Abstract
Background
The real impact of anti-tumor necrosis alpha (TNF) therapy in postoperative complications after intestinal resections in Crohn’s disease (CD) still needs to be determined.
Aims
To compare the postoperative complication rates after elective intestinal resections in CD patients, with or without previous exposure to anti-TNF therapy.
Methods
This was a retrospective and observational study, with elective intestinal resections for CD (emergency procedures were excluded). Patients were allocated in two groups according to preoperative anti-TNF status. Surgical and medical complications were analyzed and subsequently compared between the groups.
Results
A total of 123 patients were included (71 with and 52 without preoperative anti-TNF). The groups were considered homogeneous, except for perianal CD, previous azathioprine, and stomas. There was no significant difference between the groups regarding overall surgical complications (32.69% in anti-TNF− vs. 39.44% in anti-TNF+ patients, p = 0.457) or overall medical complications (21.15 vs. 21.13%, respectively, p = 1.000). In univariate analysis, previous steroids, perianal CD, and stomas were considered risk factors for surgical complications, and previous steroids and hypoalbuminemia for medical complications. In multivariate analysis, previous steroids were associated with higher rates of surgical and medical complications, while hypoalbuminemia was associated with higher medical complication rates.
Conclusions
There was no influence of the previous use of anti-TNF agents in postoperative surgical and medical complication rates in elective intestinal resections for CD. Previous steroids and hypoalbuminemia were associated with higher complication rates. This was the first case series of the literature describing outcomes in exclusively elective operations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Crohn’s disease (CD) is a progressive systemic entity, caused by genetic predisposition, associated with an increased intestinal permeability that allows luminal antigens to enter the lamina propria resulting in an uncontrolled inflammatory process. This inflammation usually causes ulcers at the mucosal layer of the affected areas that can progress transmurally over the muscularis propria and serosa. As a consequence, CD can often result in fibrosis, stenosis, abscesses, and fistulas. Due to this possibility to cause complications, CD is considered to be an aggressive disease that frequently needs surgery as an important therapeutic option [1].
Over the last years, a remarkable evolution in medical therapy of inflammatory bowel diseases (IBD) was achieved, mainly with the development and overspread use of anti-TNF (tumor necrosis factor alpha inhibitors) [2]. In CD, clinical response and remission were observed in some randomized clinical trials with these agents, and later on, other studies demonstrated reduction in hospitalization and surgical rates, with consequent change in the natural course of the disease [3–5].
The indications for biological agents in CD are mainly focused on the severe forms of the disease [3, 4]. Frequently, a patient needs some form of surgical treatment during the use of this class of medications [5]. It is well established that previous steroids and poor nutritional status can increase postoperative complications in CD [6]. As biologicals result in significant immunosuppression, there is evident concern if this reduction in the mechanisms of defense can lead to higher rates of surgical and medical (mainly infectious) complications after intestinal resections in CD, directly caused by these drugs. Indeed, there is also controversy regarding the effects of anti-TNF agents in tissue healing, at the level of intestinal anastomoses [7–9].
Data from different studies demonstrated controversial results regarding the impact of anti-TNF agents in surgical outcomes in CD. Some outlined that these agents can represent a negative impact in surgical outcomes in CD, increasing postoperative complications [10, 11]. Other studies demonstrated opposite results, suggesting that anti-TNF can be considered safe in the perioperative period [12–15]. Mostly, these are all retrospective case series from IBD referral centers, with similar methodology. Moreover, some meta-analysis that referred almost the same studies also demonstrated opposite results, making this topic significantly controversial in the IBD management [16–19].
The first prospective study that highlighted the relation between an anti-TNF agent, infliximab (IFX), and surgical outcomes in CD was published in 2014 [20]. In this study, a possible relation of higher serum levels of the drug with higher rates of overall complications and readmissions was identified in CD patients, but not in ulcerative colitis (UC). A second prospective study was also recently presented, with more than 500 patients from several centers from France, and anti-TNF therapy was associated with higher postoperative morbidity after ileocaecal resections for CD [21]. Currently, a multicenter prospective study is being conducted in the USA, in order to better clarify the relation of serum levels of anti-TNF agents in surgical outcomes in IBD (PUCCINI trial), possibly with a higher level of evidence than we currently have [22].
