Abstract
Background
Thymoquinone (TQ) is the major constituent of Nigella sativa seed and has shown biological activity in various human carcinomas. However, few studies have reported its effect on esophageal carcinoma (EC).
Aims
To explore the chemosensitive effect and mechanism of TQ in augmentation of cisplatin (DDP)-induced apoptosis of EC, both in vitro and in vivo.
Methods
The viability and apoptosis of esophageal carcinoma cells were detected by the Cell Counting Kit-8 assay, flow cytometry, and Hoechst 33258 staining. The expression levels of JAK2, p-JAK2, STAT3, p-STAT3, Bax, Bcl-2, Cyclin D1, Survivin, and caspase-3, 7, 9 were evaluated by western blot analysis. The histological changes were examined by TUNEL technique and immunohistochemical analysis.
Results
TQ enhanced the proapoptotic effect of DDP in human esophageal carcinoma cell line Eca-109, while blocking the activation of JAK2/STAT3 signaling pathway. The apoptosis of esophageal carcinoma cells was induced via blocking the activation of JAK2/STAT3 by using a molecular inhibitor (WP1066). Consistent with the in vivo and in vitro results, TQ increased cellular apoptosis and enriched the chemosensitivity of DDP.
Conclusions
TQ along with DDP may regulate the progression of EC and has potential to be a chemotherapeutic agent in EC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Esophageal carcinoma (EC) is the eighth most common type of malignant tumor (249,923 new cases in East Asia, 17,273/4242 in eastern/southern Africa) and the sixth leading cause of cancer-related mortality (215,122 deaths in East Asia, 15,804/3902 in eastern/southern Africa) [1, 2]. Moreover, it has been documented that esophageal adenocarcinoma (AC) predominates in the developed countries compared to esophageal squamous cell cancer (SCC) in eastern Asia and Africa [3, 4]. Unfortunately, EC has a poor prognosis with the 5-year survival rate of 18% [5]. Most of the EC progress to an advanced stage and require chemotherapy. Cisplatin (DDP) is one of the first-line chemotherapeutic agents for EC, which could be used as a sole agent or in combination [6]. However, the development of resistance and other drug-related side effects are the prime reasons for the failure of treatment [6, 7]. Hence, this has driven the research for the anticancer agent with natural properties, low-toxicity, and high-efficacy. Furthermore, it was also desirable for the agent to demonstrate an increase in therapeutic efficacy should it be combined with DDP.
Thymoquinone (TQ), an active constituent of the volatile oil, has been identified and isolated from the black seed of Nigella sativa [8]. Laboratory studies have demonstrated, in vivo and in vitro, that TQ possesses anti-inflammatory, anti-migration, pro-apoptosis, and it also augments anti-carcinogenic properties in various cells [9,10,11,12]. The biological activity of TQ is related to the following pathways, such as NF-kB, STAT3, PI3K/AKT, p53, PPAR, and MAPK [10,11,12,13,14,15]. So far, there is not any literature available to report the systemic toxicities with TQ treatment in either human or animals [16,17,18]. Moreover, there is a limited literature available which notably explored the role of TQ in augmenting the anti-carcinogenic effects of DDP on EC. Hence, we utilized the opportunity to study the effect and underlying mechanism employed by TQ on the DDP-induced apoptosis and growth inhibition of EC.
Studies have documented that multiple signaling pathways participate in regulating the apoptosis and proliferation of tumor cells, like JAK/STAT pathway [10, 19]. Our previous study reported that TQ induced the significant antitumor effect in gastric cancer via JAK2/STAT3 signaling pathway [10]. Signal transducers and activators of transcription (STAT) proteins contained a family of transcription factors [20]. STAT3 is the prominent member of this family and a major cell survival signal in a variety of malignancies, including gastric cancer, ovarian cancer, lung cancer, diffuse large B cell lymphoma, and others [10, 21,22,23]. However, few studies outlined the relationship between STAT3 and tumor progression in EC. In our study, we investigated whether TQ could enhance the anti-carcinogenic effect of DDP on EC through blocking the activation of JAK2/STAT3 signaling pathway. WP1066 is a JAK2/STAT3 inhibitor which deactivates STAT3 by directly acting on Janus kinases (JAKs) [24]. Therefore, we used WP0166 to explore the mechanism employed by TQ and DDP on the proliferation and apoptosis of esophagus cancer cells.
