Abstract
Background
Disparities in receipt of hepatocellular carcinoma (HCC) surveillance contribute to disparities in overall survival outcomes.
Aim
We aim to evaluate disparities in receipt of routine HCC surveillance among patients with cirrhosis in a large urban safety-net hospital.
Methods
Consecutive adults (age ≥ 18) with cirrhosis from July 1, 2014, to December 31, 2015, were retrospectively evaluated to determine rates of receiving appropriate HCC surveillance within 6 months and 1 year after diagnosis of cirrhosis. Rates of HCC surveillance were stratified by sex, race/ethnicity, and liver disease etiology. Multivariate Cox proportional hazards models were utilized to evaluate for predictors of receiving appropriate HCC surveillance.
Results
Among 157 cirrhosis patients enrolled [hepatitis C virus (HCV): 29.9%, hepatitis B virus: 13.4%, alcoholic cirrhosis: 44.6%, nonalcoholic steatohepatitis (NASH): 8.9%], mean age of cirrhosis diagnosis was 53.8 ± 9.0 years. Among these patients, 49% received (n = 77) HCC surveillance within 6 months and 78% (n = 123) were surveyed within 1 year of cirrhosis diagnosis. On multivariate analyses, patients with NASH cirrhosis were significantly less likely to receive HCC surveillance compared with chronic HCV cirrhosis patients (HR 0.44, 95% CI 0.19–0.99, p < 0.05). No significant sex-specific or race/ethnicity-specific disparities in receipt of HCC surveillance were observed.
Conclusion
Among a diverse safety-net hospital population, sub-optimal HCC surveillance rates were observed: Only 49% of cirrhosis patients received HCC surveillance within 6 months, and 78% of cirrhosis patients received HCC surveillance within 1 year. Differences in rates of HCC screening by liver disease etiology were observed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the second leading cause of cancer mortality worldwide [1]. HCC accounts for roughly 85–90% of all primary liver cancers. Each year, approximately half a million people worldwide are newly diagnosed with HCC. In the USA, HCC is currently the fastest growing cause of cancer-related deaths [2]. HCC has a very poor prognosis with 5-year overall survival rate of less than 30% [3, 4]. However, prognosis depends on tumor stage, with curative therapies available for patients who are detected at an early stage [5]. A 2014 meta-analysis by Singal et al. evaluated 15,158 patients with cirrhosis, of whom 6284 (41.4%) had HCC detected by surveillance. The study demonstrated that HCC surveillance was associated with improved early stage detection [odds ratio (OR) 2.08, 95% CI 1.80–2.37] and curative treatment rates (OR 2.24, 95% CI 1.99–2.52) as well as significantly prolonged survival (OR 1.90, 95% CI 1.67–2.17) even after adjusting for lead-time bias [6]. AASLD guidelines recommend HCC surveillance for cirrhotic patients and high-risk chronic HBV patients. Patients at high risk for developing HCC should be entered into surveillance programs. Surveillance for HCC should be performed using ultrasonography. The recommended interval between HCC surveillance tests is 6–12 months [7, 8]. The interval is based on the median doubling time of HCC, which is estimated to range between 80 and 117 days [9, 10]. Despite these recommendations, surveillance rates still remain low, with rates varying from 50% to less than 20% [11,12,13].
Early detection through routine HCC surveillance affords greater treatment options, thereby improving survival outcomes. Variations in receipt of HCC surveillance contribute to disparities in overall HCC survival. In this study, we aim to evaluate disparities in receipt of routine HCC screening among patients with cirrhosis in a large urban safety-net hospital system.
