Abstract
Background
Esophageal motility disorders and possibly gastroesophageal reflux disease are common in patients with diabetes mellitus.
Aims
We aimed to investigate both the electrophysiological characteristics of the esophageal epithelium and the contractility of the lower esophageal sphincter (LES) muscle in alloxane-induced diabetic rabbits.
Methods
Electrophysiological properties were measured using an Ussing chamber method. An acid-pepsin model was employed with pH 1.7 or weakly acidic (pH 4) Ringer and/or pepsin. Smooth muscle strips of the LES were mounted in an isolated organ bath. Contractile responses to an electrical field stimulation and cumulative concentrations of acetylcholine were recorded. Contractility of the muscle strips were tested in the presence of Rho-kinase inhibitor (Y-27632) and nonspecific nitric oxide inhibitor (L-NAME).
Results
The resistance of diabetic tissue perfused in the pH 1.7 Ringer decreased 17 %; pepsin addition decreased it by 49 %. The same concentrations caused a more distinct loss of resistance in the control tissues (22 and 76 %, p < 0.05). The perfusion of tissues in increased concentrations of luminal and serosal glucose did not change the tissue resistance and voltage. Diabetes significantly increased both the electrical field stimulation and acetylcholine-induced contractions in the LES muscle strips (p < 0.01). Incubation with Y-27632 significantly decreased the acetylcholine-induced contractions in a concentration-dependent manner (p < 0.01).
Conclusions
The acid-pepsin model in the diabetic rabbit esophageal tissue had less injury compared with the control. The diabetic rabbit LES muscle had higher contractility, possibly because of the activation of the Rho-Rhokinase pathway. Our results show that in a chronic diabetic rabbit model the esophagus resists reflux by activating mechanisms of mucosal defense and increasing the contractility of the LES.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of gastroesophageal reflux disease (GERD) is higher than it was a decade ago. The evident increase in GERD within last few years seems to be related to an increase in the body mass index. Risk increases even more with obesity (approximately 15 % increase) [1–4].
Patients with diabetes mellitus suffer from different gastrointestinal motility disorders in general. Dysmotility problems are mainly related to motor dysfunctions and cause severe problems, such as delayed gastric emptying [5, 6]. Esophageal motor function is very much affected by chronic diabetes, and delayed esophageal transit is a frequent problem that affects the quality of life in diabetic patients [7, 8] as well as patients with GERD [9]. Some studies researching the relationship between diabetes and GERD had results contrary to expectations [10]. Lluch et al. [11] showed that pathological reflux was present in 38.7 % of diabetic patients with abnormal cardiovascular tests, whereas only 10.5 % of diabetic patients without signs of autonomic neuropathy had pathological reflux. This figure is lower than the actual population-based prevalence rate of GERD.
Research on diabetic patients focused on whether GERD is a motility and abnormal esophageal transit problem that results in delayed esophageal emptying or whether GERD occurs because of an increase of acid reflux episodes [12] or reduction of LES pressure [13]. Most gastrointestinal symptoms of diabetics are the result of motor dysfunction and neuropathy [14]. Diabetic patients may suffer from GERD because of delayed gastric emptying. However, other mechanisms are also possible such as esophageal epithelial permeability and muscle contractility and tension.
The effects of diabetes on esophageal epithelia in animal models have not been studied well. Our rabbit model of chronic diabetes enables us to investigate in vitro electrophysiological parameters of esophageal epithelia as well as LES muscle kinetics. Our aim was to evaluate the esophageal epithelial resistance and LES muscle contractions in diabetic rabbit esophagus and to determine hyperglisemia-induced alterations in the esophageal wall.
Materials and Methods
Induction of Diabetes in Rabbits
Experiments were performed on male New Zealand White rabbits (2.5–3 kg). The experimental protocol was approved by the Institutional Animal Welfare Committee of Ege University (approval no. 2013-45). Diabetes was induced by a single intravenous injection of alloxane (100 mg/kg) [15]. Fasting blood glucose levels were measured 72 h after injection using glucose strips. Blood glucose levels higher than 126 mg/dl were considered positive for diabetes. Blood glucose levels and body weights were recorded every week for 10 weeks to follow the diabetic status. Control rabbits did not receive any alloxane injection.
All chemicals used in the study were obtained from Sigma-Aldrich Co., MO, USA.
