Abstract
Background
Gastrointestinal graft-versus-host-disease (GI-GVHD) is a major cause of nonrelapse mortality after hematopoietic stem cell transplantation (HSCT) necessitating endoscopic examinations and biopsies for diagnosis. Fecal calprotectin (CPT) has been widely used in gastrointestinal inflammation, but comprehensive data in GI-GVHD are lacking.
Aims
We aimed to identify an association of CPT with endoscopic findings, mucosal damage and symptoms for diagnosing and monitoring acute GI-GVHD.
Methods
Symptoms were prospectively evaluated in 110 consecutive HSCT recipients by standardized questionnaires and Bristol Stool Scale (BSS). CPT was assayed by ELISA. Symptom assessment and CPT were performed weekly and with onset of first symptoms. GVHD was diagnosed according to the Glucksberg criteria and by endoscopic biopsies. Patients with GI-GVHD received standard high-dose corticosteroid therapy and follow-up CPT, and symptom evaluation was performed after 28 days. Patients not responding to steroid treatment were re-evaluated by colonoscopy.
Results
GI-GVHD was diagnosed in 40 patients. Twelve patients with GI symptoms and CMV colitis and 24 patients with isolated skin GVHD were included as control subjects. CPT was significantly higher in GI-GVHD compared to skin GVHD and CMV colitis. Endoscopic findings, histological grading, abdominal cramps, diarrhea, urgency and BSS correlated with CPT. At follow-up, CPT correlated with abdominal cramps, diarrhea, urgency and BSS. In steroid refractory patients, CPT level was still significantly associated with severity of mucosal damage.
Conclusion
CPT predicts endoscopic and histological findings in GI-GVHD and correlates with lower GI symptoms. It enables to discriminate GVHD from CMV colitis and to monitor therapeutic success.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) offers a curative treatment option for management of a variety of hematologic malignancies. Intestinal symptoms frequently occur after HSCT. In particular, diarrhea is an ambiguous symptom. Its main causes are drug-induced side effects, conditioning regime-induced toxicity, infections and graft-versus-host-disease. Acute graft-versus-host-disease (aGVHD) is a major cause of nonrelapse mortality, but also induces substantial morbidity, which can severely affect quality of life after HSCT. The incidence of acute GVHD ranges from 35 to 80 % [1]. Early diagnosis and timely initiation of immunosuppressive therapy play a pivotal role for patient’s survival as mortality exceeds 60 % for patients not responding to first-line corticosteroid therapy [2]. The assessment of aGVHD activity is based on a combination of symptoms and histological findings. However, there is often an insufficient correlation between these diagnostic elements. Although ileocolonoscopy is considered the gold standard for assessment of intestinal inflammation, it has the disadvantage of being invasive, time-consuming and uncomfortable for patients.
Fecal calprotectin (CPT) increasingly serves as surrogate marker of disease activity in inflammatory bowel disease. There are some data indicating that fecal CPT may differentiate aGVHD from infectious colitis [3, 4] but fails to discriminate patients with aGVHD from non-GI-GVHD controls. However, these observations are based on few numbers, heterogeneous patients including pediatric patients and lack of widely available endoscopic biopsies. Furthermore, standardized symptom characterization has not been performed as yet. We therefore aimed to prospectively identify an association of fecal CPT with clinical, endoscopic and histological manifestation of acute intestinal GVHD in HSCT recipients.
Methods
Study Population
A total of 110 patients undergoing HSCT between February 2012 and February 2013 were prospectively evaluated after obtaining informed consent. The study was approved by the University Hospital Essen Human Ethics Committee and conducted in accordance with the World Medical Association’s Declaration of Helsinki.
Fecal calprotectin and gastrointestinal symptoms were assessed in all subjects weekly over a period of 120 days after HSCT and at any time of GVHD manifestation. Diagnosis of acute GVHD was assessed according to the modified Glucksberg criteria [5]. At time of symptom manifestation, patients with suspected acute GI-GVHD underwent colonoscopy to obtain endoscopic biopsies. Stool samples were obtained prior to colonoscopy preparation. Patients were tested for cytomegalovirus, adenovirus, rotavirus and Clostridium difficile as potential cause of symptoms. Patients presenting with skin GVHD in the absence of gastrointestinal symptoms were included as controls. Acute GVHD was treated by corticosteroids (1–2 mg/kg/d) after confirming diagnosis by colonoscopy.