Methods
We retrospectively evaluated all consecutive adults (age ≥ 18) with cirrhosis from July 1, 2014, to December 31, 2015, seen in gastroenterology/hepatology clinics to determine the rates of receiving appropriate HCC surveillance within 6 months and 1 year after the diagnosis of cirrhosis at a large urban safety-net hospital. Our cohort is particularly unique in that the majority of this underserved population lives at or below the national poverty level and the majority are non-White minorities. The diagnosis of cirrhosis was determined through a thorough review of the electronic medical records and incorporated data from the clinical history from inpatient and outpatient chart notes, laboratory data, radiographic data, and liver biopsy data if available. Our diagnosis was not based on diagnosis coding or billing coding, but rather based on thorough chart review by a clinician to interpret and identify the diagnosis of cirrhosis. Based on this compilation of data, we estimated the earliest date at which cirrhosis was diagnosed for each patient. In a similar fashion, we identified the age at first gastroenterology/hepatology clinical encounter, and time from cirrhosis diagnosis to first receipt of HCC screening. Child–Pugh score and MELD score were calculated based on available clinical and laboratory data. Appropriate HCC surveillance was defined as undergoing imaging for evaluation of liver mass via ultrasound, computed tomography (CT), or magnet resonance imaging (MRI) as indicated in the clinical notes and radiography reports.
Overall rates of appropriate HCC surveillance were presented as proportions and frequencies, and additional comparisons between groups were performed using Chi-square testing and stratified by sex, race/ethnicity, and liver disease etiology. Continuous variables were presented as mean and standard deviation. Multivariate logistic regression models were utilized to evaluate for predictors of receiving appropriate HCC surveillance. Statistical analyses were performed using Stata statistical software package (version 13.0), and statistical significance was met with p value <0.05. This study was approved by Alameda Health System Institutional Review Board.
Results
Among 157 cirrhosis patients enrolled in the study, the mean (±SD) age of cirrhosis diagnosis was 53.8 ± 9.0 years (Table 1). The study consisted of 65% males (n = 102). When stratified by race/ethnicity, 18% of the population consisted of non-Hispanic White, 22% African-American/Black, 20% Asian, 34% Hispanic, and 5% other race/ethnicity. The etiology of cirrhosis was hepatitis C virus (HCV): 29.9%, hepatitis B virus (HBV): 13.4%, alcoholic cirrhosis: 44.6%, nonalcoholic steatohepatitis (NASH): 8.9%, and other etiologies 3%. Ascites was present in 47% (n = 68) of patients at time of first gastroenterology encounter, history of hepatic encephalopathy was present in 45% (n = 63), and esophageal varices were present in 54% (n = 72). HCC was present in 17% (n = 23) of the patients at time of presentation. 30% (n = 43) had diabetes mellitus, and 40% (n = 57) had hypertension. Among all cirrhosis patients, liver disease severity breakdown included Child–Pugh A: 50% (n = 76), Child–Pugh B: 35% (n = 53), and Child–Pugh C: 15% (n = 22). Mean MELD score was 9.4 ± 5.2 (Table 1).
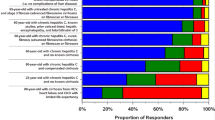
Among these patients, 49% received HCC surveillance within 6 months and 78% were surveyed within 1 year of cirrhosis diagnosis. No significant difference in receipt of HCC surveillance within 1 year was observed when stratified by sex (females: 76% vs. males: 79%, p = 0.66) or race/ethnicity (White non-Hispanic: 72%, African-American/Black: 83%, Asian: 81%, and Hispanic: 74%, p = 0.39). However, when stratified by etiology of liver disease, significant disparities were observed. Patients with NASH cirrhosis were significantly less likely to receive HCC surveillance when compared to patients with HCV, HBV, and alcoholic cirrhosis (HCC screening rates stratified by diagnosis: HCV 77%, HBV 86%, alcoholic cirrhosis 84%, and NASH 50%, p < 0.05) (Fig. 1). However, this difference observed was based on a small sample size of NASH patients.
The mean time from cirrhosis diagnosis to first HCC surveillance was 255 ± 411 days for the combined cohort. There was no statistically significant difference in the time from cirrhosis diagnosis to receipt of HCC surveillance when stratified by sex, race/ethnicity, or etiology of cirrhosis diagnosis (Table 2). No significant differences were observed when stratified by presence of ascites, hepatic encephalopathy, or esophageal varices (Table 2).