Materials and Methods
Cell Culture
Human esophageal carcinoma Eca-109 cells were generously donated by China Center for Type Culture Collection (CCTCC). The cells were cultured in DMEM/F-12 (1:1) (HyClone) medium supplemented with heat-inactivated 10% fetal bovine serum (FBS) (GIBCO, USA). The cells were maintained in a humidified incubator at 37 °C and 5% CO2.
Reagents and Antibodies
Thymoquinone (TQ) (> 99% pure; molecular weight of 164.2) was purchased from Santa Cruz (Santa Cruz Biotechnology, Inc, USA). TQ was dissolved in 100% ethyl alcohol to yield a concentration of 10 mM stock solution, which was stored at − 20 °C. Cisplatin (DDP) was obtained from the pharmacy at Renmin Hospital of Wuhan University. The DDP stock solution was prepared at the concentration of 4 mg/ml and stored at − 20 °C.
Antibodies against STAT3, Phospho-Stat3 (Tyr705), JAK2, Phospho-Jak2 (Tyr1007/1008), Bax, Bcl-2, Cyclin D1, Survivin, cleaved caspase-3, cleaved caspase-9, cleaved caspase-7, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were purchased from Cell Signaling Technology (Danvers, MA, USA). The effective concentrations of all the antibodies were 1:1000. The secondary antibodies were obtained from LI-COR and diluted to 1:10,000. WP1066, JAK2/STAT3 inhibitor, was purchased from Selleckchem (Houston, TX, USA).
Cell Viability Assay In Vitro
Eca-109 cells viability and inhibition were evaluated by the Cell Counting Kit-8 assay (CCK-8; Beyotime, China). The cells were seeded in a 96-well microtiter plate at a density of 5 × 103 (100 μl) per well. After 24 h, the medium was replaced with TQ (20, 40, 80, 160, or 320 μM), DDP (3, 6, 12, 24, or 48 μg/ml), TQ (100 μM), DDP (10 μg/ml), or TQ + DDP (100 μM + 10 μg/ml). Subsequently, the cells were incubated for another 24 h at 37 °C with 5% CO2. Then, 10 μl CCK-8 dye was added to each well and cultured for an additional 2 h. Then optical density was measured at a wavelength of 450 nm using an iMark microplate reader (Victor3 1420 Multilabel Counter, PerkinElmer). GraphPad Prism software (GraphPad Software Inc. v.5.01) was employed to calculate the half maximal inhibitory concentration (IC50), and each experiment was repeated thrice to enhance the reliability.
Hoechst 33258 Assay for Apoptosis
Eca-109 cells were cultured in a sterile 6-well plate with DMEM for 24 h. Subsequently, TQ (100 μM), DDP (10 μg/ml), or TQ + DDP (100 μM + 10 μg/ml) was added for another 24 h. Then, the apoptosis of cells was detected by using Hoechst 33258 staining (Beyotime Institute of Biotechnology, Shanghai, China; C0003). Afterward, the cells were fixed in 0.5 ml methanol for 30 min and rinsed with phosphate-buffered saline (PBS) thrice. Next, the cells were incubated with 0.5 ml Hoechst 33258 dye for 10 min at room temperature in the dark and washed for three times. The apoptotic cells were identified by their morphological changes, such as chromatin condensation, nuclear fragmentation, and bright-blue fluorescent. In each group, ten random microscopic fields were examined and photographed under a fluorescence microscope (OLYMPUS, Japan). Subsequently, the apoptosis ratio was quantified.
