Abstract
Background
Budd–Chiari syndrome (BCS) with hepatic vein (HV) occlusion is manifested by severe liver damage in acute cases and esophageal variceal bleeding or refractory ascites in chronic cases, which is difficult to differentiate from cirrhotic portal hypertension.
Aims
To evaluate the clinical efficacy and safety of HV angioplasty and transjugular intrahepatic portosystemic shunt (TIPS) in the treatment of BCS with HV occlusion.
Methods
Between May 1995 and December 2014, 60 patients with HV occlusive BCS underwent HV angioplasty or TIPS. BCS was subacute or chronic in 55 patients and acute in 5 patients. HV angioplasty was performed in 18 patients with HV occlusion, combined HV and IVC angioplasty in 9 patients with HV and IVC occlusion, TIPS in 12 patients with HV occlusion, and modified TIPS in 21 patients with extensive HV occlusion.
Results
The interventional procedure was successfully performed in all 60 patients. The portal pressure decreased from 41.23 ± 10.46 cmH2O preoperatively to 26.68 ± 6.46 cmH2O postoperatively, while the portal flow velocity increased from 14.31 ± 10.43 to 52.16 ± 13.68 cm/s in patients undergoing TIPS or modified TIPS. During hospitalization, two patients died from hepatic failure, and acute shunt occlusion occurred in two other patients during subsequent treatment with repeated intervention. During 82.25 ± 46.16 months of follow-up, three patients underwent re-intervention with a stenotic shunt, and other three with repeated dilation of the stenotic HV.
Conclusion
HV angioplasty and TIPS yield excellent long-term outcomes in patients with HV occlusive BCS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Budd–Chiari syndrome (BCS), defined as a hepatic venous outflow obstruction, usually occurs as a result of thrombosis of the hepatic veins (HVs) and/or the suprahepatic inferior vena cava (IVC). The exact incidence and prevalence of BCS in the general population are unknown. The high pressure in the occluded HV leads to an enlarged liver and spleen, impaired liver function, refractory ascites, esophageal variceal bleeding, and even acute liver failure in severe cases [1]. BCS associated with IVC occlusion is more common than BCS with HV occlusion [2]. HV occlusive BCS is difficult to diagnose and often mistaken for hepatic cirrhosis, liver carcinoma, or BCS with IVC occlusion. Recently, HV angioplasty, transjugular intrahepatic portosystemic shunt (TIPS), and modified TIPS have become the main treatments for HV occlusive BCS [3–5]. Rapid and effective recovery of HV and portal vein (PV) blood flow can promote the control of bleeding and ascites as well as the recovery of liver function. The aim of this study was to evaluate the clinical efficacy and safety of HV angioplasty and TIPS in the treatment of BCS with HV occlusion.
Materials and Methods
All procedures involving human participants were performed in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. Additional informed consent was obtained from all individual participants for whom identifying information is included in this article.
Patients
Between May 1995 and December 2014, a total of 60 patients with HV occlusive BCS (27 males and 33 females, with a mean age of 38.82 ± 11.45 years) were admitted into our institute. The clinical presentation was ascites in all 60 cases, with concomitant upper gastrointestinal bleeding in 12 patients, hepatorenal syndrome in 6 patients, and impaired liver function in all patients. Acute occlusion occurred in 5 cases, and subacute or chronic occlusion in the remaining 55 cases. Among cases with hepatic venular occlusion, 6 cases were associated with oral sedum aizoon and 5 with oral stauntoniae. The average Child–Pugh score was 9.65 ± 2.31. In addition to routine examination, Doppler ultrasound and computed tomography angiography (CTA)/magnetic resonance angiography (MRA) were required in all patients after admission, followed by IVC and HV angiography to help select the interventional procedure. The diagnosis was established by Doppler ultrasound imaging, CTA, MRA, and IVC or HV angiography. Patients had hepatic outflow obstruction at any point from the small hepatic veins to proximal ostial occlusion. Of the 60 enrolled patients, 18 had proximal ostial occlusion of the HV, 9 had concomitant IVC stenosis, 21 had extensive HV occlusion, and 12 had hepatic venular occlusion (Table 1).