On multivariate analyses (Fig. 2), patients with NASH cirrhosis were significantly less likely to receive HCC surveillance compared to chronic HCV cirrhosis patients (HR 0.445, 95% CI 0.19–0.99, p < 0.05), whereas patients with ascites were significantly more likely to be surveyed compared to patients without ascites (HR 1.55, 95% CI 1.04–2.31, p < 0.05). No significant sex-specific or race/ethnicity-specific disparities in receipt of HCC surveillance were observed.
Discussion
Hepatocellular carcinoma is a leading cause of cancer-related death worldwide, and the burden of this devastating disease is expected to continue rising [14]. Despite AASLD recommendations for routine HCC surveillance as well as data supporting improved survival with routine surveillance [6], overall HCC screening rates throughout the USA remain sub-optimal.
In our study, among a diverse safety-net hospital population, sub-optimal HCC surveillance rates were observed. This study observed that 49% (n = 77) of cirrhotic patients received HCC surveillance within 6 months and 78% (n = 123) received surveillance within 1 year of cirrhosis diagnosis. Our findings are in line with previous reports that HCC surveillance in cirrhotic patients is sub-optimal [11, 12, 15, 16]. In 2011, AASLD and EASL established guidelines recommending ultrasound imaging of cirrhotic patients every 6–12 months [7, 8, 17]. Our study cohort is at particular high risk of not receiving appropriate surveillance as per these guidelines given the safety-net population, including higher proportion of non-English speaking minorities and lower socioeconomic status, both of which contribute to barriers in timely access to appropriate medical care. However, all of these patients made contact with subspecialty gastroenterology/hepatology clinics in our system, and this may have selected for a more engaged population. Furthermore, we have 4 full time clinic providers in our gastroenterology clinics which have very similar practice patterns, ensuring consistency of HCC surveillance recommendations for our patients. An informal survey of our providers demonstrates strict adherence to AASLD guidelines for HCC surveillance. However, it should also be noted that actual HCC surveillance rates may be significantly lower than our observations. As previously stated, the study cohort included patients that were linked to GI clinic and thus may be more engaged into care. It is possible that cirrhosis is under diagnosed among our safety-net population, and these undiagnosed cirrhosis patients are also missing much needed HCC surveillance. While our chart review attempted to best identify imaging exams that were specifically ordered for HCC surveillance, it is also possible that some tests were ordered for other reasons (e.g., evaluation of ascites) and if not for these other reasons, HCC surveillance may not have been performed.
Our study also demonstrates that patients with NASH cirrhosis were significantly less likely to receive HCC surveillance compared with chronic HCV cirrhosis patients (HR 0.445, 95% CI 0.19–0.99, p < 0.05). However, these observations among the NASH patients were based on relatively small sample size and need to be interpreted in this context. Our cohort included 14 patients with NASH cirrhosis with only 50% (n = 7) receiving appropriate HCC surveillance. Of the 7 patients who did not receive timely surveillance, 3 patients had surveillance imaging ordered but did not complete the study, 2 patients did not follow-up in GI clinic after the initial GI consult, 1 patient had multiple admissions for variceal bleed and HCC surveillance was never ordered, and 1 patient did not have surveillance imaging ordered until 1.5 years after cirrhosis diagnosis. A 2015 study by Mittal et al. [18] showed that a significantly higher proportion of patients with nonalcoholic fatty liver disease (NAFLD)-related HCC did not receive HCC surveillance in the 3 years before their HCC diagnosis, compared to patients with alcohol or HCV-associated HCC. These observations were noted among a cohort of 1500 patients who developed HCC from 2005 to 2010 within the Veterans Affairs Healthcare System. NAFLD diagnosis was based on histological evidence or presence of metabolic syndrome in the absence of HCV, HBV, or alcoholic liver disease. Patwardhan, et al. analyzed patients with cirrhosis in outpatient gastroenterology and primary care settings and found that patients with NASH cirrhosis were less likely to receive surveillance recommendations compared with cirrhosis from viral hepatitis, alcoholic cirrhosis, or nonviral, nonalcoholic, non-NASH cirrhosis. They also observed that NASH patients were less likely to receive gastroenterology clinic referrals [15].