Symptom Assessment
Symptoms of the upper and lower gastrointestinal tract were evaluated by standardized questionnaires [6] consisting of a 5-point Likert scale. Stool consistency was characterized utilizing the Bristol Stool Scale (BSS) [7]. The following symptoms were considered key symptoms and were preselected prior to data analysis: abdominal pain, cramps, bloating, diarrhea, urgency, upper abdominal pain, fullness, early satiety, nausea and vomiting.
Fecal Calprotectin
Fecal calprotectin was quantified by a quantitative enzyme-linked immunosorbent assay (CalproLab AS, Oslo, Norway) according to the manufacturer’s instructions with minor modifications. The scientist carrying out the analysis was blinded to clinical manifestation and endoscopic findings. Optical density was measured at a wavelength of 450 nm and a reference wavelength of 590 nm. Density values were linearly correlated with the concentrations of calprotectin standards. The limit of sensitivity of the assays was 25 µg calprotectin/g feces.
Steroid Response
In patients with acute intestinal GVHD, abdominal symptoms and fecal CPT were re-evaluated 28 days after initiation of corticosteroid treatment. Steroid response was defined as “no response” in patients presenting with same grade of GVHD or progression after 28 days. “Partial response” was defined as improvement in GVHD grade, while “complete response” was defined as complete resolution of GVHD. In patients not responding to steroids follow-up colonoscopy was performed.
Endoscopic Scoring
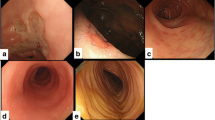
Endoscopic scoring was performed by an experienced physician applying the Freiburg criteria [8]. Grade 1 indicates non-GVHD ≥ 2. Grade 2 consists of spotted erythema and initial aphthous lesions. Grade 3 shows aphthous lesions or focal erosions, while grade 4 is characterized by confluent defects, ulcerations or complete denudation of the mucosa.
Histopathology
Histologic grading of endoscopically obtained biopsies was performed by a blinded pathologist applying a modified system for colonic GVHD described by Lerner et al. [9]. Briefly GVHD grade 1 is characterized by isolated apoptotic epithelial cells without crypt loss; grade 2 demonstrates loss of isolated crypts without loss of contiguous crypts; grade 3 shows two or more contiguous crypts; and grade 4 depicts extensive crypt loss with complete mucosal denudation.
Data Analysis
Results are expressed as mean ± SE and n = number of subjects throughout the manuscript. Paired and unpaired Student’s t test or two-way ANOVA determined the significance of changes in calprotectin levels. Unpaired Student’s t test determined the significance of differences in CPT levels between acute intestinal GVHD, skin GVHD and non-GVHD controls, with one-way ANOVA used to determine differences between aGVHD groups. The relationship between calprotectin levels and the intensity of symptoms was assessed by Spearman’s rank correlations.
The accuracy and appropriate cutoff value of calprotectin for identifying patients with acute intestinal GVHD were analyzed using receiver operator characteristic (ROC) statistics in order to determine whether there was a significant difference between the area under the ROC curves of aGVHD and sGVHD/non-GVHD patients. In addition, cutoff value of calprotectin for predicting 1-year mortality was analyzed using ROC.
In all cases, p values <0.05 were considered significant. For the statistical analysis, SPSS version 12 (Statistical Package for Social Sciences, Chicago IL) was used.
Results
Patient Characteristics
Out of 110 HSCT recipients, 40 patients developed acute GI-GVHD consequently diagnosed by endoscopic biopsies. GI-GVHD stages at the time of manifestation were I° n = 10, II° n = 12, III° n = 10 and IV° n = 8. In 12 patients presenting with GI symptoms, GVHD was excluded and CMV colitis is confirmed by colonoscopy and patients were enrolled as control subjects. Twenty-four patients with skin GVHD in the absence of GI-GVHD were also enrolled as control subjects. Patient demographics are displayed in detail in Table 1.