Most of these studies were performed in patients with intestinal resections due to CD, including the elective and urgent settings. It is known that emergency operations can have higher morbidity rates per se, and this was even demonstrated in a retrospective study from the Mayo Clinic [23]. To date, there are no data regarding this topic exclusively in elective operations. Moreover, there are also scarce published data in Latin American CD patients.
The primary aim of this study was to verify and compare the postoperative complication rates after elective intestinal resections in CD, in patients with and without previous exposure to anti-TNF agents. Secondary objectives were to define possible risk factors for medical and surgical postoperative complications.
Methods
Study Design
This was a retrospective longitudinal and observational study, with CD patients submitted to intestinal resections due to complications or failure to medical therapy, from 2 different IBD referral units from Brazil, in a 7-year period (January 2007–July 2014).
Inclusion and Exclusion Criteria
Patients with an established diagnosis of CD with clinical, imaging, endoscopic, and histological criteria, over 18 years old at the time of surgery, submitted to any elective abdominal surgical procedure with intestinal resection, were included in the study. We excluded from the analysis patients with undetermined IBD or UC, those submitted to strictureplasties or to other procedures without intestinal resection (diverting stomas) and operated in the emergency setting.
Variables Analyzed
The following variables were analyzed: age at diagnosis and at surgery, gender, disease duration until the surgical procedure, and smoking status. Patients’ phenotype was described according to the Montreal classification. Surgical characteristics were also evaluated: type of procedure according to the location, approach (conventional or laparoscopic) and type of anastomosis. Perianal disease, hypoalbuminemia (albumin <3.0 mg/dL), and preoperative medication (steroids, immunomodulators, or/and biological agents) were also checked. Preoperative use of steroids was defined as any exposure to 20 mg of prednisone or equivalent during any period for at least six weeks before surgery.
Early postoperative surgical and medical complications (up to 30 days after the surgical procedure) were evaluated. We considered as a surgical complication any occurrence different from a normal postoperative period that needed intervention (abdominal abscesses, anastomotic dehiscence, surgical site infection, reoperation, or small bowel obstruction). Medical complications were defined as any postoperative occurrences apart from the surgical site per se. We checked as medical complications: pneumonia, urinary tract infections, infections and other complications in general, readmissions, and death.
Regarding preoperative exposure to medication, patients with previous anti-TNF agents (IFX or adalimumab [ADA]) up to 8 weeks from the surgical procedure were considered exposed. This was in accordance with the definitions of most of the similar studies from the literature [12–14]. Previous steroids and thiopurines were also checked.
The specific protocols with the variables previously described were retrospectively completed, after electronic chart review. The postoperative medical and surgical complications were identified and then compared between the 2 different groups.
Group Definition
After patient identification and selection, they were allocated in 2 groups, according to the previous exposure to anti-TNF agents (ADA or IFX) in the preoperative period. The study group was composed by patients with preoperative use of anti-TNF (anti-TNF+), while the control group by patients with previous conventional therapy (anti-TNF−).
Statistical Analysis
The results were expressed as median and standard deviation (SD). For the analysis of group homogeneity, in relation to quantitative variables, the Student’s t test or Mann–Whitney method was used. For qualitative variables, Fisher’s exact test and the Chi-square method were chosen. Log-rank test was used for the comparison of the groups in univariate analysis. For multivariate analysis, in order to define risk factors for postoperative complications, a Cox regression model was used in addition to the Wald test, including as reference variables those that were significant in the univariate analysis. p values <0.05 were considered significant. The analysis was performed using SPSS version 14.0 software (Chicago, IL).
Ethical Considerations
The study was approved by the board of the ethical committees of both institutions, according to protocol 63324/2012 and CAAE 03687612.8.1001.5404, from the ministry of health Web site “plataforma Brasil”.
Results
Initially 144 patients were identified from the surgical databases of both units, and had their records accessed. From those, 12 were excluded for having emergency operations and 9 for having abdominal procedures without intestinal resection (strictureplasties and stomas). Therefore, 123 patients composed the final population analyzed (Fig. 1). From this sample, 52 had no previous exposure to anti-TNF agents, while 71 used these agents preoperatively (39 on IFX and 32 on ADA).