Electrophysiological Studies in Esophageal Epithelium
At the end of 10 weeks, rabbits were killed using anesthesia with ketamine (100 mg/kg) and xylazine (10 mg/kg). The esophagus of each animal was excised and opened lengthwise, and the mucosal surface was pinned down on a paraffin tray containing ice-cold oxygenated normal Ringer solution. The submucosa was dissected from the underlying mucosa with a scalpel. This yielded a sheet of stratified squamous epithelium. The epithelium was cut into four sections. Each piece was mounted into a Lucite half-chamber (with a diameter of 1.2 cm2) in a Ussing system, which enables tissues to be bathed in separate luminal and basolateral solutions during a complete experiment protocol. To stabilize the tissues, they were bathed with normal Ringer solution [composed of 140 Na+, 119.8 Cl−, 5.2 K+, 25 HCO3 −, 1.2 Ca+, 1.2 Mg2+, 2.4 HPO4 2−, 0.4 H2PO4 − (in mmol/l)] with an osmolality of approximately 275–285 mOsmol/kg H2O and pH 7.5. The solution was gassed with 95 % O2–5 % CO2 at 37 °C. Osmolalities of all solutions were measured with an Osmometer (Advanced Instruments Micro-Osmometer Model 3300, USA). Calomel and Ag–AgCl electrodes with Ringer agar bridges were connected to luminal and basolateral components of the Ussing chambers to measure the potential difference (PD) and short-circuit current (Isc) using a Multi-Channel Voltage/Current Clamp (EVC4000—World Precision Instruments Inc., Sarasota, FL, USA). Tissues were continuously open-circuited except for 5–10 s when the Isc was recorded. The junctional potential of acidic Ringer solutions was measured, and calculations were performed before each experiment [16]. Electrical resistance (R) was calculated using Ohm’s law (PD = Isc × R). After mounting, tissues were bathed in normal Ringer solution for 45 min to reach equilibration. Experiments were initiated if a tissue had an R value >1000 ohms.cm2 and PD value >10 volts. Measurements were collected every 10 min during an 1-h period. Data collected prior to beginning an experiment were used as the reference point, and the reference point was accepted as 100 %. The only exception was the 0-min measurements of all tissues. Absolute values were taken and used to compare two groups for R, PD and Isc. All PD, Isc and R values measured after addition of the agents were calculated from a reference point and defined as percentage change. Four sheets of tissue were obtained from each rabbit, enabling us to repeat experiments with four different bathing solutions.
Tissues were exposed to concentrations of acid and pepsin to simulate the acid-peptic model previously used by Tobey et al. [17]. One tissue from each rabbit was kept in a neutral Ringer solution as the control tissue. Other Ringer solutions were titrated luminally with an HCl (3 M) solution to make unnoxious concentrations of weakly acidic Ringer (pH 4) and acidic Ringer (pH 1.7) solutions. Pepsin (0.25 mg/ml) in dH2O was added only to the acidified luminal side (pH 1.7) to create a noxious effect.
Glucose perfusion of epithelial tissues was used to simulate the effects of acute hyperglycemia. The effects of luminal and serosal glucose (l-glucose and S-glucose) (MW = 180.16) were investigated by perfusing diabetic or control EE tissues with different concentrations of glucose (1, 2, 4 and 6 mg/ml). The same amount of mannitol (MW = 182.17) was added to the opposite sides to balance the osmolality on both sides of tissues. All agents were introduced in a manner that would not change the final volume of the 10 ml perfusion solutions on either side of an epithelial tissue sample. Control tissues were exposed to a Ringer solution.
Determination of Isolated LES Muscle Contractility
While tissue samples were used for Ussing chamber experiments, after the squamocolumnar junction, four muscle strips of 5–8 mm near the LES area were dissected transversely and mounted in 20 ml organ chambers containing a Krebs-Henseleit solution gassed with a 95 % O2/5 % CO2 mixture and maintained at 37 °C. Each strip was connected to a force displacement transducer to measure isometric force, which was continuously displayed and recorded via an eight-channel transducer data acquisition system.
