Abstract
Background
Acute pancreatitis (AP) is a common acute gastrointestinal disorders. Increasing evidence indicated that autophagy is involved in the development of AP. Resolvin D1 is an endogenous pro-resolving lipid mediator, which can protect mice from cerulein-induced acute pancreatitis and facilitate autophagy in macrophage, but its mechanism remians unclear.
Aims
To investigate the effect of resolvin D1 on autophagy in mouse models of cerulein-induced AP.
Methods
C57BL/6 mice were randomly divided into control group, AP group and resolvin D1 group. The models of cerulein-induced AP were constructed by intraperitoneally cerulein. Resolvin D1 group was established by intraperitoneally resolvin D1 based on AP models, simultaneously, control group received normal saline. The severity of AP, the level of inflammatory cytokines, the number of autophagic vacuoles, and the expression of autophagy-related markers were evaluated among three groups.
Results
The AP models were established successfully. Compared to control group, the number of autophagic vacuoles and expressions of autophagy-related markers including Beclin-1, p62 and LC3-II were increased in AP models, In contrast, the degree of inflammation and levels of inflammatory cytokines in AP models were reduced after resolvin D1 treatment. Moreover, resolvin D1 attenuated the number of autophagic vacuoles and expressions of autophagy-related markers.
Conclusions
Autophagic flux is impaired in cerulein-induced AP. Resolvin D1 ameliorate the severity of mice with cerulein-induced acute pancreatitis, possible attributing to its reducing impaired autophagy and restoring autophagic flux.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute pancreatitis (AP) is a common clinical hospitalized illness. Despite significant progress achieved in medical knowledge and effective treatments, AP remains considerable incidence and mortality, which may attribute to its obscure pathophysiological mechanism [1]. Trypsinogen activation and excessive inflammation are considered as key factors in the onset and progression of AP [2]. Increasing evidence indicates that autophagy is implicated in trypsinogen activation, besides, regulating autophagy in AP models can reduce inflammation of pancreas [3,4,5,6,7].
Autophagy is an evolutionarily conserved cellular process, characterized by double-membrane vesicles sequestering damaged organelles, misfolded protein aggregates, and pathogens into autophagosomes, which then fusing with lysosomes and maturating autolysosomes for degradation to maintain cell homeostasis [8]. There are three major autophagic pathways: macroautophagy, chaperone-mediated autophagy and microautophagy. Macroautophagy has been most extensively studied, which usually is referred to as autophagy and also used as such in this study [9]. Current studies confirmed that autophagy is linked to diverse biological processes, including mammalian development, differentiation, and aging [10]. Autophagy is a double-edged sword, physiological autophagy can promote health, whereas beyond this range it will result in cell damage and pathological conditions [11]. Basal autophagy maintains pancreatic acinar cell homeostasis [12]. In contrast, defective autophagy mediates acinar cell vacuole formation and trypsinogen activation in models of AP, aggravating the inflammation in the pancreas [4, 6, 7].
Resolvin D1 (RvD1) is an endogenous anti-inflammatory and pro-resolving lipid mediator and derived from omega-3 polyunsaturated fatty acid. RvD1 has been proved to be promising therapeutic role in inflammatory diseases [13]. It is demonstrated that the level of RvD1 is negatively related to incidence of post-ERCP pancreatitis in patients [14]. It is also reported that RvD1 can protect mice against cerulein-induced AP [15], but the mechanism of which remains poorly understood. Previously, RvD1 has been identified to activate autophagy in macrophage, inducing formation of autophagosomes, facilitating fusion of autophagosomes with lysosomes and supporting process of autophagic flux [16]. Therefore, we speculated that RvD1 can attenuate severity of AP by regulating autophagy in cerulein-induced AP.
