate analyses, we used t test and Wilcoxon rank-sum test for continuous variables and χ2 test for proportion of discrete variables. Logistic regression modeling was used to calculate the odds ratios (ORs) and 95% confidence intervals (CIs) for the colectomy group compared with the non-colectomy group to identify predictors of response. All p values were two-tailed, and p values < 0.05 were considered statistically significant.
Results
Patient Characteristics
A total of 72 patients (45 men, 62.5%) ranging in age from 18 to 81 years (median = 38.5 years, IQR 27–54) were included. The patient demographics are summarized in Table 1. Twenty-eight patients (38.9%) were on an immunomodulator prior to hospitalization, with ten (35.7%) of those patients remaining on immunomodulators post-discharge. Based on the Montreal Classification, 6 had left-sided (8.3%), and 66 (91.7%) had pancolitis prior to hospitalization. Two patients (2.8%) were on a biologic agent at admission. Fifty-seven patients (79.2%) were on 5-ASA. Median disease duration was 3 years (IQR 0.3–9.8).
The median Mayo score was 12 (IQR 11–12), and the median endoscopic Mayo score was 3 (IQR 3–3), while the median CRP was 46.5 mg/L (IQR 30.5–100.1). Median hemoglobin at induction was 106 g/L (IQR 93–125), and median albumin was 30 g/L (IQR 27–36). All patients completed full induction therapy other than the 5 patients who underwent colectomy within the first 30 days. The median follow-up time was 12 months (IQR 3–72).
Baseline Characteristics of 5 mg/kg Versus 10 mg/kg Group
Table 2 displays the demographic, clinical, laboratory, and endoscopic features of the UC patients stratified by standard 5 mg/kg dose (N = 37) versus 10 mg/kg high-dose induction dosage (N = 35). There was no significant baseline characteristics difference between the different regimens, particularly the clinical, biochemical, and endoscopic assessment of disease severity. Magnitude of CRP reduction following IV steroid therapy prior to IFX induction was also not different between the two groups (p = 0.309). Higher proportions of patients were given 10 mg/kg dosing from 2014 onwards compared to prior to 2014 (70.6% versus 28.9%, p < 0.001) which is likely to represent a change in the management of hospitalized UC patients.
Colectomy Rates
The 30-day colectomy rate in our cohort was 6.9%, 90-day colectomy rate was 9.7%, and 2 year-colectomy rate was 15.2%. There were no mortality events.
Different IFX Induction Doses on Colectomy Rates
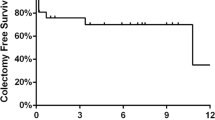
The rate of colectomy at 3 months was not significantly different between the standard 5 mg/kg and high-dose 10 mg/kg IFX induction (p = 0.205) (Fig. 1). The colectomy rates at 3 months in the 5 mg/kg and 10 mg/kg groups were 5.4% and 14.3%, respectively. Similarly, no difference in colectomy rate was found between these two groups at 1 month and 24 months.
Propensity score matching was undertaken to control for potential selection bias in this non-randomized cohort. While no difference between the two groups were found when matched for gender, age and disease extend (p = 0.455, caliper 0.01), additional pertinent parameters such as CRP, albumin and Hb were unable to be included to achieve adequate matching as this was limited by the patient sample size.
Predictors of Colectomy
The univariate regression analysis (Table 3) demonstrated that a Hb ≤ 90 g/L (OR 15.6, 95% CI 2.61–62.66, p = 0.003), albumin < 30 g/L (OR 9.4, 95% CI 1.06–83.14, p = 0.044) and CRP ≥ 60 mg/L (OR 10.9, 95% CI1.23–96.5, p = 0.032) at induction were associated with increased risk of colectomy at 3 months. These cutoff values were selected based on sensitivity analysis. In multivariate analysis, there was a trend of CRP ≥ 60 and Hb ≤ 90 g/L in predicting colectomy although this was statistically insignificant (Table 4).
Clostridium Difficile Infection
A total of 9 cases of C. Difficile occurred over 2 years following IFX therapy, with the first 5 cases in the first 3 months, 3 further cases by 6 months and 1 additional case by 24 months. High-dose IFX was associated with a reduced risk of subsequent C. Difficile, compared to standard dose within a 2-year interval (OR 0.1; 95% CI 0.01–0.88, p = 0.038) in multivariate analysis. Conversely, Hb of ≤ 90 g/L was associated with increased risk of C. Difficile infection (OR 5.3; 95% CI1.04–27.38, p = 0.045).