Briefly, 39 male and 37 female patients with a median age of 52 (range 24–75) were included. Diseases were distributed as follows: acute leukemia 57 %, chronic leukemia 12 %, lymphoma 9 %, myelodysplasia 8 %, chronic myeloproliferative disease 9 %, multiple myeloma 4 % and aplastic anemia 1 %. Myeloablative conditioning regime was applied in 74 %. The stem cell source was peripheral blood in 96 % and bone marrow in 4 %. Donors were HLA-matched unrelated 56 %, HLA-matched sibling 25 % and mismatched unrelated in 19 %. GVHD prophylaxis consisted of antithymocyte globulin 62 %, cyclosporine plus methotrexate 82 %, cyclosporine plus mycophenolate mofetil 11 %, steroids plus mycophenolate mofetil 1 %, tacrolimus 2 % or tacrolimus plus mycophenolate mofetil 4 %.
Fecal Calprotectin Levels
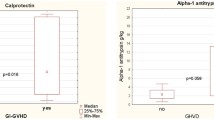
Symptomatic subjects without GVHD (CMV colitis) or sGVHD showed (Fig. 1) significantly lower CPT level (53.1 ± 10.9 mg/kg) compared to gastrointestinal GVHD I° (193.9 ± 24.3 mg/kg; p = 0.017; F 6.5), GVHD II° (248.5 ± 32.7 mg/kg; p = 0.04; F 5.1), GVHD III° (646.6 ± 207.7 mg/kg; p = 0.002; F 14.8) and GVHD IV° (1290.6 ± 352.2 mg/kg; p < 0.001; F 21.7).
GI-GVHD I° showed significantly higher CPT level (193.9 ± 24.3 mg/kg) compared to sGVHD (p < 0.001; F 15.8), lower level compared to GVHD III° (p < 0.001; F 19.5) and GVHD IV° (p < 0.001; F 35.7) but failed to reach significance compared to GVHD II° (p = 0.58; F 0.3).
While GvHD II° showed significantly lower CPT level compared to GVHD III° (p = 0.02; F 8.0) and GVHD IV° (p = 0.003; F 13.3), no difference was observed between GVHD III° and GVHD IV° (p = 0.121; F 2.8).
In subjects with sGVHD fecal CPT level was 51.7 ± 6.5 mg/kg and did not differ significantly (p = 0.91; F 0.26) to non-GVHD controls 53.1 ± 10.9 mg/kg but were significantly lower compared to GVHD I° (p < 0.001; F 15.8), GVHD II° (p = 0.02; F 11.4), GVHD III° (p < 0.001; F 34.8) and GVHD IV° (p < 0.001; F 50.4).
Utilizing a cutoff point value for CPT of 96.5 mg/kg sensitivity was 92.7 %, specificity 90.6 % with a positive predictive value 93 % and a negative predictive value 91 %. Area under receiver operating characteristic (ROC) curve was 0.962 (confidence interval 0.916–1.000).
Histopathology
CPT level was significantly associated with severity of histopathologic (Fig. 2) abnormalities (r = 0.69; p < 0.001) at time of manifestation and at follow-up in steroid refractory patients (r = 0.75; p = 0.02).
Endoscopic Findings
CPT level was significantly associated with endoscopic alterations (Fig. 3) quantified by Freiburg criteria (r = 0.62; p < 0.001). Patients not responding to steroids (GVHD II°: n = 2, GVHD III°: n = 2, GVHD IV°: n = 5) were re-evaluated by colonoscopy. Freiburg criteria at follow-up correlated with CPT (r = 0.673; p = 0.04).
Gastrointestinal Symptoms
At onset of acute GVHD calprotectin level was correlated with severity of abdominal cramps (Fig. 4) r = 0.39 (p = 0.001), diarrhea r = 0.53 (p < 0.001), urgency r = 0.53 (p < 0.001) and stool consistency according to BSS (r = 0.43, p < 0.001). No correlation was observed with upper abdominal symptoms (data not shown). At day 28 follow-up CPT level were still correlated with abdominal cramps r = 0.68 (p < 0.001), diarrhea p = 0.72 (p < 0.001), urgency p = 0.67 (p < 0.001) and BSS r = 0.54 (p = 0.005).