Annexin V-PE/7-AAD Assay for Apoptosis
Eca-109 cells were grown in a germfree 6-well plate for 24 h at 37 °C. Then, the plate was supplemented with TQ (100 μM), DDP (10 μg/ml), or TQ + DDP (100 μM + 10 μg/ml) as described above. Then the percentage of apoptotic cells was evaluated by Annexin V-PE/7-AAD kit (BD. USA). Firstly, the adherent cells were harvested and rinsed twice with cold PBS of around 4 °C. Next, we resuspended the cells in binding buffer at a concentration of 1 × 106 cells/ml. Then, 100 μl of the cell suspension was transferred into a tube of 5 ml, followed by addition of 5 μl Annexin V-PE and 10 μl 7-AAD, and incubated in the dark for 15 min at room temperature. Subsequently, the tube was centrifuged, mixed with 200 μl of binding buffer before flow cytometric analysis (FACSCalibur, Becton-Dickinson).
Western Blot Analysis
All the proteins were extracted after 24 h treatment with TQ (100 μM), DDP (10 μg/ml), TQ + DDP (100 μM + 10 μg/ml), or TQ + DDP + WP1066 (100 μM + 10 μg/ml + 6 μM). Protein concentrations were quantified using bicinchoninic acid (BCA) Protein Assay Kit (Beyotime, China). Proteins were separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and were transferred onto polyvinylidene difluoride (PVDF) membrane (Millipore, Billerica, MA, USA). The membranes were blocked by 10% nonfat milk for 1.5 h at room temperature and incubated with various primary antibodies (STAT3, Phospho-Stat3, JAK2, Phospho-Jak2, Bax, Bcl-2, Cyclin D1, Survivin, cleaved caspase-3, cleaved caspase-9, cleaved caspase-7, and GAPDH, the concentrations were 1:1000) overnight at 4 °C. Then, the membranes were incubated with secondary antibodies for 1 h at the ambient temperature. Odyssey Infrared Imaging System (Odyssey, Lincoln, NE, USA) was utilized to visualize protein band intensity. GAPDH was used as a protein loading control.
Xenograft Tumor Model
All the procedures were approved by Institutional Animal Care and Use Committee and performed in compliance with National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. Male BALB/c nude mice, 5–6 weeks of age, were obtained from Beijing HFK Experimental Animal Center (Beijing, China). All the mice were randomly assigned into four groups, and there were six mice in each cluster. All housed in plexiglass cages in a temperature-controlled environment given autoclaved food, sterile water, and filtered air. All the mice were subcutaneously inoculated in the lower right flank with Eca-109 cells, which were harvested and resuspended in PBS. When the xenograft tumors reached the size of 80–120 mm3, the mice were intraperitoneally injected with normal saline, TQ, DDP, TQ + DDP thrice a week. The tumor volume (TV) was calibrated after every 3 days. TV was calculated using the formula: TV (mm3) = d 2 × D/2 (d and D are the shortest and longest diameters, respectively).
HE Staining and TUNEL Assay
The xenograft tumors were fixed in 4% paraformaldehyde for histological examination and to keep the tissue fresh. Subsequently, in series, the samples were dehydrated with an ethanol gradient, embedded in paraffin, dewaxed, and rehydrated with gradient ethanol. Then the tissues were examined by H&E staining and TUNEL technique. The positive cells were observed, counted, and analyzed under a light microscope (OLYMPUS, Japan).
Immunohistochemistry
The previously described paraffin sections were deparaffinized, rehydrated, and incubated with 3% hydrogen peroxide (H2O2) in methanol for 15 min at room temperature. Subsequently, the sections were blocked with 5% goat serum at ambient temperature for 10 min. Then, the sections were incubated with primary antibodies of STAT3 (1:600) and Phospho-STAT3 (1:100) overnight at 4 °C. Afterward, the samples were washed and incubated with secondary antibody for 30 min at room temperature. The sections were washed again and treated with a 3,3′-diaminobenzidine solution and counterstained with hematoxylin. Positive immunoreactivity was detected as a brown staining. The treated specimens were observed and analyzed by using an upright microscope (OLYMPUS, Japan).
Statistical Analysis
All the results were expressed as the mean ± standard deviation (SD) and analyzed with the unpaired Student’s t test. The P value was less than 0.05 (P < 0.05), which denoted statistically significant difference.