Discussion
This study has shown that the use of higher 10 mg/kg IFX induction as rescue therapy for ASUC did not lead to improved colectomy rate outcome at 3 months as compared to the standard 5 mg/kg in this cohort. This result is consistent with the recently published Mount Sinai New York Hospital cohort with 146 adult patients that also showed that no difference in the 30- and 90-day colectomy rate was demonstrable between 10 mg/kg dose and standard dose of IFX [15]. This study also utilized propensity score matching to control for potential patient- and disease-related confounders as well as selection bias inherent in a non-randomized cohort. Interestingly, the standard dose, however, had significantly higher rate of needing additional IFX dose within 7 days of the initial dose (i.e., accelerated induction regimen). Additionally, the need for shortened induction regimen was itself an independent variable associated with higher 30-day colectomy rate. Gibson et al. in a retrospective analysis have shown that in patients with ASUC, an accelerated induction strategy administrating 5 mg/kg IFX within a median period of 24 days reduced the need for early colectomy (rate of colectomy was 6.7% in those treated with the shortened induction regimen, compared to 40% in those with the standard induction regimen) [16], as well as longer time to colectomy. However, the colectomy rates remain similar between the two groups up to two years in this study. Despite the increasingly widespread use of intensified IFX rescue therapy, observational studies continue to show conflicting results. Ongoing randomized prospective trials such as the Australian PREDICT-UC study (NCT02770040) should help to establish the optimal management approach in this high-risk population. Higher IFX was associated with reduced Clostridium difficile infection in this cohort; however, interpretation is limited by the small patient number.
Better selection of patients needing more aggressive therapy may also act as one of the important factors to improve outcome. This will also minimize unnecessary exposure and cost to patients. The Mayo score by itself for these patients on the severe end of disease activity was limited in its ability to predict those likely not to response to standard IFX rescue therapy in this study. Additional factors, such as multiorgan dysfunction and colonic dilatation which are associated with poorer outcomes, may potentially assist to further stratify these high-risk patients [17, 18]. Baseline albumin, C-reactive protein, and hemoglobin levels, which reflect severity of disease activity, were predictive of colectomy and/or subsequent C-difficile infection in our study. Arias et al. [19] showed similar results that predicted long-term outcomes of therapy. These biochemical parameters may potentially be used in conjunction with other clinical factors as selection criteria for those that may benefit from more aggressive IFX rescue therapy; although this also needs to be validated by further prospective studies. Biochemical response during induction therapy, such as CRP progression, may also serve as selecting criteria for those requiring more intensified therapy.
Many studies have shown the association between higher serum concentrations of anti-TNF agents and better outcomes. Detectable trough levels are associated with higher rates of remission in acute UC and undetectable trough levels with ninefold increased rate of colectomy [20]. Similarly, early week-2 trough levels of the IFX biosimilar CTP-13 of 11.5 μg/ml and 15 μg/ml were predictive of week-14 clinical response and remission, respectively, in a Hungarian study [21]. Anti-TNF pharmacokinetics is a dynamic and complex process. There are many issues that may influence anti-TNF levels. High TNF burden in ASUC forms immune complexes with anti-TNF agents and are cleared through Fc receptor-mediated endocytosis and proteolytic degradation by mononuclear phagocytes of the reticuloendothelial system (RES) [22]. RES activity is suspected to be influenced by the degree of inflammation [23]. Furthermore, IFX is lost into the stools of patients with ASUC via the diseased colon mucosa. Low serum albumin, patient weight, and male sex have all been associated with lower trough levels [22]. Potentially, dose optimization based on therapeutic drug monitoring during the early induction phase may help to enhance the effective anti-TNF level and thus lead to improved outcomes. In addition, this approach will also depend on expedited report of the anti-TNF testing results. Unfortunately, we did not have anti-TNF trough and anti-drug antibody measurements during induction, which would have significantly enhanced our understanding of this complex process as higher IFX dosing does not necessarily equate to high trough levels.
The main limitation of this retrospective study relates to the lack of randomization and potential selection bias for those receiving standard versus high IFX induction. Dosing regimen was determined by the treating gastroenterologist, who responded to clinical or laboratory evidence of refractory inflammation, and hence it was not standardized. However, baseline disease characteristics, clinical, biochemical and endoscopic disease activity were all well matched between the two cohorts thus limiting inherent underlying differences contributing to the clinical outcomes. The median CRP in the high IFX dose group was numerically higher; however, it was not statistically significant. Adequate propensity score matching to control for potential confounders was unable to be performed due to the sample size in this patient cohort. We also did not have endoscopic outcome assessment which may have shown differences between these IFX dosing regimen. Furthermore, the 3-month colectomy rate in this study is lower compared to other retrospective cohorts. This difference is unlikely to be explained by disease severity in view of the comparable baseline clinical and biochemical parameters of the patient population in this study. Perhaps the difference may involve the inclusion of a more recent cohort of which 83.3% of the cases presented between 2013 and 2016, shorter disease duration, use of rescue therapy in patients with first episode of ASUC, prompt recognition and early use of medical rescue therapy in accordance with guidelines may have lowered poor outcomes. Finally, in contrast, this study is strengthened by the inclusion of relatively large cohort size of 72 consecutive well-characterized patients with severe hospitalized ASUC.
In conclusion, use of high-dose IFX rescue therapy was not associated with a reduced colectomy rate in our cohort. Tailored use in high-risk patients such as those with low albumin, low hemoglobin and high CRP may be beneficial although this needs to be validated in prospective clinical trials.
References
Truelove SC, Jewell DP. Intensive intravenous regimen for severe attacks of ulcerative colitis. Lancet (Lond Engl). 1974;1:1067–1070.