Materials and Methods
Animals and Reagents
Adult male C57BL/6 mice, weighing 18–22 g, were provided by Animal Center of Anhui Province. Mice were adapted for a week before entering the experimental period and housed in the environment with a room temperature and a 12–12 h light–dark cycle. Cerulein was purchased from Sigma (USA), and Resolvin D1 was from Cayman (USA). α-Amylase assay kit, lipase assay kit and myeloperoxidase (MPO) enzyme-linked immunosorbent assay (ELISA) kit were purchased from Nanjing Jiancheng Bioengineering institute (China). IL-1β, IL-6, IL-8, and TNF-α ELISA kit were purchased from Shanghai Yuanye Bio-Technology Co., Ltd (China). Anti-Beclin-1 antibody and anti-LC3-II antibody were purchased from Abcam Ltd (Cambridge, UK). Anti-P62 antibody and anti β-actin were from Bioss Ltd (China).
AP Induction and Resolvin D1 Administration
A total of 30 Male C57BL/6 mice were randomly divided into control group, AP group and RvD1 group. AP models were induced by intraperitoneally cerulein (50 μg/kg/h) for seven times. Resolvin D1 (50 ug/kg) was given intraperitoneally to the mice 1 h before and 4 h after the induction of AP. Mice in control group received same dose of normal saline. The mice were killed after completion of AP induction. Serum were harvested and stored at − 80 °C for analysis; besides, pancreatic and lung tissue were rapidly removed and stored in liquid nitrogen or fixed in 2.5% glutaraldehyde or fixed in 10% formalin for analysis. The protocol was approved by the Ethics Committee of the First Affiliated Hospital of Anhui Medical University.
Amylase and Lipase Measurement
Serum Amylase and lipase activities were determined using α-Amylase assay kit and lipase assay kit according to the manufacturer’s instructions.
Inflammatory Cytokine
Pancreas and lung were homogenized in cold saline and centrifuged for supernatant. The levels of IL-1β, IL-6, IL-8, TNF-α, MPO and PGE2 were measured in the supernatant using ELISA kits according to the manufacturer’s instructions.
Histological Examination
Formalin-fixed and paraffin-embedded pancreatic and lung tissue were sectioned. Sections were stained with hematoxylin and eosin (H&E) for histological analysis to evaluate the inflammatory injury degree of pancreatic and lung tissue. All samples were scored by two experienced pathologist who were blinded to experimental group. According to the criteria described by Schmidt et al. [17], pancreatic histopathological damage were estimated on edema, acinar necrosis, hemorrhage and fat necrosis, and inflammation and perivascular infiltrate scored from 0 to 4. Lung histopathological damage was quantified on alveolar congestion, hemorrhage, aggregation of neutrophils in airspace, and thickness of the alveolar wall scored from 0 to 4 according to the criteria described by Imanaka et al. [18].
Transmission Electron Microscope
To assess autophagic vacuoles in acinar cells, fresh pancreatic tissue were cut into small pieces (approximate 1 mm3) and immediately fixed in a 2.5% glutaraldehyde at 4 °C overnight. After being postfixed in 1% osmic acid and dehydrated with a step-wise gradient ethanol (30, 50, 70, 80, 95, 100%), samples were embedded in an epoxy resin. Samples then were cut on a LKB-NOVA ultramicrotome into 70 nm sections, which were examined under a JEM-1230F electron microscope.
Reverse Transcription Quantitative Real-Time PCR (RT-qPCR) Analysis
Total RNA was extracted from pancreatic tissue using the TRIzol Reagent (Invitrogen, USA). RNA then were reverse transcribed into cDNA using the RevertAid™ first Strand cDNA Synthesis Kit (Thermo Scientific, USA) according to the manufacturer’s instructions. Quantitative PCR was performed using QuantiFast SYBR Green PCR kit (Qiagen, Hilden, Germany) and at reaction conditions of 95 °C for 2 min followed by 35 cycles of 95 °C for 10 s, 60 °C for 20 s. Relative expression of the target gene was quantified using the 2−ΔΔCt method and normalized with β-actin gene. The primer sequences (Shanghai Bocai Biotechnology Co., Ltd., China) were as follows: Beclin-1: (forward) 5′-GCACCATGCAGGTGAGCTTC-3′, (reverse) 5′-TTTCGCCTGGGCTGTGGTAA-3′; P62: (forward) 5′-AGGGAACTGCAGCACACACT-3′, (reverse) 5′-TGCCTGCCACCTTTCACTCA-3′; LC3II: (forward) 5′-CATGCCGTCCGAGAAGACCT-3′, (reverse) 5′-GTGGTCAGGCACCAGGAACT-3′; β-actin: (forward) 5′-AGTGTGACGTTGACATCCGT-3′, (Reverse) 5′-TGCTAGGAGCCAGAGCAGTA-3′.