IVC and HV Angioplasty
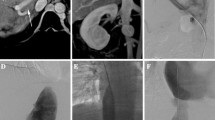
All patients underwent right internal jugular vein puncture and catheterization under ultrasonic guidance. Subsequently, a Rosch-Uchida Transjugular Liver Access Set device (COOK, Bloomington, IN, USA) was inserted into the IVC for IVC angiography via the right internal jugular vein under digital subtraction angiography guidance. If the guidewire and catheter could not enter the HV, the HV stump was identified by careful review of the IVC angiogram and used as a puncture point. After successful puncture into the right HV from the stump to the right HV, contrast injection showed the dilated HV and collaterals. Then, the guidewire and catheter were inserted, and right HV angiography was performed. After dilating the occlusive segment of the right HV using an 8-mm-diameter balloon, a bare mesh stent (BARD Corporation) of 8–12 mm diameter and 6 cm length was implanted, followed by repeated HV angiography to confirm that the HV function was being restored and that the intrahepatic collaterals disappeared. Then, the Rosch-Uchida Transjugular Liver Access Set catheter device was removed, and a 5F catheter was inserted into the right HV via the right internal jugular vein for local anticoagulant therapy and repeat angiography (Figs. 1, 2).
TIPS and Modified TIPS
A Rosch-Uchida Transjugular Liver Access Set device (COOK, Bloomington, IN, USA) was inserted in the IVC via the right internal jugular vein for IVC angiography. Subsequent HV angiography was performed after selective right HV catheterization. TIPS creation was performed in the right HV according to the standard TIPS procedure. A bare metal stent of 8 mm diameter and 8 cm length was used in nine cases, and a stent–graft of 8 mm diameter and 6 cm length was used in three cases. Modified TIPS creation was performed in patients with extensive HV occlusion. After the Rups-100 device was inserted into the IVC, the HV stump and post-liver IVC position were carefully identified, and the HV stump was used as the puncture point. Once the intrahepatic branches of the PV were successfully punctured from the HV stump, a guidewire was inserted into the PV and superior mesenteric vein or splenic vein, and the vascular sheath was then pushed into the main vessel of the PV for portal angiography and pressure measurement. A balloon dilatation device of 8 mm diameter was used to dilate the PV and the anterior wall of the IVC, and portal angiography was repeated to demonstrate no significant spillover in the intrahepatic shunt tract, followed by stent implantation. A bare metal stent of 8 mm diameter and 8 cm length was used in 21 cases, and a stent–graft of 8 mm diameter and 6 cm length was used in six cases (Figs. 3, 4). Pressure in the PV and IVC was measured again after shunt creation. A 5F catheter was inserted below the stent through the jugular vein for local anticoagulant therapy, after which PV pressure measurement and angiography were repeated.
To prevent thrombosis of the PV and shunt, heparin solution (500 U/h) was continuously administered for 2 weeks through the indwelling catheter using a micro-volume injection pump. Shunt patency was evaluated by Doppler ultrasound and direct portography 2 weeks after the procedure. Re-evaluation of shunt function was performed by Doppler ultrasound every 6 months thereafter, with simultaneous examination of blood flow velocity in the PV and intrahepatic shunt (Fig. 5).
Statistical Analysis
All quantitative variables are presented as mean values with standard deviation. Statistical comparisons of quantitative data obtained before and after the procedure were made using t tests. Statistical analysis was performed with SAS software.
Results
Short- and Long-Term Clinical Efficacy
The interventional procedure was performed successfully in all 60 patients. TIPS creation was terminated after PV bleeding in the hepatic hilus was found in two patients. These patients underwent successful stent implantation in the shunt tract 1 week later. During hospitalization, color Doppler and angiography showed acute occlusion of the intrahepatic shunt in two patients, and repeated shunt dilation and stenting were performed later to restore shunt patency. Two patients died of acute liver failure. In the remaining 58 patients who received successful treatment, bleeding stopped, ascites subsided, and the liver function and renal function were significantly improved. During a mean follow-up period of 82.25 ± 46.16 months after procedure, there was concomitant recurrence of bleeding in 2 cases, and recurrence of ascites in 5 cases. Doppler ultrasound and angiography demonstrated shunt occlusion of intrahepatic PV in three cases and HV re-occlusion in another three cases. Three cases underwent repeated shunt dilation and stent implantation, other three cases underwent repeated HV dilation, and all cases are still being monitored (Table 2).