After an initial equilibration period of 60–90 min, strips were stimulated with platinum wire electrodes placed longitudinally on both sides with pulse trains of 60 V in amplitude and 1 s in duration, with a pulse duration of 10 ms at an increasing frequency of 0.5, 1, 2 and 4 Hz using a stimulator. Because no frequency-response relationship was observed in either the control or diabetic LES muscle strips, a frequency of 4 Hz was chosen based on previous reports [18]. The strips were washed four times at 15-min intervals and allowed to equilibrate for 45 min to return a basal resting tone. After equilibrium was reached, EFS responses were recorded at a frequency of 4 Hz after incubating the strips in either Y-27632 (0.01–10 µM) or L-NAME (100 µM) for 30 or 20 min, respectively. Cumulative contractile responses to acetylcholine (0.001–30 µM) were also obtained on different LES muscle strips. To confirm the effect of Y-27632 (0.01–10 µM) on acetylcholine-induced contractions, muscle strips were preincubated in Y-27632 for 30 min before adding acetylcholine to the organ chambers.
Statistical Analysis
All data were reported as the mean ± standard errors mean (SEM). Statistical significance was determined using Student’s t test for electrophysiological studies and repeated measures of ANOVA for studies of LES muscle contractility. A p < 0.05 denoted a statistically significant difference between groups.
Results
Injection of alloxan induced a marked increase in blood glucose levels of rabbits (p < 0.0001; 112 ± 11.87 mg/dl in control and 447 ± 22.75 mg/dl in diabetic rabbits during sacrifice). Measurement of baseline electrophysiological parameters in the epithelium revealed that induction of diabetes resulted in significantly higher tissue resistance in the epithelium; however, neither PD nor Isc showed any difference between diabetic and control epithelium (Table 1).
The PD of EE tissues from diabetic rabbits perfused in Ringer and weakly acidic Ringer (pH 4) solutions decreased gradually (83 ± 1.6 and 87 ± 1.9 %) during the 1-h perfusion, respectively, similar to control tissues (Fig. 1a). The most distinct decrease in PD (25 ± 1.4 %) in the diabetic tissues perfused in the acidic Ringer (pH 1.7) was observed in the first 10 min (Fig. 1b). After the first 10 min, the values remained stable during the experimental period. The PD of the diabetic EE tissues perfused in the acidified Ringer (pH 1.7) + pepsin decreased 20 ± 4.2 % in the first 10 min and then recovered slightly (10 ± 7.1 %) at the end of the experiments. The deleterious effect of the acidic Ringer (pH 1.7) + pepsin was significantly reduced in the diabetic EE tissues. The PD of the control group of the EE tissues perfused in the normal Ringer did not change. However, the decrease was significantly lower than the diabetic tissues at 50 and 60 min. The most significant change was observed in the tissues perfused in the acidified Ringer (pH 1.7) + pepsin in which the PD decreased 37 ± 11.2 %. This change in voltage was significantly more profound compared to the diabetic tissues (PD decrease 10 ± 7.1 %; p < 0.05).
a, b Potential difference (PD) values of diabetic and control rabbit tissues in Ussing chambers. Effects of Ringer, weakly acidic Ringer (pH 4), acidic Ringer (pH 1.7) and pepsin (0.25 mg/ml) in acidified Ringer (pH 1.7) solution. Data are expressed as % of initial values prior to acid-pepsin exposure (0–60 min). The tissues perfused in the acidified Ringer (pH 1.7) + pepsin was significantly different compared to the diabetic tissues (*p < 0.05). The values are mean ± SEM; N = 7/group
The tissue resistance of the diabetic EE tissues perfused in the Ringer solution did not change as much as the tissues perfused in the weak acidic Ringer solution (Fig. 2a). These results are possibly related to the slight decrease in ion transport through the tissues. The acidification of the luminal Ringer solution of the EE tissues of the diabetic rabbits to pH 1.7 decreased the resistance (9 ± 3.2 %) over time (Fig. 2b). This group of tissues was more resistant to acid-peptic damage compared to the control EE tissues. The tissue resistance decreased 49 ± 5.1 % in the diabetic EE tissues when pepsin was luminally added to the acidified Ringer (pH 1.7). The same concentrations caused a more distinct loss of resistance, abolishing the tissue resistance of the control EE tissues (22 ± 7.2 % decrease with the pH 1.7 perfusion and 76 ± 5.9 % decrease with the pH 1.7 + pepsin perfusion). This change in tissue resistance (49 ± 5.1 vs. 76 ± 5.9 % decrease in resistance) was the result of a twofold increase in ion transport through the control tissues compared to the diabetic tissues. The values were statistically significant throughout the duration of the experiment (p < 0.05, R values, diabetic group vs. control group).