Results
TQ Sensitizes DDP-Mediated Growth Inhibition in Eca-109 Cell Line
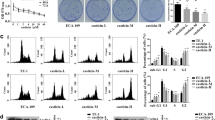
Exposure to TQ or DDP for 24 h clearly inhibited the viability of cells, in a dose-dependent manner. Figure 1 depicted the effect of each drug on the Eca-109 cell line at the TQ concentrations of 20 and 320 μM; the mean inhibition rate was 6.67 and 85.10%, respectively (Fig. 1a). Meanwhile, 3 μg/ml DDP had an inhibition rate of 26.39% and it approached to 83.69% at 48 μg/ml (Fig. 1b). The IC50 value of TQ and DDP in Eca-109 cells at 24 h were 100 μM and 10 μg/ml, respectively. To further verify whether TQ could enrich the DDP-induced apoptosis, these two drugs were admixed with Eca-109 cells for 24 h, which resulted in a remarkably reduced proliferation of Eca-109 cells in contrast to either TQ or DDP alone (P < 0.01) (Fig. 1c). Hence, the results suggested that TQ might sensitize DDP-mediated growth inhibition in Eca-109 cell line.
Effect of TQ or/and DDP on Eca-109 cell viability were evaluated by CCK-8. a Eca-109 cells were incubated with 20, 40, 80, 160, or 320 μM TQ for 24 h, b cells were treated with DDP for 24 h at several concentrations (3, 6, 12, 24, and 48 μg/ml), c cells were assessed with 100 μM TQ, 10 μg/ml DDP, and 100 μM TQ + 10 μg/ml DDP for 24 h. Compared with the control group, *P < 0.05; **P < 0.01
TQ Sensitizes DDP-Mediated Apoptosis in Eca-109 Cell Line
Hoechst 33258 staining was designed to detect the morphological alterations in Eca-109 cell line, and it exhibited the characteristics of apoptosis, such as cell shrinkage, nuclear fragmentation, and chromatin condensation. The results proved that the cells treated with TQ and DDP, either as a sole agent or in combination, showed significant apoptosis compared to the control group (P < 0.01). Furthermore, the rate of cell apoptosis was significantly higher in the combined treatment than individual drug (P < 0.01) (Fig. 2a).
Esophageal cancer cells were exposed to 100 μM TQ, 10 μg/ml DDP, and 100 μM TQ + 10 μg/ml DDP for 24 h, and then, apoptosis was detected. a Apoptosis was evaluated by Hoechst 33258 straining, and apoptotic features were identified by chromatin condensation, nuclear fragments; the arrow showed the apoptotic cell. Scale bar represents 50 μm. Original magnification: × 200. Data are represented as mean ± SE (n = 10 for each group) (*P < 0.05; **P < 0.01), b apoptosis was detected by Annexin V-PE/7-AAD kit. The rate of apoptosis in united group were significantly increased than respective groups (*P < 0.05; **P < 0.01)
Annexin V-PE/7-AAD staining was used to detect TQ and DDP-induced apoptosis. The proportion of apoptotic cells was distinct in the TQ or DDP group from the control group, whereas the apoptotic rate was remarkably higher with TQ plus DDP compared to either TQ or DDP (P < 0.01, respectively) (Fig. 2b).
TQ Sensitizes DDP-Mediated Eca-109 Cell Line Growth Suppression Through Blocking the Activation of JAK2/STAT3 Pathway
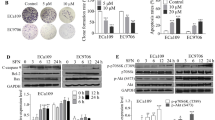
The western blot analysis was performed to elucidate the molecular mechanism involved in between STAT3 and TQ combined with DDP. The results indicated significantly decreased expression of p-STAT3 and p-JAK2 in the TQ with DDP group (P < 0.01) (Fig. 3a). Furthermore, Eca-109 cells treated with TQ and DDP resulted in the increase in proapoptotic protein (Bax, P < 0.01) and the decrease in antiapoptotic proteins (Bcl-2, Survivin; P < 0.01, P < 0.01), proliferative protein (Cyclin D1, P < 0.01) (Fig. 3b). Subsequently, mitochondrial-mediated apoptosis was initiated by activating caspase-3, 7, 9 (Fig. 3c).