Steroid Response
In all subjects with endoscopic confirmed acute intestinal GVHD standard corticosteroid treatment was initiated with 1–2 mg/kg/d and calprotectin level re-evaluated after 28 days. Compared to initial levels CPT decreased at day 28 by 80.6 ± 3.1 % in GVHD I°, 66.3 ± 8.7 % in GVHD II°, 51.5 ± 12.9 % in GVHD III° and 19.4 ± 15.1 % in GVHD IV°. The steroid-induced CPT level decrease was significantly reduced in GVHD III° (p = 0.04) and GVHD IV° (p < 0.01) compared to GVHD I° and GVHD II° compared to GVHD IV° (p = 0.01) (Fig. 5). Overall complete remission was achieved in 57.5 %, partial remission in 20 % and no remission in 22.5 %. One-year survival rates were 88.8 % for GVHD I°, 71.4 % for GVHD II°, 40 % for GVHD III° and 22.2 % for GVHD IV°. Utilizing a cutoff point value for CPT of 266.5 mg/kg sensitivity was 75 %, specificity 86.3 % with a positive predictive value 68 % and a negative predictive value 90 %. Area under ROC curve was 0.806 (confidence interval 0.68–0.933).
Discussion
Appropriate and timely diagnosis of acute gastrointestinal GVHD is indispensable to implement adequate immunosuppressive therapy, which is pivotal in hematopoietic stem cell recipients. GVHD is a major contributing cause of nonrelapse-related death [2] and mortality of uncontrolled GVHD not responding to first-line corticosteroid therapy exceeds 60 % [10]. Although diagnosis of GVHD is considered appropriate if the classical constellation of symptoms is present [11], endoscopy with biopsies for histologic confirmation is desirable and remains the gold standard for the diagnosis of GI-GVHD.
This prospective study demonstrates a strong correlation of a noninvasive marker, namely fecal calprotectin with endoscopic alterations classified by the Freiburg criteria [8], histological grading, symptom severity of standardized assessed abdominal key symptoms and stool consistency according to BSS in patients with acute intestinal GVHD. Fecal samples for the quantification of CPT are easy to obtain before initiating immunosuppressive therapy. This in particular important as endoscopic biopsies should not delay immunosuppressive therapy in patients with classical clinical features and endoscopic results might be impaired if performed after initiation of high-dose corticosteroid treatment.
In line with recent data by O’Meara et al. [12], we were able to confirm in a larger sized control group that CPT enables to discriminate between patients with GI-GVHD and patients with isolated skin GVHD who do not differ significantly from non-GVHD controls with regard to CPT level. However, non-GVHD control groups consist of very heterogeneous patients among different studies including bacterial, viral and unspecific enteritis [4, 12]. We therefore choose symptomatic patients identified by repetitive standardized symptom assessment with isolated and histopathologically proven CMV enteritis as non-GVHD controls.
Distinction between GVHD and CMV infection can be difficult as patients present with similar abdominal symptoms potentially leading to false diagnosis of GI-GVHD if clinical criteria are applied exclusively. In our study, all patients with clinical suspicion of GI-GVHD and/or CMV enteritis underwent colonoscopy. We found that CPT allows discriminating patients with CMV enteritis without GI-GVHD and patients with GI-GVHD. However, both may occur simultaneously and GVHD is known to be a risk factor for refractory CMV enteritis [13, 14]. Thus, biopsies are still needed to exclude alternative or coexisting CMV. However, endoscopic diagnostic procedures may be limited to sigmoidoscopy as it has been shown that sigmoidoscopy and colonoscopy are equally effective for the diagnosis of GI-GVHD and CMV enteritis [15, 16].
The severity of intestinal GI-GVHD according to our results is depicted by CPT level as we were able to discriminate between lower grades I°/II° and higher grades III°/IV° GI-GVHD not necessitating further confirmation by endoscopy. These findings are in contrast to other studies which might be due to smaller numbers [4] or the lack of IV° GVHD [17].