Western Blot Analysis
Protein was extracted from pancreatic tissue by being homogenized with lysis buffer containing phenylmethylsulfonyl fluoride and centrifuged at 12,000 rpm for 15 min at 4 °C. The protein was separated on SDS-PAGE and transferred to PVDF membrane after electrophoresis. Blocked with 5% skim milk in TBST for 2 h at room temperature, membranes were then incubated with antibody against Beclin-1 (1:1000), P62 (1:500), LC3-II (1:2000) and β-actin (1:1000) at 4 °C overnight. After being washed with TBST, membranes were incubated with HRP-conjugated secondary antibodies for 2 h at room temperature. Washed with TBST again, target protein was detected with SuperSignal West Femto Trial Kit (Thermo Scientific, USA). Band intensity was quantified with Image J software and normalized with β-actin protein.
Statistical Analysis
All data were analyzed using SPSS 17.0 software and expressed as the mean ± standard deviation. Statistical significance was evaluated using one-way analysis of variance, followed by a Student–Newman–Keuls test. P < 0.05 was thought to be significant.
Results
Resolvin D1 Protects Mice from Cerulein-Induced Acute Pancreatitis
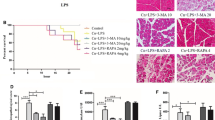
The model of cerulein-induced acute pancreatitis was constructed successfully, characterized by a severer pathological damage of pancreas and lung, heavier relative pancreatic weight and higher serum amylase and lipase activities than control group (P < 0.05). As we expected, mice in RvD1 group exhibited less inflammatory injury of pancreas and lung in histopathologic examination compared with AP group (P < 0.05). Corresponding to histological manifestation, histological scores were markedly lower in the pancreas and lung of mice in RvD1 group than AP group (P < 0.05). Similar to histological results, pancreatic and lung relative weight were lighter in RvD1 group than AP group (P < 0.05). In addition, serum amylase and lipase activities were significantly decreased in RvD1 group than AP group (P < 0.05). These results indicated that the severity of AP was obviously reduced by RvD1, as shown in Fig. 1.
Resolvin D1 can reduced the severity of cerulein-induced AP in mice. a, b Activities of amylase and lipase in serum (n = 10). c, d Pancreatic and lung weight relative to body weight (n = 10). e, f Histological scores of pancreas and lung (n = 6). g, h Representative images of pancreatic and lung histological manifestation. *P < 0.05
Resolvin D1 Down-Regulates Inflammatory Cytokine in Cerulein-Induced Acute Pancreatitis
It has been proved that increasing inflammatory cytokine plays an important role in progression of AP [19]. Inflammatory cytokine can amplify inflammatory response of AP. Thus, IL-1β, IL-6, IL-8, TNF-α, MPO and PGE2 were analyzed in pancreatic and lung tissue. Compared with control group, levels of IL-1β, IL-6, IL-8, TNF-α, MPO and PGE2 in pancreatic tissue were significantly elevated in AP group (P < 0.05), as well as lung tissue (P < 0.05). In contrast, these inflammatory cytokine levels were dramatically reduced in RvD1 group in pancreatic tissue (P < 0.05) and lung tissue (P < 0.05), as shown in Fig. 2.
Resolvin D1 reduced inflammatory cytokine of pancreas and lung in cerulein-induced AP in mice (n = 8). a Levels of IL-1β in pancreatic and lung tissue. b Levels of IL-6 in pancreatic and lung tissue. c Levels of IL-8 in pancreatic and lung tissue. d Levels of TNF-α in pancreatic and lung tissue. e Levels of MPO in pancreatic and lung tissue. f Levels of PGE2 in pancreatic and lung tissue. *P < 0.05
Resolvin D1 Restores Autophagic Flux in Cerulein-Induced Acute Pancreatitis
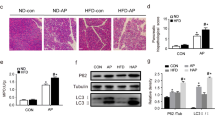
Based on autophagy involvement in the onset and development of AP, we aimed to determine whether Resolvin D1 acts in resolution of AP inflammation through regulation of autophagy. We successively performed transmission electron microscopy, RT-qPCR and Western Blot analysis for autophagic changes among three groups. Transmission electron microscopy results displayed accumulation of autophagic vacuoles and trypsinogen in both AP group and RvD1 group according to previous described method [20]; furthermore, more vacuoles were showed in AP group than RvD1 group (P < 0.05), and size of autophagic vacuoles and the number of trypsinogen was reduced in RvD1 group relative to AP group. Both autophagy induction and autophagy impairment can lead to accumulation of autophagic vacuoles [3, 4]. The difference between autophagic induction and impairment is efficiency of autophagic flux.