Portal Hemodynamics
In patients undergoing HV angioplasty, preoperative Doppler ultrasound showed the HV blood flow to be patent, the HV flow velocity to be increased from 12.24 ± 8.12 cm/s preoperatively to 34.23 ± 12.56 cm/s postoperatively, and the portal blood flow to be switched from hepatofugal to hepatopetal. In patients undergoing TIPS creation, the portal pressure was 41.23 ± 10.46 cmH2O and the PV blood flow velocity was 14.31 ± 10.43 cm/s before the procedure. The main vessel of the PV was slightly widened, and the intrahepatic branches were tenuous with stiff vessel walls. After shunt creation, the PV pressure was 26.68 ± 6.46 cmH2O. Portal blood flow velocity was 52.16 ± 13.68 cm/s, and the blood flow in the shunt was 136.2 ± 26.4 cm/s (Table 3).
Discussion
BCS refers to post-hepatic portal hypertension caused by occlusion of the HV and/or post-hepatic IVC. Etiology and classification of BCS are very complex [6, 7]. In China and elsewhere, BCS associated with IVC occlusion is common, while BCS with HV occlusion is rare. HV occlusion may lead to limited or extensive disease, and the change in coagulant status is a major factor in the formation of HV occlusion. According to the speed and extent of thrombosis, BCS with HV occlusion can be divided into acute, subacute, and chronic forms. In patients with acute BCS, HV thrombosis is always associated with a wide range, a short time, concomitant hepatic venular occlusion, and various clinical manifestations of liver dysfunction, severe ascites, hepatorenal syndrome, and hepatic encephalopathy. Liver failure is the major cause of death. Medical treatment with anticoagulants, diuretics, and thrombolytics can help control symptoms and establish collateral circulation. In patients with subacute or chronic BCS, the consequent portal hypertension and esophageal variceal bleeding have a serious impact on survival. The slow development of thrombosis may create conditions promoting the formation of rich collateral vessels, a reduction in liver congestion, and protection of liver function [8].
Clinical diagnosis of HV occlusive BCS is complex due to the lack of specific clinical manifestations [9]. This disease is manifested by severe liver damage in acute cases and esophageal variceal bleeding or refractory ascites in chronic cases, which is difficult to differentiate from cirrhotic portal hypertension. Patients with HV occlusive BCS typically have no history of viral hepatitis, whereas those with hepatic venular occlusion always have a history of oral sedum aizoon or stauntoniae, which may cause endothelial dysfunction and thrombosis in hepatic venules. Doppler ultrasound, CTA, MRA, and percutaneous HV and IVC angiography are effective methods for diagnosing BCS with HV occlusion [10, 11]. In these patients, Doppler ultrasound imaging always shows HV enlargement with stenotic origin, hypoechoic lumen with no or reverse blood flow, and large collateral vessels in the liver, under the liver membrane or in the third hepatic hila. The diameter of the PV does not increase and flow velocity to the liver, decreases or even reverses CT and MR imaging show an enlarged caudate lobe with hyperenhancement due to separate direct drainage into the IVC, nutmeg liver in the PV phase and HV phase, high parenchymal density in the liver center, delayed emptying of contrast agent, porphyritic sign in the perihepatic parenchyma, and nodular hyperplasia in chronic cases [12]. CTA and MRA show an enlarged hepatic artery, small lumen, and stiff walls of hepatic branches of the PV and the dilated or indiscernible HV. CTA and MRA are effective methods for diagnosing BCS with HV occlusion [13]. Patients with ostial occlusion of the HV present with ostial stenosis or obstruction, distal expansion of the HV, and rich intrahepatic collateral vessels. The HV in those with whole occlusion cannot be observed, with a spider web-like change in intrahepatic collateral vessels. The main vessel of HV is patent in those with hepatic venular occlusion. HV angiography can be performed percutaneously via the liver or the internal jugular vein access. In the present study, HV angiography was performed using the internal jugular vein access in order to avoid intra-abdominal hemorrhage, and the treatment options were decided by imaging classification.