The baseline characteristics of the patients, according to the study groups, are described in detail in Table 1, and the groups were not fully homogeneous. There was no significant difference between the groups in terms of gender, age at diagnosis, age at surgery, disease duration until the procedure, smoking, hypoalbuminemia, and previous steroids. As observed, patients on the anti-TNF+ group were predominantly younger (Montreal A1) and had colonic location of CD (Montreal L2) with perianal disease and more exposure to previous thiopurines as compared to controls. Moreover, in terms of surgical characteristics, patients from the anti-TNF+ group also had more stomas and less primary anastomoses than patients from the control group.
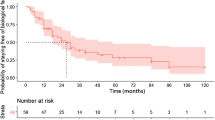
In relation to the main outcomes of the study, there was no significant difference between the two groups regarding overall surgical and medical complications. According to the type of surgical complications, no difference between the groups was also found regarding abdominal abscesses, anastomotic dehiscence, bowel obstruction, surgical site infection, or reoperations. In terms of the detailed medical complications, no difference was also found between the groups in relation to pneumonia, urinary tract infections, other complications in general, readmissions, and death. There was a tendency to higher rates of abdominal abscesses, surgical site infections and readmissions on the anti-TNF+ patients, without statistical significance. These data are described in detail in Figs. 2 and 3.
In relation to possible risk factors for an impact in postoperative morbidity, in univariate analysis, preoperative anti-TNF exposure was not a risk factor for surgical postoperative complications. Perianal CD, previous exposure to steroids, lower rates of primary anastomoses, and higher rates of stomas were associated with higher rates of surgical complications. These data are illustrated in detail in Table 2.
In multivariate analysis (logistic regression model and Wald test), it was confirmed that preoperative anti-TNF agents were not associated with an increase in surgical complications (OR 0.90; 95% CI 0.37–2.18; p = 0.815). However, previous steroids were confirmed as a risk factor associated with these types of complications (Table 3).
Regarding overall medical complications, in univariate analysis, previous anti-TNF agents equally were not considered an associated risk factor for higher morbidity. There was an association of higher complication rates with hypoalbuminemia and previous steroids, as illustrated in Table 4.
In multivariate analysis, preoperative anti-TNF agents were also not confirmed as a risk factor for medical complications (OR 0.98; IC 0.32–3.06; p = 0.976). Again, in accordance with the univariate analysis findings, hypoalbuminemia and previous steroids were associated with higher postoperative medical complications, as illustrated in Table 5.
Discussion
Several studies were published regarding the complex relation between surgery and biological therapy in CD management. The majority of these data come from retrospective cohort studies, and opposite results were described. Indeed, currently there is still controversy over the real impact of preoperative anti-TNF agents in postoperative outcomes after abdominal surgical procedures in CD [24].
In the present study, preoperative anti-TNF exposure was not associated with an increase in postoperative medical and surgical complications. These findings are in accordance with the majority of previous retrospective cohort studies published in the literature. Moreover, most of the data from experimental studies also suggested that anti-TNF agents are not related to higher postoperative complication rates.
A more detailed analysis of the previous reports shows some differences in the methodology used on the publications. Some studies included CD and UC patients in the same sample [14, 19]. Thus, even the meta-analyses that were published including the same studies came to different results, what brings even more controversy over the real impact of these agents in the postoperative outcomes in CD patients [17–19].
The population included in our study reflects real-world practice of surgical management of CD: young patients, with median age at surgery of 35–38 years old, with long disease duration between diagnosis and surgery (100–114 months) and with similar distribution regarding gender. The groups were not fully homogeneous, but comparable in most of the variables analyzed. However, the patients with preoperative exposure to anti-TNF agents were considered to have special characteristics that are usually seen in more severe cases: higher prevalence of perianal CD, more frequent use of azathioprine, higher rates of stomas, and less primary anastomoses. With the retrospective and observational design of the study, these differences between the groups could be expected, as biological agents are mostly used in more severe cases, reflecting current practice of CD. Even with more severe cases in the anti-TNF+ group, higher complication rates were not observed, what emphasizes the findings of our analysis.