To evaluate the efficiency of autophagic flux [21, 22], expression of autophagy-related markers were detected in pancreas. By RT-qPCR analysis, mRNA levels of Beclin-1, P62/SQSTM1 and LC3-II were higher in AP group compared to control group (P < 0.05). In contrast, mRNA levels of Beclin-1, P62 and LC3-II were dramatically decreased in RvD1 group (P < 0.05). In accord with RT-qPCR results, higher protein levels of Beclin-1, P62 and LC3-II were observed in AP group than control group in Western Blot analysis (P < 0.05). RvD1 group exhibited lower protein levels of Beclin-1, P62 and LC3-II than AP group (P < 0.05), as shown in Fig. 3. Autophagic vacuoles and Beclin-1 alteration, together with an increase expression in both P62 and LC3-II in AP group, suggested that autophagy is activated but inefficient and impaired autophagic flux in cerulein-induced AP. Thus, RvD1 reduce the number of autophagic vacuoles and trypsinogen and these autophagy-related markers, indicating that RvD1 reduces impaired autophagic flux and restores autophagic flux in cerulein-induced AP.
Resolvin D1 reduced impaired autophagy and restoring autophagic flux in cerulein-induced AP in mice. a Representative autophagic vacuoles in acinar cells under electron microscopy, asterisks standing for autophagic vacuoles, arrows standing for zymogen granules, M standing for mitochondria; N standing for nucleus. Scale Bar: 200 nm. b The quantification of autophagic vacuoles (n = 4). c Band intensity of Beclin-1, P62, LC3-II in pancreatic tissue among three groups. d, e Relative expression Beclin of pancreas (n = 6). f, g Relative expression LC3-II of pancreas (n = 6). h, i Relative expression P62 of pancreas (n = 6). Besides, data were expressed as fold changes relative to β-actin. *P < 0.05; **P < 0.01
Discussion
This study demonstrated that Resolvin D1 can effectively ameliorate the severity of cerulein-induced AP in mice, down-regulating inflammatory cytokine. Besides, Resolvin D1 attenuates the impaired autophagy and restore autophagic flux in cerulein-induced AP.
AP accounts for considerable proportion of critical digestive system disease, severe to systemic inflammatory response syndrome and multiple organ failure including acute lung injury [1]. It is proved that the prognosis of pancreatitis is related to the extent of inflammatory response [23]. RvD1 is an member of the omega-3 PUFA docosahexaenoic acid, possessing anti-inflammatory and pro-resolving properties [13]. Zhao et al. [14] found that patients with higher levels of Resolvin D1 have lower risk of post-ERCP pancreatitis. Liu et al. [15] reported that RvD1 exhibits potency in suppressing inflammatory injury in mouse models of AP, including decreasing serum amylase and lipase activity, reducing pancreatic and lung tissue pathological damage and suppressing the expressions of pro-inflammatory cytokines, such as IL-6, TNF-α, which is consistent with our observations in this study. Although study has demonstrated that RvD1 can protect mice against cerulein-induced AP, down-regulating expression of pro-inflammatory cytokine, the mechanism of which remains elusive.