a, b Resistance (R) values of diabetic and control rabbit tissues in Ussing chambers. Effects of Ringer, weakly acidic Ringer (pH 4), acidic Ringer (pH 1.7) and pepsin (0.25 mg/ml) in acidified Ringer (pH 1.7) solution, tissue resistance (R). Data are expressed as % of initial values prior to acid-pepsin exposure (0–60 min). The values are mean ± SEM; N = 7/group. (*p < 0.05 control vs. diabetic)
The effects of luminal and serosal glucose were investigated by perfusing the diabetic or control EE tissues with increased concentrations of luminal or serosal glucose (1, 2, 4 and 6 mg/ml). The same concentration of mannitol was added to the opposite sides of the tissues to balance osmolality. The PD of diabetic tissues perfused in l-glucose steadily decreased to approximately 12 ± 1.3 − 19 ± 5.6 % in all the concentrations at the end of the experiments without dose dependency. The ion transfer through the tissues decreased (21 ± 2.9 − 26 ± 0.6 %) similarly in all the tissues independent of the concentration change; thus, the tissue resistance remained stable (103 ± 1.0 − 114 ± 2.3 %) for the entire 60 min (Fig. 3).
a, b Effects of different concentrations of l-glucose (1–6 mg/ml) on the diabetic rabbit and healthy rabbit esophageal epithelial tissue resistance (R). Data are expressed as % of initial values prior to glucose exposure (0–60 min). The values are mean ± SEM; N = 3/group. No difference has been shown
The diabetic tissues were also serosally perfused with glucose to mimic hyperglycemia in the blood with the same concentrations (1, 2, 4 and 6 mg/ml). Mannitol was added to luminal Ringer solution. The voltage (PD) did not change in any of the groups (4 ± 6.7 − 11 ± 4.1 % decrease). The perfusion with S-glucose did not drastically change the tissue resistance (10 ± 3.6 − 25 ± 2.3 % increase) as a result of simultaneous decrease in ISC (short circuit current) matching the decrease in PD. As a result of the similar changes in the Isc in the tissues perfused with l-glucose, there were no significant differences in electrical parameters throughout the experiments in the diabetic EE tissues serosally and luminally perfused with glucose.
The EE tissues from the healthy rabbits were luminally perfused with the same concentrations (1, 2, 4 and 6 mg/ml) of glucose. The PD as well as the tissue resistance changed slightly (9 ± 1.0 − 12 ± 0.9 % drop in PD and 12 ± 0.5 − 19 ± 1.1 % increase in R) during 1 h of luminal perfusion without any significant effect in the bathing solutions. This was similar to the diabetic EE tissues.
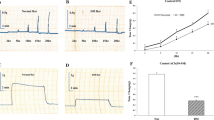
The electrical stimulations induced LES muscle contractions in the control and diabetic rabbits. However, this contractility was not frequency-dependent (Fig. 4). The EFS contractions of the LES muscles were significantly higher in the diabetic group than in the control group (p < 0.01). Incubation of the strips with Y-27632 or L-NAME (alone or in combination) did not significantly change the EFS responses in the control or diabetic LES muscles (data not shown).
The acetylcholine-induced contraction was significantly higher in the diabetic LES muscle compared to the controls (p < 0.01; Fig. 5a). The preincubation of the strips with Y-27632 did not affect the acetylcholine-induced contractions in the LES muscles of the control group (Fig. 5b). However, it significantly decreased acetylcholine contractions in the diabetic group (p < 0.01 and p < 0.001; Fig. 5c).
Acetylcholine-induced contractions of the control and diabetic (DM) lower esophageal sphincter muscle incubated with or without increasing concentrations of Y-27632, a ROCK inhibitor. Data are presented as the mean ± SEM, N = 8–11. **p < 0.01 compared with the control; ## p < 0.01 and ### p < 0.001 compared with the DM
Discussion
Data obtained in this study revealed that inducing diabetes in rabbits produces a higher resistance in the tissue of esophageal epithelium compared to controls at the baseline. Adding the different noxious agents into the chambers produce a decrease in the resistance, as expected. However, this loss of resistance was significantly lower in the diabetic rabbit tissues than the loss of resistance in the control group. The perfusion of the diabetic EE tissue with increased concentrations of luminal and serosal glucose also did not change the tissue integrity. The electrophysiological data indicate a self- (or possibly adaptive)protective mechanism against mucosal damage in the diabetic milieu. Furthermore, these data were supported by muscle functional experiments showing that induction of diabetes results in increased contractility of LES muscles in rabbits and suggesting that LES may adapt to prevent reflux into the esophagus from the stomach.