TQ and DDP regulated the JAK2/STAT3 pathway in esophageal carcinoma. a Effects of TQ and DDP on the JAK2/STAT3 pathway were analyzed by Western blotting, b JAK2/STAT3 targets (Bax, Bcl-2, Cyclin D1, and Survivin) were assayed by western blotting, c caspase pathway was detected by western blotting. GAPDH was used as a loading control. Compared with the control group, *P < 0.05; **P < 0.01
WP1066 Inhibits the Growth of Eca-109 Cell Line Through Blocking the Activation of JAK2/STAT3 Pathway
To further confirm the relationship between the apoptosis of esophageal carcinoma cells and JAK2/STAT3 pathway, Eca-109 cells were treated with JAK2/STAT3 inhibitor (WP1066). Western blot analysis revealed that treatment with WP1066 decreased the expression of phosphorylated JAK2 and STAT3 (P < 0.01; P < 0.01) (Fig. 4a). Simultaneously, the expression levels of Bcl-2, Cyclin D1, and Survivin were markedly reduced after treatment of WP1066 in Eca-109 cells (P < 0.01) (Fig. 4b). All the results support the finding that TQ may enhance DDP-induced apoptosis of esophageal carcinoma cells through mitigating the activation of JAK2/STAT3 pathway.
Effect of WP1066 on the JAK2/STAT3 pathway. a The protein expression levels of JAK2/STAT3 pathway were evaluated by western blotting, b the pathway downstream targets (Bax, Bcl-2, Cyclin D1, and Survivin) were assessed by western blotting. GAPDH was used as an internal control. Compared with the control group, *P < 0.05; **P < 0.01
TQ Sensitizes DDP-Mediated Eca-109 Cell Line Growth Suppression In Vivo
The xenograft tumor model was used to elucidate the role of TQ in augmenting the antitumor effect of DDP. The data indicated that TQ and DDP administered individually or in combination presented potent inhibitory effects in vivo, which was exhibited by markedly diminished tumor volume and weight in the treatment groups (P < 0.01) (Fig. 5a). Furthermore, volume and weight were significantly lower in combined group than TQ or DDP alone group (P < 0.01) (Fig. 5a). In Fig. 5b, TUNEL staining suggested that efficient apoptosis occurred in the tumor mass from treatment groups, whereas the degree of apoptosis differed in each cluster. Also, the tumor of combined group exhibited a distinct apoptosis compared with the TQ or DDP (P < 0.01, respectively) (Fig. 5b). Furthermore, we evaluated the level of STAT3 in tumor tissue by immunohistochemical analysis, and the results revealed that the cell apoptosis inhibition of TQ was associated with STAT3 phosphorylation in vivo (Fig. 5c). What’s more, the ALT, AST, BUN, and Cr levels in the serum were measured to evaluate liver and renal injury and there were no significant variations among groups (P > 0.05; Table 1).
Antitumor effect of TQ and DDP in vivo. a Each time point represents the mean tumor volume for each group. Tumor weight was obtained at the end of the experiment. Error bars represent the standard error of the mean (SEM), b detection of apoptotic cells in tumor tissue by TUNEL assay. The apoptotic cells appeared with nut-brown nuclei is shown by the arrows, c STAT3 and p-STAT3 were detected by immunohistochemical staining. Scale bar represents 50 μm. Original magnification: × 400. Compared with the control group. *P < 0.05; **P < 0.01
Discussion
Esophageal carcinoma is one of the malignant tumors with an estimated 398,000 and 52,000 cases of SCCs and ACs, respectively, in 2012 [4]. In the coming decades, the incidence and mortality rate of EC are expected to rise, particularly in Asia [2]. It is hard to completely resect the tumor once the EC has progressed to late-stage or metastasized. Hence, chemotherapy remains the primary treatment. It is vital to develop novel natural drugs due to the resistance and severe side effects associated with the existing chemotherapeutic drugs [6, 7].
Few studies have confirmed the anticancer effect of TQ both in vitro and in vivo [13, 17, 18]. What’s more, TQ is not just limited to a potential anticancer drug, but also a chemosensitizer [9, 18, 25, 26]. Zhang and colleagues reported that TQ could serve as a booster for the anticancer effects of cisplatin in COLO205 and HCT116 cells [26]. TQ was reported to potentiate the antitumor activity of cisplatin, not only improved the therapeutic index in Ehrlich ascites carcinoma but also reduced the toxic effects [27]. However, whether TQ exerts chemosensitive effect and the potential mechanism on carcinoma have not been clarified yet. In our study, the results indicated that TQ might inhibit proliferation and enhance DDP-induced apoptosis in esophagus cancer via suppressing the activation of JAK2/STAT3 pathway.