Prieto et al. [16] previously revealed that RvD1 promotes autophagy in macrophage, supporting process of autophagic flux. With significant advances in science research, growing study revealed autophagy is dysfunctional in pancreatitis, and regulating autophagic pathway will contribute to reduce the severity of pancreatitis [3, 6, 7, 22, 24]. Mareninova et al. [4] showed that trypsinogen activation and acinar cell vacuole formation were related to impaired autophagic flux in models of AP. On the other hand, autophagy was demonstrated to regulate the transcription, processing and secretion of cytokine, e.g. IL-1, TNF-a [25]. Defective autophagy can promote inflammation through blockade of P62-dependent NF-κB activation, clearance of damaged mitochondria, elimination of inflammasomes and apoptotic bodies [5]. Thus, it is reasonable to explore effect of RvD1 on autophagy in cerulein-induced AP.
It is long-noted that accumulation of large vacuoles in acinar cells in pancreatitis, which is later demonstrated to be autophagic [4]. Transmission electron microscopy displayed that RvD1 decreased the number and size of autophagic vacuoles in acinar cells, contrasted with acinar cells of mice administration of cerulein alone. Hashimoto et al. [3] revealed that autophagic vacuoles accumulate in acinar cells when autophagy is induced. In addition, Mareninova et al. [4] revealed that acinar cell vacuole formation result from impaired autophagic flux. Therefore, both autophagy inducement and autophagic flux impairment can lead to vacuoles accumulation in acinar cell.
To better understand autophagic changes in cerulein-induced pancreatitis, autophagy-related markers were detected in pancreas. This study showed that the expression of Beclin-1, P62 and LC3-II was evaluated in cerulein-induced AP, which is consistent with previous study [4, 7, 26]. Beclin-1 is involved in autophagosome nucleation by interacting with the class III phosphatidylinositol 3-kinase (PI3K) and Vps34 [21]; thus, hyperexpression of Beclin-1 indicated that autophagy was activated in models of pancreatitis. P62 which binds to LC3 and ubiquitinated protein aggregates forming into autophagosome is degraded in autophagy flux [21]. Accordingly, P62 is taken as a indicator of autophagic degradation. Mammalian has two forms of LC3, and LC3-I localized in the cytoplasm is transformed into LC3-II. Amount of LC3-II is associated with the number of autophagosomes, regarded as a indicator of autophagosome formation [21]. Therefore, over-expression of P62 in models of pancreatitis implied that efficiency of autophagic degradation decreased. The tendency of P62 and LC3-II changes are in accordance with electron microscopy results. As previously reported [20, 22, 27], dramatic increase in P62 and LC3-II expression, together with accumulation of vacuoles, suggested that autophagic flux was impaired in cerulein-induced AP, which is in accord with previous studies [4, 7, 28]. Hence, RvD1 reduced expression of p62 and LC3-II, number and size of autophagic vacuole, indicating that RvD1 restored autophagic flux in cerulein-induced AP.
In conclusion, our results show that autophagy was activated but autophagy flux was impaired in cerulein-induced AP in mice, and RvD1 attenuates impaired autophagic flux. Based on these results, we speculate that RvD1 can attenuate the severity of AP correlated with its reducing impaired autophagy and restoring autophagic flux, and more efforts should be paid to investigate the mechanism of RvD1 reducing impaired autophagy in acinar cell.
References
Xiao AY, Tan MLY, Wu LM, et al. Global incidence and mortality of pancreatic diseases: a systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol Hepatol. 2016;1:45–55.
Singh P, Garg PK. Pathophysiological mechanisms in acute pancreatitis: current understanding. Indian J Gastroenterol. 2016;35:153–166.
Hashimoto D, Ohmuraya M, Hirota M, et al. Involvement of autophagy in trypsinogen activation within the pancreatic acinar cells. J Cell Biol. 2008;181:1065–1072.
Mareninova OA, Hermann K, French SW, et al. Impaired autophagic flux mediates acinar cell vacuole formation and trypsinogen activation in rodent models of acute pancreatitis. J Clin Investig. 2009;119:3340.
Gukovsky I, Li N, Todoric J, et al. Inflammation, autophagy, and obesity: common features in the pathogenesis of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1199–1209.
Chinzei R, Masuda A, Nishiumi S, et al. Vitamin K3 attenuates cerulein-induced acute pancreatitis through inhibition of the autophagic pathway. Pancreas. 2011;40:84–94.