While we demonstrated similar findings, observing these disparities among our specific population distinguishes it from prior studies. Specifically, while our cirrhosis cohort was in some ways more engaged into care given that all were seen and evaluated by gastroenterology/hepatology providers, our cohort consisted of a diverse group of patients with a high percentage of non-English speaking minorities and patients living at or below the poverty line. Furthermore, there are inherent barriers in timely access to medical care among our safety-net population that result from patient-specific factors (e.g., lack of education and awareness of the importance of cirrhosis follow-up, difficult with public transportation to make it to appointments), provider-specific factors (e.g., inadequate clinic time to address all medical issues requiring need to prioritize some items or others), and system-level factors (e.g., patient’s difficulty in reaching clinic staff and providers, hospital-centered localization of specialty care such that patients need to travel significant distances from their home and from their primary care providers to access specialty care and/or diagnostic imaging (e.g., HCC surveillance). A recent study by Goldberg et al. retrospectively looked at identifying barriers to HCC surveillance using the Veterans Health Administration with a primary outcome of a continuous measure of the percentage of time up-to-date with HCC surveillance based on abdominal ultrasound. They concluded the strongest predictor of HCC surveillance was the number of specialist visits within the first year of cirrhosis diagnosis as well as lead time of appointments and surveillance tests. They also observed patients with alcohol and NAFLD cirrhosis to have lower rates of surveillance compared to HCV cirrhosis [19]. In a large behavioral survey, Farvardin et al. explored the patient’s perspective regarding factors that pose barriers to HCC surveillance in a socioeconomically disadvantaged cohort of patients who are at risk for HCC. Nearly half of the patients reported barriers to receiving HCC surveillance including difficulties in scheduling, cost of tests, as well as transportation [20].
This observed trend of lower rates of HCC surveillance among NASH cirrhosis patients is concerning given the rising prevalence of NAFLD along with the growing obesity epidemic [21]. It is estimated that between 75 and 100 million individuals in the USA have NAFLD, and this large disease burden may translate into major consequences for the future HCC burden in the USA [22,23,24]. Furthermore, Younossi, et al. demonstrated that patients with NAFLD-related HCC have a worse prognosis compared to patients with HCC secondary to HCV, HBV, and alcoholic liver disease. NAFLD-HCC was associated with shorter survival time, more advanced tumor stage, and lower probability of receiving liver transplant [25]. However, as previously stated, our observations were based on a small sample size of NASH patients and thus must be interpreted with caution.
The absence of reliable serologic biomarkers to diagnose NASH combined with the perception that NAFLD is a benign and indolent disease may contribute to the low rates of awareness and implementation of timely HCC surveillance in NASH cirrhotic patients. Another factor that may challenge the success of HCC surveillance for early detection and treatment in patients with NASH cirrhosis relates to the underlying risk factors that contribute to NASH. Obesity and metabolic syndrome while increasing the risk of developing NASH may also limit the sensitivity of current imaging modalities for accurate HCC detection. A study in 2014 by Del Poggio et al. [26] observed that a body mass index (BMI) >25 kg/m2 was associated with a high degree of surveillance failure when using ultrasound. This may be particularly concerning for the subset of patients with central obesity. Although highly sensitive and specific biomarkers for NASH are lacking, alpha-fetoprotein (AFP) may be useful in conjugation with ultrasound for HCC surveillance in patients with NASH cirrhosis. In 2013, Gopal et al. [27] demonstrated the AFP better sensitivity and specificity for HCC detection in NAFLD patients with cirrhosis compared to HCV patients with cirrhosis (sensitivity 89.7% and specificity 85.1%). However, current AASLD guidelines do not recommend routine evaluation of AFP for HCC surveillance in all patients.