While we found CPT as a useful diagnostic marker for the initial diagnosis of GI-GVHD with a positive predictive value of 93 % and a negative predictive value 91 % in addition, CPT correlates with clinical course and mucosal damage during treatment with corticosteroids. This is in particular important as avoidance of repetitive endoscopic procedures is warranted due to potential complications, patient’s inconvenience and efficient resource utilization. Thus, the present study suggests that there is limited need for repetitive endoscopic procedures once GVHD diagnosis has been confirmed by fecal CPT and concomitant CMV enteritis excluded by initial biopsies. Only patients with persisting abdominal symptoms and decreased fecal CPT levels should be re-evaluated by colonoscopy as up to one-third of patients with acute intestinal GVHD acquire additional CMV colitis over time [14].
There are some data indicating CPT is able to predict steroid response [17] in acute GI-GVHD when re-evaluating GVHD after a 14-day treatment period. However, it is well known that patients with initial high-risk GVHD consisting of grade 3 + [18] are less likely to respond to steroid treatment [19]. Indeed, after 28-day follow-up, we observed a higher number of steroid-resistant patients initially presenting with III°/IV° GVHD compared to initial I°/II° patients. In all subjects not responding to steroids, persisting GI-GVHD and exclusion of concomitant CMV enteritis were again confirmed by colonoscopy and biopsies. In these patients persisting high CPT levels, intensity of diarrhea, urgency, abdominal cramps and stool consistency according to BSS at follow-up identifies patients not sufficiently responding to first-line steroid treatment. Thus, abdominal key symptoms, stool consistency and fecal CPT may be sufficient to initiate second-line therapy in GVHD if symptom intensity and CPT elevation persist during corticosteroid treatment.
While CPT nicely correlates with lower GI-GVHD and symptom severity, no correlation was observed with upper abdominal symptoms. To our knowledge, there are no data clearly investigating fecal CPT and small bowel GVHD manifestation. Thus, there is a need for further studies investigating the use of this noninvasive marker for diagnosis of small bowel inflammation. Extrapolating from data available in patients with Crohn’s disease appears to be difficult as conflicting results have been published. There are some data indicating a correlation of CPT with ileitis [20] assessed by MRI, while others failed to observe an association utilizing capsule endoscopy [21]. Excessive high CPT level might be indicative of small bowel inflammation. Interestingly, Hoog et al. [22] reported a correlation of increased CPT levels with the presence of small bowel ulcerations but no correlation with symptoms. Therefore, the lack of correlation with upper abdominal symptoms and CPT in patients with GI-GVHD does not allow exclusion of an association of fecal CPT with small bowel GVHD and needs to be addressed in future studies.
We therefore conclude that fecal CPT allows diagnosing acute intestinal GVHD as it nicely correlates with endoscopic alterations quantified by Freiburg criteria and allows to discriminate between lower I°/II°, III° and IV° GI-GVHD. This correlation remains stable over time reflected by our prospective evaluation at multiple time points and follow-up investigations once diagnosis has been confirmed. CPT enables to discriminate patients with CMV colitis in the absence of concomitant GVHD. It may serve as a monitoring tool of therapeutic success of first-line steroid treatment and may decrease the need for repetitive endoscopic procedures.
References
Ferrara JL, Levine JE, Reddy P, et al. Graft-versus-host disease. Lancet. 2009;373:1550–1561.
Weisdorf D, Haake R, Blazar B, et al. Treatment of moderate/severe acute graft-versus-host disease after allogeneic bone marrow transplantation: an analysis of clinical risk features and outcome. Blood. 1990;75:1024–1030.
Bastos OM, Castilla-Llorente C, de la Guia AL, et al. Fecal calprotectin in allogeneic stem cell transplantation for the diagnosis of acute intestinal graft versus host disease. Bone Marrow Transplant. 2012;47:1241–1242.
Chiusolo P, Metafuni E, Giammarco S, et al. Role of fecal calprotectin as biomarker of gastrointestinal GVHD after allogeneic stem cell transplantation. Blood. 2012;120:4443–4444.
Przepiorka D, Weisdorf D, Martin P, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–828.
Adam B, Liebregts T, Saadat-Gilani K, et al. Validation of the gastrointestinal symptom score for the assessment of symptoms in patients with functional dyspepsia. Aliment Pharmacol Ther. 2005;22:357–363.