Travis SP, Farrant JM, Ricketts C, et al. Predicting outcome in severe ulcerative colitis. Gut. 1996;38:905–910.
Jarnerot G, Rolny P, Sandberg-Gertzen H. Intensive intravenous treatment of ulcerative colitis. Gastroenterology. 1985;89:1005–1013.
Cima RR. Timing and indications for colectomy in chronic ulcerative colitis: Surgical consideration. Dig Dis (Basel, Switz). 2010;28:501–507.
de Silva S, Ma C, Proulx MC, et al. Postoperative complications and mortality following colectomy for ulcerative colitis. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2011;9:972–980.
Mortensen C, Caspersen S, Christensen NL, et al. Treatment of acute ulcerative colitis with infliximab, a retrospective study from three Danish hospitals. J Crohn’s Colitis. 2011;5:28–33.
Jarnerot G, Hertervig E, Friis-Liby I, et al. Infliximab as rescue therapy in severe to moderately severe ulcerative colitis: a randomized, placebo-controlled study. Gastroenterology. 2005;128:1805–1811.
Gardiner KR, Halliday MI, Barclay GR, et al. Significance of systemic endotoxaemia in inflammatory bowel disease. Gut. 1995;36:897–901.
Kevans D, Murthy S, Iacono A, Silverberg MS, Greenberg GR. Sa2031 Accelerated clearance of serum infliximab during induction therapy for acute ulcerative colitis is associated with treatment failure. Gastroenterology. 2012;142:S-384-S-5.
Brandse JF, van den Brink GR, Wildenberg ME, et al. Loss of infliximab into feces is associated with lack of response to therapy in patients with severe ulcerative colitis. Gastroenterology. 2015;149:350-5.e2.
Herfarth HH, Rogler G, Higgins PD. Pushing the pedal to the metal: should we accelerate infliximab therapy for patients with severe ulcerative colitis? Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2015;13:336–338.
Shah S, Naymagon S, Sands BE, Cohen BL, Dubinsky M. Mo1908 colectomy free survival is independent of initial infliximab dosing strategy in hospitalized ulcerative colitis patients. Gastroenterology. 2016;150:S813.
Sjoberg M, Magnuson A, Bjork J, et al. Infliximab as rescue therapy in hospitalised patients with steroid-refractory acute ulcerative colitis: a long-term follow-up of 211 Swedish patients. Aliment Pharmacol Ther. 2013;38:377–387.
Shah SC, Naymagon S, Panchal HJ, Sands BE, Cohen BL, Dubinsky MC. Accelerated infliximab dosing increases 30-day colectomy in hospitalized ulcerative colitis patients: a propensity score analysis. Inflamm Bowel Dis. 2018;24:651–659.
Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Canadian journal of gastroenterology = Journal canadien de gastroenterologie. 2005;19 Suppl A:5a-36a.
Gibson DJ, Heetun ZS, Redmond CE, et al. An accelerated infliximab induction regimen reduces the need for early colectomy in patients with acute severe ulcerative colitis. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2015;13:330-5.e1.
Caprilli R, Latella G, Vernia P, Frieri G. Multiple organ dysfunction in ulcerative colitis. Am J Gastroenterol. 2000;95:1258–1262.
Lennard-Jones JE, Ritchie JK, Hilder W, Spicer CC. Assessment of severity in colitis: a preliminary study. Gut. 1975;16:579–584.
Arias MT, Vande Casteele N, Vermeire S, et al. A panel to predict long-term outcome of infliximab therapy for patients with ulcerative colitis. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2015;13:531–538.
Seow CH, Newman A, Irwin SP, Steinhart AH, Silverberg MS, Greenberg GR. Trough serum infliximab: a predictive factor of clinical outcome for infliximab treatment in acute ulcerative colitis. Gut. 2010;59:49–54.
Gonczi L, Vegh Z, Golovics PA, et al. Prediction of short- and medium-term efficacy of biosimilar infliximab therapy. Do trough levels and antidrug antibody levels or clinical and biochemical markers play the more important role? J Crohn’s Colitis. 2016;11:697–705.
Rosen MJ, Minar P, Vinks AA. Review article: applying pharmacokinetics to optimise dosing of anti-TNF biologics in acute severe ulcerative colitis. Aliment Pharmacol Ther. 2015;41:1094–1103.
Fasanmade AA, Adedokun OJ, Ford J, et al. Population pharmacokinetic analysis of infliximab in patients with ulcerative colitis. Eur J Clin Pharmacol. 2009;65:1211–1228.
Acknowledgments
The co-authors would like to thank Janie Coulombe for her kind assistance with the statistical analysis of the study data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None declared by all co-authors.
Additional information
Co-First Authors: Che-Yung Chao, Alex Al Khoury
Rights and permissions
About this article
Cite this article
Chao, CY., Al Khoury, A., Aruljothy, A. et al. High-Dose Infliximab Rescue Therapy for Hospitalized Acute Severe Ulcerative Colitis Does Not Improve Colectomy-Free Survival. Dig Dis Sci 64, 518–523 (2019). https://doi.org/10.1007/s10620-018-5358-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-018-5358-z