While our study distinguishes itself with the inclusion of an underserved safety-net cohort with a large proportion of non-English speaking ethnic minorities, we also specifically evaluated patients that were seen and established in gastroenterology and hepatology clinics. This attempted to identify the most engaged patients such that we could evaluate a “best case” scenario among our patient population that already faces multiple systems-level and socioeconomic barriers in timely access to medical care. Even so, our low rates of successful completion of HCC surveillance are alarming. There are certain limitations to our study that need to be acknowledged. Our small sample size, specifically among NASH patients should be noted, and thus, our NASH-related observations regarding HCC surveillance need to be interpreted with caution. Our calculation of appropriate HCC surveillance was based on review of medical records, including radiology reports within our hospital system. It is possible that patients received HCC surveillance at other facilities that were not reported in our medical records, which could have contributed to lower than actual rates of HCC surveillance. However, this scenario is less likely, given that if patients were to receive HCC surveillance at other facilities, this would have been noted in the clinical encounter notes, which were reviewed. Furthermore, our hospital system serves as the primary source of medical care for the majority of our underserved patients, and thus, it is unlikely that routine care including HCC surveillance would have been completed at other hospital systems outside of our network. It is also possible that our date of cirrhosis diagnosis may not have captured the earliest point at which a patient had cirrhosis, and patients may have had cirrhosis for a significant period prior to presentation to care. However, our assumptions of cirrhosis diagnosis are biased toward the null hypothesis and if in fact patients had cirrhosis earlier than we had identified, the magnitude of our findings would have been even more concerning. In addition, our ascertainment of data such as comorbidities or concurrent high-risk behavior (e.g., alcohol use) was based on retrospective review of medical records, and no attempt was made to contact patients directly to confirm any details, and thus, potential underreporting or misreporting of data could have occurred.
In summary, among a cohort of underserved, ethnically diverse, safety-net patients with cirrhosis that have established care with gastroenterology/hepatology specialists, sub-optimal rates of HCC surveillance were observed, with less than half receiving HCC surveillance within 6 months of cirrhosis diagnosis. Particularly concerning, patients with NASH cirrhosis were significantly less likely to receive timely HCC surveillance compared to patients with HCV cirrhosis, an alarming observation given the rising rates of obesity and metabolic syndrome in USA that contribute to rising rates of NAFLD and NASH. Given the importance of early detection of HCC, which improves treatment options and overall HCC survival, more awareness is needed among both patients and providers about the need for routine HCC surveillance among NASH patients with cirrhosis. Furthermore, better diagnostic tools, both radiographic and serological biomarkers, are needed to improve the sensitivity and specificity of HCC surveillance programs.
Abbreviations
- AASLD:
-
American association for the study of liver disease
- AFP:
-
Alpha-fetoprotein
- EASL:
-
European association for the study of the liver
- HBV:
-
Hepatitis B virus
- HCC:
-
Hepatocellular carcinoma
- HCV:
-
Hepatitis C virus
- MELD:
-
Model for end stage liver disease
- NAFLD:
-
Nonalcoholic fatty liver disease
- NASH:
-
Nonalcoholic steatohepatitis
References
Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol.. 2001;2:533–543.
El-Serag HB. Hepatocellular carcinoma. N Engl J Med.. 2011;365:1118–1127.
Liu JH, Chen PW, Asch SM, Busuttil RW, Ko CY. Surgery for hepatocellular carcinoma: does it improve survival? Ann Surg Oncol.. 2004;11:298–303.
Njei B, Rotman Y, Ditah I, Lim JK. Emerging trends in hepatocellular carcinoma incidence and mortality. Hepatology. 2015;61:191–199.
Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35:519–524.
Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med.. 2014;11:e1001624.
Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236.
European Association For The Study Of The Liver. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943.