Heaton KW, Radvan J, Cripps H, et al. Defecation frequency and timing, and stool form in the general population: a prospective study. Gut. 1992;33:818–824.
Kreisel W, Dahlberg M, Bertz H, et al. Endoscopic diagnosis of acute intestinal GVHD following allogeneic hematopoietic SCT: a retrospective analysis in 175 patients. Bone Marrow Transplant. 2012;47:430–438.
Lerner KG, Kao GF, Storb R, et al. Histopathology of graft-vs.-host reaction (GvHR) in human recipients of marrow from HL-A-matched sibling donors. Transplant Proc. 1974;6:367–371.
Weisdorf DJ, Snover DC, Haake R, et al. Acute upper gastrointestinal graft-versus-host disease: clinical significance and response to immunosuppressive therapy. Blood. 1990;76:624–629.
Dignan FL, Clark A, Amrolia P, et al. Diagnosis and management of acute graft-versus-host disease. Br J Haematol. 2012;158:30–45.
O’Meara A, Kapel N, Xhaard A, et al. Fecal calprotectin and alpha1-antitrypsin dynamics in gastrointestinal GvHD. Bone Marrow Transplant. 2015;50:1105–1109.
Liu J, Kong J, Chang YJ, et al. Patients with refractory cytomegalovirus (CMV) infection following allogeneic haematopoietic stem cell transplantation are at high risk for CMV disease and non-relapse mortality. Clin Microbiol Infect. 2015;21:1121.e9–1121.e15.
Martinez C, Rosales M, Calvo X, et al. Serial intestinal endoscopic examinations of patients with persistent diarrhea after allo-SCT. Bone Marrow Transplant. 2012;47:694–699.
Johansson JE, Nilsson O, Stotzer PO. Colonoscopy and sigmoidoscopy are equally effective for the diagnosis of colonic acute graft-versus-host disease in patients with diarrhea after allogeneic stem cell transplantation: a prospective controlled trial. Biol Blood Marrow Transplant. 2015;21:2086–2090.
Liu A, Meyer E, Johnston L, et al. Prevalence of graft versus host disease and cytomegalovirus infection in patients post-haematopoietic cell transplantation presenting with gastrointestinal symptoms. Aliment Pharmacol Ther. 2013;38:955–966.
Rodriguez-Otero P, Porcher R, de Peffault LR, et al. Fecal calprotectin and alpha-1 antitrypsin predict severity and response to corticosteroids in gastrointestinal graft-versus-host disease. Blood. 2012;119:5909–5917.
MacMillan ML, Defor TE, Weisdorf DJ. What predicts high risk acute graft-versus-host disease (GVHD) at onset? Identification of those at highest risk by a novel acute GVHD risk score. Br J Haematol. 2012;157:732–741.
MacMillan ML, Robin M, Harris AC, et al. A refined risk score for acute graft-versus-host disease that predicts response to initial therapy, survival, and transplant-related mortality. Biol Blood Marrow Transplant. 2015;21:761–767.
Cerrillo E, Beltran B, Pous S, et al. Fecal calprotectin in ileal crohn’s disease: relationship with magnetic resonance enterography and a pathology score. Inflamm Bowel Dis. 2015;21:1572–1579.
Olsen PA, Fossmark R, Qvigstad G. Fecal calprotectin in patients with suspected small bowel disease—a selection tool for small bowel capsule endoscopy? Scand J Gastroenterol. 2015;50:272–277.
Hoog CM, Bark LA, Brostrom O, et al. Capsule endoscopic findings correlate with fecal calprotectin and C-reactive protein in patients with suspected small-bowel Crohn’s disease. Scand J Gastroenterol. 2014;49:1084–1090.
Acknowledgments
The authors wish to thank Ursel Hill (Essen, Germany) for valuable help with data entry and management.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Adam, B., Koldehoff, M., Ditschkowski, M. et al. Endoscopic and Histological Findings Are Predicted by Fecal Calprotectin in Acute Intestinal Graft-Versus-Host-Disease. Dig Dis Sci 61, 2019–2026 (2016). https://doi.org/10.1007/s10620-016-4112-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-016-4112-7