STAT3 is well known for its critical role in the development and progression of various cancers. Increasing evidence indicates that JAK2/STAT3 pathway is involved in a great number of tumors [9, 28, 29]. STAT3 transcription factor could be activated via a variety of ways, like JAK2 [19, 30]. When JAK2 is activated by cytokine via its combination to JAK2 receptor, the phosphorylated Y residues will become STAT3 proteins docking elements. STAT3 is phosphorylated on a single Y residue on the receptor to form the heterodimers or homodimers, which will translocate into nuclear and bind DNA, then regulate many essential genes, such as Bax, Cyclin D1, Survivin, Bcl-2 related to cell survival and proliferation [20]. Cyclin D1 associated with STAT3 can form a transcriptional repressor complex which will arrest the cell cycle. [31]. Moreover, the anti-apoptosis protein prevents the release of mitochondrial Cyt-c and restrains the activity of caspase-3, 7, 9, then inhibits mitochondria-dependent cell death [32,33,34].
In this study, we adopted a novel chemotherapeutic strategy to confirm the chemosensitization of TQ combined with DDP in EC in vitro and in vivo through blocking the activation of JAK2/STAT3 pathway and to assess the side effects of it. We found that TQ combined with DDP could inhibit cell proliferation and was superior to using them alone to inhibit the Eca-109 cells growth in vitro and in vivo, without obvious increase in adverse effects.
We found that TQ inhibited the growth of esophageal carcinoma cells in a dose-dependent fashion, and the inhibitory effect of TQ combined with DDP was more distinct. Meanwhile, we also found that TQ could boost DDP-induced apoptosis in esophageal carcinoma cells, which was evaluated by Hoechst 33258 staining and Annexin V-PE/7-AAD staining. Apoptosis plays a significant role in antitumor activity in various therapeutic strategies, including TQ induced chemotherapy [35]. However, particular mechanism of Eca-109 cells apoptosis induced by TQ with DDP has not been researched clearly. JAK2/STAT3 pathway has been demonstrated as a critical signaling pathway in carcinogenesis [36]. In order to determine whether TQ and DDP could induce apoptosis through suppressing the activation of JAK2/STAT3 pathway, we examined the upstream protein kinases JAK2 and revealed that TQ along with DDP inhibited the phosphorylation of JAK2, so presumably the restraint of STAT3 phosphorylation was due to the inhibition of JAK2 activity. Moreover, we detected STAT3-regulated mitochondrial apoptotic pathway and found that the treatment of TQ with DDP increased the proapoptotic protein (Bax) and decreased the antiapoptotic proteins (Bcl-2, Survivin), proliferative protein (Cyclin D1), and caspase-3, 7, 9 proteins. In addition, when pretreated with WP1066, we found that the levels of p-JAK2, p-STAT3, Cyclin D1, Bcl-2, and Survivin were decreased. So, it could be considered that TQ may augment DDP-induced apoptosis via suppressing the activation of JAK2/STAT3 pathway, and p-STAT3 may change the expression of Bcl-2, Bax, Cyclin D1, Survivin, then activate caspase-3, 7, 9 and stimulate the caspase-dependent pathway induced apoptosis [37].
The in vivo studies further support the hypothesis that TQ could be considered as a adjunctive treatment with DDP. The therapeutic effect of TQ was confirmed by the TUNEL staining of tumor sections, which presented remarkable cell apoptosis in tumor mass. Furthermore, the expression of p-STAT3 was decreased after the treatment of TQ with DDP.
In conclusion, our study suggests that TQ sensitizes DDP-induced anticancer effect in esophageal carcinoma cells via blocking the activation of JAK2/STAT3 pathway. This study suggests the potential usefulness of thymoquinone, a safe and chemosensitized antitumor agent, in the management of esophageal carcinoma. However, some points remain unclear: How does thymoquinone with cisplatin induce the decrease in levels of p-STAT3, p-JAK2, and the abnormal expressions of Bax, Bcl-2, Cyclin D1? What is the precise relationship between JAK2/STAT3 and Bax, Bcl-2, Cyclin D1? These issues should be addressed in the future.