The treatment of HV occlusive BCS is aimed at restoring the PV and HV blood reflux and depends on the location and extent of HV disease [14]. With the development of endovascular therapy, surgical elimination of HV ostial stenosis or occlusion has become rarely used [15]. Percutaneous HV angioplasty can effectively remove the obstruction and restore HV flow and is an effective treatment for local HV occlusion with small trauma and low complication rates [16–18]. HV angioplasty includes balloon angioplasty and stent implantation. Stent implantation can reduce the rate of HV restenosis; however, an appropriate stent should be selected to avoid stent migration to the heart in case of short HV ostial disease. Portosystemic shunt is the main method for treating extensive HV occlusion or hepatic venular occlusion. By PV decompression, hepatic artery blood flow increases and perfuses the first and second zones of the portal interlude area in hepatic lobules through the vascular bed of the PV, to reduce liver cell hypoxia and restore the form and function of liver cells [8]. Moreover, shunt creation can reduce the symptoms of portal hypertension and abdominal venous stasis and is conducive to the control of esophageal variceal bleeding and elimination of ascites. A shunt can be created by surgical and interventional methods. Several variants of surgical side-to-side shunting have been used. Porto- or meso-caval shunts have been used for those with a patent IVC and meso-atrial shunts for those with IVC occlusion. Surgical shunt creation is associated with large trauma and high complications [19, 20]. In patients with BCS, the increased caudate lobe severely affects the surgical procedure, and shunt occlusion is prone to occur after long graft implantation with poor long-term efficacy [20]. In recent years, the interventional technique has become the main method for restoring IVC patency and establishing intrahepatic shunt for treatment of BCS [2, 21]. TIPS is not only an important treatment for portal hypertension with esophageal variceal bleeding, but also an effective treatment for HV occlusive BCS. With improvements in the TIPS creation experience and technology as well as in the understanding of the anatomy of the post-liver IVC and liver parenchyma, extensive HV occlusive BCS has become the indications for TIPS [22]. In the classic TIPS procedure, the intrahepatic shunt is established between the right HV and intrahepatic branches of the PV, and the puncture point is always located in the right HV 2–3 cm away from the IVC. In patients with extensive HV occlusive, the puncture point is located in the anterior wall of post-liver IVC. The anatomic basis for puncture from the post-liver IVC to the intrahepatic branches of the PV is that the anterior wall of the post-liver IVC is closely apposed with liver parenchyma [23]. The right HV stump or notch of caudate lobe on the IVC shown by IVC angiography is the anatomic landmark of the IVC puncture point. In BCS, the compensatory increase in liver changes the anatomic association between the IVC and intrahepatic branches of the PV. TIPS is technically more difficult in BCS treatment. The selection of the intrahepatic shunt becomes difficult, and abdominal bleeding may occur if the puncture point of the IVC or PV is located outside the liver. Nowadays, the use of a stent–graft supports effective prevention of bleeding from the IVC or PV puncture site, the hepatic artery and PV fistula, and the biliary and shunt fistula and significantly reduces procedural complications. Moreover, the stent–graft can inhibit intimal hyperplasia of the shunt, prevent shunt stenosis and occlusion, and thus significantly improve the clinical efficacy of BCS therapy [24].
TIPS creation for the treatment of portal hypertension is prone to increase the risk of hepatic encephalopathy and liver dysfunction [25]. In patients with liver cirrhosis, the liver reserve function is poor. A small shunt diameter can reduce the negative impact of TIPS on liver function, but it cannot effectively reduce portal pressure. In addition, the rate of bleeding recurrence is high. A large shunt diameter can significantly reduce portal pressure and effectively prevent bleeding events, but it may easily induce hepatic encephalopathy and liver function. BCS is different from liver cirrhosis in terms of liver cell damage and portal hemodynamic characteristics [9]. After successful shunt creation in HV occlusive BCS, the portal venous blood flow returns to the IVC and right atrium, leading to the hepatic sinus and PV decompression. At the same time, vascular resistance of the hepatic artery is reduced, and increased arterial blood flow perfuses the liver and improves liver function. This is especially important in the treatment of acute patients [26]. The impact of TIPS on portal hemodynamics and liver function has fully demonstrated that the establishment of an intrahepatic shunt is very useful for the treatment of BCS [27]. Before treatment, the PV blood flow in BCS patients is slow, bidirectional, and even hepatofugal, with increased portosystemic pressure gradient, severe clinical manifestations of liver dysfunction, and significantly abnormal blood biochemical parameters. After PV decompression, portal flow speed is increased, whereas the portosystemic pressure gradient is reduced and clinical manifestations and blood parameters of liver dysfunction are significantly improved [28].