Kubota K, Ina H, Okada Y, Irie T. Growth rate of primary single hepatocellular carcinoma: determining optimal screening interval with contrast enhanced computed tomography. Dig Dis Sci. 2003;48:581–586. doi:10.1023/A:1022505203786.
Okada S, Okazaki N, Nose H, et al. Follow-up examination schedule of postoperative HCC patients based on tumor volume doubling time. Hepatogastroenterology. 1993;40:311–315.
Leykum LK, El-Serag HB, Cornell J, Papadopoulos KP. Screening for hepatocellular carcinoma among veterans with hepatitis C on disease stage, treatment received, and survival. Clin Gastroenterol Hepatol.. 2007;5:508–512.
Davila JA, Morgan RO, Richardson PA, Du XL, McGlynn KA, El-Serag HB. Use of surveillance for hepatocellular carcinoma among patients with cirrhosis in the United States. Hepatology. 2010;52:132–141.
Davila JA, Weston A, Smalley W, El-Serag HB. Utilization of screening for hepatocellular carcinoma in the United States. J Clin Gastroenterol.. 2007;41:777–782.
Venook AP, Papandreou C, Furuse J, de Guevara LL. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist. 2010;15:5–13.
Patwardhan V, Paul S, Corey KE, et al. Hepatocellular carcinoma screening rates vary by etiology of cirrhosis and specialist involvement. Dig Dis Sci. 2011;56:3316–3322. doi:10.1007/s10620-011-1836-2.
Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol.. 2004;130:417–422.
Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022.
Mittal S, Sada YH, El-Serag HB, et al. Temporal trends of nonalcoholic fatty liver disease-related hepatocellular carcinoma in the veteran affairs population. Clin Gastroenterol Hepatol.. 2015;13:e591.
Goldberg DS, Taddei TH, Serper M, et al. Identifying barriers to hepatocellular carcinoma surveillance in a national sample of patients with cirrhosis. Hepatology. 2017;65:864–874.
Farvardin S, Patel J, Khambaty M, et al. Patient-reported barriers are associated with lower hepatocellular carcinoma surveillance rates in patients with cirrhosis. Hepatology. 2017;65:875–884.
Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in adults. Aliment Pharmacol Ther.. 2011;34:274–285.
Younossi ZM, Reyes MJ, Mishra A, Mehta R, Henry L. Systematic review with meta-analysis: nonalcoholic steatohepatitis: a case for personalised treatment based on pathogenic targets. Aliment Pharmacol Ther.. 2014;39:3–14.
Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–2023.
Rinella ME. Nonalcoholic fatty liver disease: a systematic review. Jama. 2015;313:2263–2273.
Younossi ZM, Otgonsuren M, Henry L, et al. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology. 2015;62:1723–1730.
Del Poggio P, Olmi S, Ciccarese F, et al. Factors that affect efficacy of ultrasound surveillance for early stage hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol.. 2014;12:1927–1933.
Gopal P, Yopp AC, Waljee AK, et al. Factors that affect accuracy of alpha-fetoprotein test in detection of hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol.. 2014;12:870–877.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Hesam Tavakoli: None. Ann Robinson: None. Benny Liu: None. Taft Bhuket: None. Zobair Younossi: Consultant—Gilead, Abbvie, Bristol Myers Squibb, Glaxo Smith Kline, Tobira. Sammy Saab: Consultant and speaker’s bureau—Gilead and Bristol Myers Squibb. Aijaz Ahmed: Consultant, advisory board, and research grants—Gilead. Robert Wong: Consultant, advisory board, research grants, and speaker’s bureau—Gilead.
Rights and permissions
About this article
Cite this article
Tavakoli, H., Robinson, A., Liu, B. et al. Cirrhosis Patients with Nonalcoholic Steatohepatitis Are Significantly Less Likely to Receive Surveillance for Hepatocellular Carcinoma. Dig Dis Sci 62, 2174–2181 (2017). https://doi.org/10.1007/s10620-017-4595-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-017-4595-x