Waterman et al. [14] in 2013 demonstrated in a retrospective cohort of a referral center in Canada that the time from the last dose of the anti-TNF agent had no influence in different rates of postoperative complications. In our study, we could not check that variable due to logistic reasons (injections and infusions were performed in different infusion units), but as no serum levels of the drugs were measured before surgery, anything different from therapeutic drug monitoring in an observational study like ours would question the real biological effect of biologics in complications.
Our study is one of the first ever published to include exclusively elective operations with intestinal resection for CD in the analysis. Moreover, it is the first study to describe the relation between anti-TNF agents and postoperative complications in Latin American CD patients. It is known that emergency procedures can have higher index of postoperative complications per se. Even with the fact that previous studies included approximately 10% of patients with emergency operations on their samples, with homogeneous distribution between the groups, this could represent a significant bias that was not present in our cohort of patients. Indeed, a retrospective study from the Mayo Clinic (USA) demonstrated, in multivariate analysis, that emergency procedures were considered a risk factor for an increase in postoperative complications [23].
In univariate analysis, preoperative anti-TNF agents were not associated with an increase in both surgical and medical (including infectious) postoperative complications. Only perianal CD, previous steroids, and stomas were associated with higher rates of surgical complications. Hypoalbuminemia and previous steroids were associated with higher rates of medical postoperative complications. In multivariate analysis, only previous steroids were associated with an increase in surgical complications (OR 2.55; CI 1.12–5.82; p = 0.024). With the same analysis, previous steroids (OR 5.88; CI 2.11–16.42; p = 0.001) and hypoalbuminemia (OR 4.11; CI 1.48–11.45; p = 0.006) were associated with higher rates of medical postoperative complications. These findings are in accordance with some studies and consensus from the literature, which demonstrated the negative impact of malnutrition and previous steroids in surgical outcomes in abdominal operations for CD [6, 25].
Clearly, in a real-world practice, patients using anti-TNF agents are being operated due to failure of medical therapy. Thus, most of them would be using steroids in various dosages at the time of surgery. Moreover, a delay in decision-making that lead to surgical indication often leads to nutritional deficit that can be linked to low albumin and an increase in postoperative complications. As previously stated, in an observational study like ours, patients using anti-TNF agents are generally considered more severe cases, with a tendency to have significant rates of postoperative complications not only for the previous medications, but mostly for their complexities. Even with the differences observed in group homogeneity, no differences in the overall rates of complications were observed.
In the analysis, there was a tendency (not statistically proved) toward higher rates of abdominal abscesses (p = 0.070), surgical site infections (p = 0.075), and readmissions (p = 0.077) in patients with preoperative exposure to anti-TNF agents. With a wider sample, maybe these variables could be significant, being more prevalent in patients in the anti-TNF+ group, as demonstrated in some retrospective studies [10, 11].
Two prospective studies analyzed the impact of anti-TNF therapy in surgical outcomes in CD. Lau et al. [20] analyzed 123 patients that had serum levels of IFX measured 7 days before the surgical procedure. There were more overall postoperative complications and more readmissions in patients with IFX levels >8μg/ml as compared to those with levels <3 μg/ml.
Similar results were observed in a prospective multicentric French study (RICCO registry), recently presented at the ECCO (European Crohn’s and Colitis Organisation) congress, 2016 [21]. From a total of 592 patients submitted exclusively to ileocecal resections for CD, prospectively followed for complications, 23.1% received previous anti-TNF therapy up to 6 months before surgery. There were higher rates of overall morbidity (41 vs. 26%, p = 0.001) and abdominal sepsis (13 vs. 7.1%, p = 0.03) in the group of patients with previous anti-TNF as compared to controls. The use of anti-TNF agents less than 6 months prior to surgery remained a strong predictor of overall postoperative morbidity (OR 2.10, 95% CI 1.60–2.75, p < 0.0001) and intra-abdominal sepsis (OR 3.13; 95% CI 2.07–4.73; p < 0.001). The results of this trial were not based on serum levels, and as it included patients with exposure to anti-TNF agents up to 6 months before the operations, some patients even could not have any circulating IFX and ADA during the procedures. In our study, previous anti-TNF for 8 weeks before the procedure was the cutoff for the definition of the groups, in order to avoid the absence of circulating drug that could bias the results. The PUCCINI (Prospective Cohort of Ulcerative Colitis and Crohn’s Disease Patients Undergoing Surgery to Identify Risk Factors for Postoperative Infection) trial is currently ongoing in the USA, with serum levels of the agents being measured [22]. Soon, its results would be available and there will be more evidence to clarify the real impact of anti-TNF agents in surgical outcomes in a big sample of patients coming from important IBD referral centers from America.