References
Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386.
Malhotra GK, Yanala U, Ravipati A, et al. Global trends in esophageal cancer. J Surg Oncol. 2017;115:564–579.
Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med. 2014;371:2499–2509.
Arnold M, Soerjomataram I, Ferlay J, Forman D. Global incidence of oesophageal cancer by histological subtype in 2012. Gut. 2015;64:381–387.
Society AC. Cancer Facts & Figure 2016. Atlanta: American Cancer Society; 2016. pp. 1–66.
So B, Marcu L, Olver I, Gowda R, Bezak E. Oesophageal cancer: which treatment is the easiest to swallow? A review of combined modality treatments for resectable carcinomas. Crit Rev Oncol Hematol. 2017;113:135–150.
Zhou XL, Wang WW, Zhu WG, et al. High expression of long non-coding RNA AFAP1-AS1 predicts chemoradioresistance and poor prognosis in patients with esophageal squamous cell carcinoma treated with definitive chemoradiotherapy. Mol Carcinog. 2016;55:2095–2105.
Worthen DR, Ghosheh OA, Crooks PA. The in vitro anti-tumor activity of some crude and purified components of blackseed, Nigella sativa L. Anticancer Res. 1998;18:1527–1532.
Lei X, Lv X, Liu M, et al. Thymoquinone inhibits growth and augments 5-fluorouracil-induced apoptosis in gastric cancer cells both in vitro and in vivo. Biochem Biophys Res Commun. 2012;417:864–868.
Zhu WQ, Wang J, Guo XF, et al. Thymoquinone inhibits proliferation in gastric cancer via the STAT3 pathway in vivo and in vitro. World J Gastroenterol. 2016;22:4149–4159.
Hsu HH, Chen MC, Day CH, et al. Thymoquinone suppresses migration of LoVo human colon cancer cells by reducing prostaglandin E2 induced COX-2 activation. World J Gastroenterol. 2017;23:1171–1179.
Hossen MJ, Yang WS, Kim D, et al. Thymoquinone: an IRAK1 inhibitor with in vivo and in vitro anti-inflammatory activities. Sci Rep. 2017;7:42995.
Linjawi SA, Khalil WK, Hassanane MM, Ahmed ES. Evaluation of the protective effect of Nigella sativa extract and its primary active component thymoquinone against DMBA-induced breast cancer in female rats. Arch Med Sci. 2015;11:220–229.
Yu SM, Kim SJ. Thymoquinone-induced reactive oxygen species causes apoptosis of chondrocytes via PI3K/Akt and p38kinase pathway. Exp Biol Med (Maywood). 2013;238:811–820.
Woo CC, Loo SY, Gee V, et al. Anticancer activity of thymoquinone in breast cancer cells: possible involvement of PPAR-gamma pathway. Biochem Pharmacol. 2011;82:464–475.
Hosseinzadeh H, Parvardeh S. Anticonvulsant effects of thymoquinone, the major constituent of Nigella sativa seeds, in mice. Phytomedicine. 2004;11:56–64.
Abukhader MM. Thymoquinone in the clinical treatment of cancer: fact or fiction? Pharmacogn Rev. 2013;7:117–120.
Attoub S, Sperandio O, Raza H, et al. Thymoquinone as an anticancer agent: evidence from inhibition of cancer cells viability and invasion in vitro and tumor growth in vivo. Fundam Clin Pharmacol. 2013;27:557–569.
Cafferkey C, Chau I. Novel STAT 3 inhibitors for treating gastric cancer. Expert Opin Investig Drugs. 2016;25:1023–1031.
Ihle JN. The Stat family in cytokine signaling. Curr Opin Cell Biol. 2001;13:211–217.
Kim B, Kim HS, Kim S, et al. Adipose stromal cells from visceral and subcutaneous fat facilitate migration of ovarian cancer cells via IL-6/JAK2/STAT3 pathway. Cancer Res Treat. 2017;49:338–349.