The role of anticoagulant and thrombolytic therapy is still controversial in patients with HV occlusive BCS after TIPS creation [29]. An underlying prothrombotic disorder (or thrombophilia), or an established risk factor for venous thrombosis, is found in most patients. HV occlusion results in liver dysfunction and bleeding esophageal varices. After treatment, the hepatic and PV decompression can lead to liver function recovery. However, the shunt patency will be affected if the associated factors of HV thrombosis still exist. Local thrombolytic and anticoagulant therapy through a retained catheter was applied in the present study. Postoperative Doppler ultrasound and direct portography did not show shunt or IVC thrombosis, so there were no thrombolysis- or anticoagulation-induced complications. This confirms that local anticoagulation/thrombolysis is effective for the prevention of acute shunt thrombosis and anticoagulant/thrombolytic complications [24].
In conclusion, HV angioplasty and TIPS are effective methods for the treatment of BCS with HV occlusion. The modified TIPS technique can be successfully used in BCS with extensive HV occlusion, with good long-term efficacy.
References
Plessier A, Valla D-C. Budd–Chiari syndrome. Semin Liver Dis. 2008;28:259–269.
Zhang Q, Xu H, Zu M, et al. Strategy and long-term outcomes of endovascular treatment for Budd–Chiari syndrome complicated by inferior vena caval thrombosis. Eur J Vasc Endovasc Surg. 2014;47:550–557.
Nunez O, de la Cruz G, Molina J, et al. Interventional radiology, angioplasty and TIPS in Budd–Chiari syndrome. Gastroenterol Hepatol. 2003;26:461–464.
Rössle M, Olschewski M, Siegerstetter V, Berger E, Kurz K, Grandt D. The Budd–Chiari syndrome: outcome after treatment with the transjugular intrahepatic portosystemic shunt. Surgery. 2004;135:394–403.
Rosenqvist K, Sheikhi R, Eriksson L-G, et al. Endovascular treatment of symptomatic Budd–Chiari syndrome-in favour of early transjugular intrahepatic portosystemic shunt. Eur J Gastroenterol Hepatol. 2016;28:656–660.
Ludwig J, Hashimoto E, McGill DB, van Heerden JA. Classification of hepatic venous outflow obstruction: ambiguous terminology of the Budd–Chiari syndrome. In: Mayo Clinic Proceedings. Amsterdam: Elsevier; 1990:51–55.
Zhou W-J, Cui Y-F, Zu M-H, Zhang Q-Q, Xu H. Budd–Chiari syndrome in young chinese: clinical characteristics, etiology and outcome of recanalization from a single center. Cardiovasc Interv Radiol. 2016;39:557–565.
Cazals-Hatem D, Vilgrain V, Genin P, et al. Arterial and portal circulation and parenchymal changes in Budd–Chiari syndrome: a study in 17 explanted livers. Hepatology.. 2003;37:510–519.
Goel RM, Johnston EL, Patel KV, Wong T. Budd–Chiari syndrome: investigation, treatment and outcomes. Postgrad Med J. 2015:postgradmedj-2015-133402.
Brancatelli G, Vilgrain V, Federle MP, et al. Budd–Chiari syndrome: spectrum of imaging findings. Am J Roentgenol. 2007;188:W168–W176.
Meng X-C, Zhu K-S, Qin J, et al. Clinical significance of multislice spiral CT scans in hepatic veins occlusion in Budd–Chiari syndrome. Chin Med J. 2007;120:100–105.