A single-center study from the Mayo Clinic (USA) described the surgical outcomes in CD patients submitted to previous use of vedolizumab, an anti-integrin alpha-4-beta-7 [26]. This was the first description of postoperative outcomes after abdominal operations in patients using a gut-selective biological agent, with a different mechanism of action than the inhibition of TNF-α. In this initial series of 94 patients, 53% had complications and 36% had surgical site infections. The role of vedolizumab in postoperative outcomes still needs to be determined, and with the increased use of this agent, more solid information regarding its effect in postoperative outcomes is expected in the future.
The present study has some limitations that must be taken into consideration in the analysis of our results. First, the retrospective and observational methodology could affect data collection. The included patients came from 2 Brazilian IBD referral centers and were not operated all by the same surgeon. The analysis was also undertaken in a convenience sample; there was no sample calculation that could power the study for the specific detection of differences in postoperative complications. Thus, the groups were not fully homogeneous. Additionally, there were no specific nutritional measurements, only serum albumin. Preoperative serum levels of IFX and ADA were also not measured. Patients with previous anti-TNF agents had lower prevalence of primary anastomoses in their procedures, what can also have influenced the results, mainly in the surgical complications. On the other hand, the exclusion of patients without resection and patients submitted to emergency procedures reduced significantly the bias on the results.
In summary, in this retrospective observational study that reflects real practice of surgical management in CD, there was no influence of preoperative anti-TNF agents in medical and surgical complications after elective intestinal resections. Perianal CD and stomas were associated with higher rates of surgical postoperative complications, while hypoalbuminemia was associated with more medical complications. Previous steroids were associated with both complications (medical and surgical), being an important risk factor for higher morbidity in this scenario. This was the first study based exclusively on elective operations.
References
Baumgart DC, Sandborn WJ. Crohn´s disease. Lancet. 2012;380:1590–1605.
Targan SR, Hanauer SB, Van Deventer SJH, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis-factor alfa for Crohn´s disease. N Engl J Med. 1997;337:1029–1035.
Hanauer SB, Feagan BG, Lichtenstein GR, et al. ACCENT I Study Group. Maintenance infliximab for Crohn´s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–1549.
Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn´s Disease. N Engl J Med. 2004;350:876–885.
Vermeire S, Van Assche G, Rutgeerts P. Review article: altering the natural history of Crohn´s disease: evidence for and against current therapies. Aliment Pharmacol Ther. 2006;25:3–12.
Dignass A, Van Assche G, Lindsay JO, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: current management. J Crohns Colitis. 2010;4:28–62.
El-Hussuna A, Andersen J, Bisgaard T, et al. Biologic treatment or immunomodulation is not associated with postoperative anastomotic complications in abdominal surgery for Crohn’s disease. Scand J Gastroenterol. 2012;47:662–668.
Papaconstantinou I, Zeglinas C, Gazouli M, et al. Effect of infliximab on the healing of intestinal anastomosis. An experimental study in rats. Int J Surg. 2014;12:969–975.
Strebel K, Nielsen SR, Biagini M, Qvist N. Effect of Humira® on intestinal anastomotic response in rabbits. J Invest Surg. 2015;28:167–172.
Appau KA, Fazio VW, Shen B, et al. Use of infliximab within 3 months of ileocolonic resection is associated with adverse postoperative outcomes in Crohn’s patients. J Gastrointest Surg. 2008;12:1738–1744.