Xiao J, Feng X, Huang XY, et al. Spautin-1 ameliorates acute pancreatitis via inhibiting impaired autophagy and alleviating calcium overload. Mol Med. 2016;22:643.
Feng Y, He D, Yao Z, et al. The machinery of macroautophagy. Cell Res. 2014;24:24.
Mizushima N, Levine B, Cuervo AM, et al. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069.
Hale AN, Ledbetter DJ, Gawriluk TR, et al. Autophagy: regulation and role in development. Autophagy. 2013;9:951–972.
Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science. 2004;306:990–995.
Antonucci L, Fagman JB, Kim JY, et al. Basal autophagy maintains pancreatic acinar cell homeostasis and protein synthesis and prevents ER stress. Proc Natl Acad Sci USA. 2015;112:E6166–E6174.
Krishnamoorthy S, Recchiuti A, Chiang N, et al. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc Natl Acad Sci USA. 2010;107:1660–1665.
Zhao X, Bao J, Hu C, et al. Effect of diclofenac on the levels of lipoxin A4 and Resolvin D1 and E1 in the post-ERCP pancreatitis. Dig Dis Sci. 2014;59:2992–2996.
Liu Y, Zhou D, Long FW, et al. Resolvin D1 protects against inflammation in experimental acute pancreatitis and associated lung injury. Am J Physiol Gastrointest Liver Physiol. 2016;310:G303–G309.
Prieto P, Rosales-Mendoza CE, Terrón V, et al. Activation of autophagy in macrophages by pro-resolving lipid mediators. Autophagy. 2015;11:1729–1744.
Schmidt J, Lewandrowski K, Fernandez-del Castillo C, et al. Histopathologic correlates of serum amylase activity in acute experimental pancreatitis. Dig Dis Sci. 1992;37:1426–1433.
Imanaka H, Shimaoka M, Matsuura N, et al. Ventilator-induced lung injury is associated with neutrophil infiltration, macrophage activation, and TGF-β1 mRNA upregulation in rat lungs. Anesth Analg. 2001;92:428–436.
Dambrauskas Z, Giese N, Gulbinas A, et al. Different profiles of cytokine expression during mild and severe acute pancreatitis. World J Gastroenterol. 2010;16:1845.
Takahashi K, Mashima H, Miura K, et al. Disruption of small GTPase Rab7 exacerbates the severity of acute pancreatitis in experimental mouse models. Sci Rep (UK). 2017;7:2817.
Klionsky DJ, Abdelmohsen K, Abe A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2016;12:1–222.
Gukovskaya AS, Gukovsky I. Autophagy and pancreatitis. AJP Gastrointest Liver Physiol. 2012;303:G993–G1003.
Mofidi R, Duff MD, Wigmore SJ, et al. Association between early systemic inflammatory response, severity of multiorgan dysfunction and death in acute pancreatitis. Br J Surg. 2006;93:738–744.
Fortunato F, Kroemer G. Impaired autophagosome-lysosome fusion in the pathogenesis of pancreatitis. Autophagy. 2009;5:850–853.
Harris J. Autophagy and cytokines. Cytokine. 2011;56:140–144.
Xu B, Bai B, Sha S, et al. Interleukin-1β induces autophagy by affecting calcium homeostasis and trypsinogen activation in pancreatic acinar cells. Int J Clin Exp Med. 2014;7:3620.
Mareninova OA, Sendler M, Malla SR, et al. Lysosome-associated membrane proteins (LAMP) maintain pancreatic acinar cell homeostasis: LAMP-2–deficient mice develop pancreatitis. Cell Mol Gastroenterol Hepatol. 2015;1:678–694.
Cao Y, Yang W, Tyler MA, et al. Noggin attenuates cerulein-induced acute pancreatitis and the impaired autophagy. Pancreas. 2013;42:301.
Acknowledgments
The authors are grateful to Department of Pathology of the First Affiliated Hospital of Anhui Medical University for providing technical support in histological studies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Wang, B., Hu, C., Mei, Y. et al. Resolvin D1 Resolve Inflammation in Experimental Acute Pancreatitis by Restoring Autophagic Flux. Dig Dis Sci 63, 3359–3366 (2018). https://doi.org/10.1007/s10620-018-5191-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-018-5191-4