Zhou P, Ren J, Han X, et al. Initial imaging analysis of Budd–Chiari syndrome in Henan province of China: most cases have combined inferior vena cava and hepatic veins involvement. PloS One. 2014;9:e85135.
Kane R, Eustace S. Diagnosis of Budd–Chiari syndrome: comparison between sonography and MR angiography. Radiology. 1995;195:117–121.
Seijo S, Plessier A, Hoekstra J, et al. Good long-term outcome of Budd–Chiari syndrome with a step-wise management. Hepatology. 2013;57:1962–1968.
Wei X, Hao X, Da-Hai Y, Mao-Heng Z, Qing-Qiao Z, Yu-Ming G. Analysis of interventional treatments of Budd–Chiari Syndrome and the related complications: a study of 1006 cases. Panminerva medica. 2013;55:371–376.
Pelage J-P, Denys A, Valla D, et al. Budd–Chiari syndrome due to prothrombotic disorder: mid-term patency and efficacy of endovascular stents. Eur Radiol. 2003;13:286–293.
Li T, Zhai S, Pang Z, et al. Feasibility and midterm outcomes of percutaneous transhepatic balloon angioplasty for symptomatic Budd–Chiari syndrome secondary to hepatic venous obstruction. J Vasc Surg. 2009;50:1079–1084.
Ding PX, Zhang SJ, Li Z, Fu MT, Hua ZH, Zhang WG. Long-term safety and outcome of percutaneous transhepatic venous balloon angioplasty for Budd–Chiari syndrome. J Gastroenterol Hepatol. 2016;31:222–228.
Mancuso A. An update on the management of Budd–Chiari syndrome: the issues of timing and choice of treatment. Eur J Gastroenterol Hepatol. 2015;27:200–203.
Langlet P, Valla D. Is surgical portosystemic shunt the treatment of choice in Budd–Chiari syndrome? Acta gastro-enterol Belgica. 2002;65:155–160.
Fu Y-F, Xu H, Wu Q, Zhang Q-Q, Cui Y-F, Wei N. Combined thrombus aspiration and recanalization in treating Budd–Chiari syndrome with inferior vena cava thrombosis. La Radiol Med. 2015;120:1094–1099.
Molmenti EP, Segev DL, Arepally A, et al. The utility of TIPS in the management of Budd–Chiari syndrome. Ann Surg. 2005;241:978–983.
Gasparini D, Del Forno M, Sponza M, et al. Transjugular intrahepatic portosystemic shunt by direct transcaval approach in patients with acute and hyperacute Budd–Chiari syndrome. Eur J Gastroenterol Hepatol. 2002;14:567–571.
Hernández-Guerra M, Turnes J, Rubinstein P, et al. PTFE-covered stents improve TIPS patency in Budd–Chiari syndrome. Hepatology. 2004;40:1197–1202.
Perry BC, Kwan SW. Portosystemic shunts: stable utilization and improved outcomes, two decades after the transjugular intrahepatic portosystemic shunt. J Am Coll Radiol. 2015;12:1427–1433.
Mancuso A, Fung K, Mela M, et al. TIPS for acute and chronic Budd–Chiari syndrome: a single-centre experience. J Hepatol. 2003;38:751–754.
Tripathi D, Macnicholas R, Kothari C, et al. Good clinical outcomes following transjugular intrahepatic portosystemic stent-shunts in Budd–Chiari syndrome. Aliment Pharmacol Ther. 2014;39:864–872.
Wu X, Ding W, Cao J, Han J, Li J. Modified transjugular intrahepatic portosystemic shunt in the treatment of Budd–Chiari syndrome. Int J Clin Pract. 2010;64:460–464.
Plessier A, Sibert A, Consigny Y, et al. Aiming at minimal invasiveness as a therapeutic strategy for Budd–Chiari syndrome. Hepatology. 2006;44:1308–1316.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Xinxin Fan and Kai Liu contributed equally to this study and share first authorship.
Rights and permissions
About this article
Cite this article
Fan, X., Liu, K., Che, Y. et al. Good Clinical Outcomes in Budd–Chiari Syndrome with Hepatic Vein Occlusion. Dig Dis Sci 61, 3054–3060 (2016). https://doi.org/10.1007/s10620-016-4208-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-016-4208-0