Syed A, Cross RK, Flasar MH. Anti-tumor necrosis factor therapy is associated with infections after abdominal surgery in Crohn’s disease patients. Am J Gastroenterol. 2013;108:583–593.
Nasir BS, Dozois EJ, Cima RR, et al. Perioperative anti-tumor necrosis factor therapy does not increase the rate of early postoperative complications in Crohn’s disease. J Gastrointest Surg. 2010;14:1859–1865.
Nørgård BM, Nielsen J, Qvist N, Gradel KO, de Muckadell OB, Kjeldsen J. Pre-operative use of anti-TNF-α agents and the risk of post-operative complications in patients with Crohn’s disease—a nationwide cohort study. Aliment Pharmacol Ther. 2013;37:214–224.
Waterman M, Xu W, Dinani A, et al. Preoperative biological therapy and short-term outcomes of abdominal surgery in patients with inflammatory bowel disease. Gut. 2013;62:387–394.
Myrelid P, Marti-Gallostra M, Ashraf S, et al. Complications in surgery for Crohn’s disease after preoperative antitumour necrosis factor therapy. Br J Surg. 2014;101:539–545.
El-Hussuna A, Krag A, Olaison G, Bendtsen F, Gluud LL. The effect of anti-tumor necrosis factor alpha agents on postoperative anastomotic complications in Crohn’s disease: a systematic review. Dis Colon Rectum. 2013;56:1423–1433.
Billioud V, Ford AC, Tedesco ED, Colombel JF, Roblin X, Peyrin-Biroulet L. Preoperative use of anti-TNF therapy and postoperative complications in inflammatory bowel diseases: a meta-analysis. J Crohns Colitis. 2013;7:853–867.
Yang ZP, Hong L, Wu Q, Wu KC, Fan DM. Preoperative infliximab use and postoperative complications in Crohn’s disease: a systematic review and meta-analysis. Int J Surg. 2014;12:224–230.
Narula N, Charleton D, Marshall JK. Meta-analysis: peri-operative anti-TNFα treatment and post-operative complications in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2013;37:1057–1064.
Lau C, Dubinsky M, Melmed G, et al. The impact of preoperative serum anti-TNFα therapy levels on early postoperative outcomes in inflammatory bowel disease surgery. Ann Surg. 2015;261:487–496.
Brouquet A, Maggiori L, Zerbib P et al. Anti-TNF therapy is associated with increased risk of postoperative morbidity after surgery for ileocolonic Crohn disease: outcome analysis in a prospective nationwide cohort of 592 patients conducted by the GETAID chirurgie group. Oral presentation 027, ECCO congress, 2016, Amsterdam [abstract].
PUCCINI trial. http://ccfacra.org/?page_id=85. Accessed 02 2016.
Indar AA, Young-Fadok TM, Heppell J, Efron JE. Effect of perioperative immunosuppressive medication on early outcome in Crohn’s disease patients. World J Surg. 2009;33:1049–1052.
Kotze PG, Coy CSR. The impact of preoperative anti-TNF in surgical and infectious complications of abdominal procedures for Crohn’s disease: controversy still persists. Am J Gastroenterol. 2014;109:139.
Aberra FN, Lewis JD, Hass D, Rombeau JL, Osborne B, Lichtenstein GR. Corticosteroids and immunomodulators: postoperative infectious complication risk in inflammatory bowel disease patients. Gastroenterology. 2003;125:320–326.
Lightner AL, Raffals LE, Mathis KL, Cima RR, Tse CS, Pemberton JH, Dozois EJ, Loftus EV. Postoperative outcomes in vedolizumab-treated patients undergoing abdominal operations for inflammatory bowel disease. J Crohns Colitis. 2016. [Epub ahead of print].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
PGK is a speaker and consultant for AbbVie, Janssen, and Takeda. All other authors have no disclosure.
Rights and permissions
About this article
Cite this article
Kotze, P.G., Saab, M.P., Saab, B. et al. Tumor Necrosis Factor Alpha Inhibitors Did Not Influence Postoperative Morbidity After Elective Surgical Resections in Crohn’s Disease. Dig Dis Sci 62, 456–464 (2017). https://doi.org/10.1007/s10620-016-4400-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-016-4400-2