Jiang J, Liu Y, Tang Y, et al. ALDH1A1 induces resistance to CHOP in diffuse large B-cell lymphoma through activation of the JAK2/STAT3 pathway. Onco Targets Ther. 2016;9:5349–5360.
Iriki T, Ohnishi K, Fujiwara Y, et al. The cell-cell interaction between tumor-associated macrophages and small cell lung cancer cells is involved in tumor progression via STAT3 activation. Lung Cancer. 2017;106:22–32.
Honda S, Sadatomi D, Yamamura Y, Nakashioya K, Tanimura S, Takeda K. WP1066 suppresses macrophage cell death induced by inflammasome agonists independently of its inhibitory effect on STAT3. Cancer Sci. 2017;108:520–527.
Banerjee S, Kaseb AO, Wang Z, et al. Antitumor activity of gemcitabine and oxaliplatin is augmented by thymoquinone in pancreatic cancer. Cancer Res. 2009;69:5575–5583.
Zhang L, Bai Y, Yang Y. Thymoquinone chemosensitizes colon cancer cells through inhibition of NF-κB. Oncol Lett. 2016;12:2840–2845.
Badary OA, Nagi MN, al-Shabanah OA, al-Sawaf HA, al-Sohaibani MO, al-Bekairi AM. Thymoquinone ameliorates the nephrotoxicity induced by cisplatin in rodents and potentiates its antitumor activity. Can J Physiol Pharmacol. 1997;75:1356–1361.
Wu J, Guo J, Cao Q, et al. Autophagy impacts on oxaliplatin-induced hepatocarcinoma apoptosis via the IL-17/IL-17R-JAK2/STAT3 signaling pathway. Oncol Lett. 2017;13:770–776.
Huang C, Yu Y. Synergistic cytotoxicity of beta-Elemene and cisplatin in gingival squamous cell carcinoma by inhibition of STAT3 signaling pathway. Med Sci Monit. 2017;23:1507–1513.
Xiong A, Yang Z, Shen Y, Zhou J, Shen Q. Transcription factor STAT3 as a novel molecular target for cancer prevention. Cancers (Basel). 2014;6:926–957.
Barré B, Vigneron A, Perkins N, Roninson IB, Gamelin E, Coqueret O. The STAT3 oncogene as a predictive marker of drug resistance. Trends Mol Med. 2007;13:4–11.
Bromberg JF, Wrzeszczynska MH, Devgan G, et al. Stat3 as an oncogene. Cell. 1999;98:295–303.
Chen M, Wang J. Initiator caspases in apoptosis signaling pathways. Apoptosis, 7, 313-9. Apoptosis. 2002;7:313–319.
Green DR. Apoptotic pathways: ten minutes to dead. Cell. 2005;121:671–674.
Ashour AE, Abd-Allah AR, Korashy HM, et al. Thymoquinone suppression of the human hepatocellular carcinoma cell growth involves inhibition of IL-8 expression, elevated levels of TRAIL receptors, oxidative stress and apoptosis. Mol Cell Biochem. 2014;389:85.
Wu KJ, Huang JM, Zhong HJ, et al. A natural product-like JAK2/STAT3 inhibitor induces apoptosis of malignant melanoma cells. PLoS ONE. 2017;12:e0177123.
Halestrap AP, Doran E, Gillespie JP, O’Toole A. Mitochondria and cell death. Biochem Soc Trans. 2000;28:170–177.
Acknowledgments
This study was supported by Grants from the National Natural Science Foundation from China (nos. 81372551, 81572426) and Natural Science Foundation Grant of Hubei Province (No. 2015CKB755).
Author information
Authors and Affiliations
Contributions
Author’s contribution
XH, JM and WD designed the research; XH, JL, DW, and YL performed the research; XH and JZ analyzed data; XH and VV wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Hu, X., Ma, J., Vikash, V. et al. Thymoquinone Augments Cisplatin-Induced Apoptosis on Esophageal Carcinoma Through Mitigating the Activation of JAK2/STAT3 Pathway. Dig Dis Sci 63, 126–134 (2018). https://doi.org/10.1007/s10620-017-4856-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-017-4856-8