The upper mucosal layer of esophageal epithelia is capable of protecting the inner cell layers from refluxed material from the stomach. Rabbit esophageal epithelia do not have submucosal glands within the esophageal wall. Thus, this epithelium cannot secrete bicarbonate or mucus. Nonetheless, it still can retain resistance to a certain point, such as with acidification of the lumen to pH 1.7 [19]. In previous studies, an increase in mucosal permeability, which can be simultaneously observed as an increase in ISC (short circuit current), is a feature of acid-peptic injury in rabbit esophageal epithelia. The mechanism of action of acid and pepsin was suggested to be through paracellular pathways increasing epithelial permeability and directly affecting the junctional complex without damaging the cells [17].
In our case, we found two aspects of protection; the first one was an increase in LES muscle contractility, and the second was an increase in mucosal resistance. Although the acid-pepsin model causes mucosal damage, the esophageal tissue of diabetic rabbits had less injury from the acid-pepsin damage compared to the control, which was shown with higher PD and R values. Furthermore, the isolated organ bath studies supported these findings. LES muscle contractions were significantly increased in the diabetic rabbits. This was probably to prevent retrograde movement of the stomach contents. Indeed, it has been clinically shown that the resting lower esophageal intrasphincteric pressure in diabetics without neuropathy tends to be higher than in controls, and the esophagus in these particular patients is more responsive to stimuli than in controls. This finding suggests that the transient hyper-responsiveness to normal stimuli may be the earliest manifestation of diabetic neuropathy in the esophagus [20]. Both the EFS- and acetylcholine-induced contractions were significantly higher in the diabetic LES muscle compared to the control. Contractions induced by direct stimulation of nerves result from the integration of intramural excitatory cholinergic nerves with inhibitory nonadrenergic, noncholinergic nerves innervating the esophagus smooth muscle stimulation [21]. We found that incubating the LES strips with L-NAME (alone or in combination) did not significantly change the EFS responses in the control or diabetic LES muscles, suggesting that excitatory contractions of rabbit LES muscle and increased EFS-contraction in diabetic LES muscles is not mediated by the ROCK pathway or nitrergic neurotransmission. However, the increase in acetylcholine-induced LES contractions in diabetic rabbits directly involves activation of the Rho-Rhokinase (ROCK) pathway in our model because preincubation of the LES muscle strips with Y-27632, a ROCK inhibitor, significantly decreased acetylcholine contractions in the diabetic group. According to our data, the failure of Y-27632 to affect EFS-induced contractions while preventing decreased acetylcholine-induced contractions in the diabetic LES muscle may be explained by a predominance of post-junctional cholinergic receptor stimulation over a pre-junctional electrical stimulation in the diabetic milieu. Indeed, it has previously been suggested that the inhibitory effect of Y-27632 is not dependent on prejunctional involvement of ROCK signaling in feline esophageal smooth muscle [18].
The epithelial barrier function is known to be maintained by the resistance of epithelial cell membranes and intercellular tight junctions. Tight junctions are composed of the transmembrane protein occludin, junctional adhesion protein, and the claudin family, which are linked to the actin cytoskeleton via zonula occludens. Inhibition of ROCK was found to increase the adhesion and expression of e-cadherin in esophageal cancer cells, which indicates the importance of the ROCK signaling pathway in cell adhesion [22].
An interesting study by Yang et al. showed that both the outer and inner perimeter and lumen area in both the muscle and mucosa-submucosa layer increased in streptozotocin-induced diabetic rats. A morphometric and biomechanical remodeling in the mucosa-submucosa layer occurs [23]. This morphological and biomechanical remodeling might explain our findings.
We conclude that in this rabbit model of chronic diabetes, the esophagus resists reflux by mucosal defense mechanisms and by increasing contractile pressure. Acid-pepsin co-acts through a paracellular pathway and somehow affects junctional complexes without damaging cells. Thus, the question in our experimental model was how could chronic diabetic EE tissue adequately cope with high concentrations of noxious agents of GERD better than healthy EE tissue. Studies need to be performed to determine whether this increase in resistance is part of an adaptive cytoprotection or a change in structure of junctional complex components.
Future studies with diabetic tissues as well as diabetic patients are required to evaluate whether there are any changes in the properties of the junctional complex such as an increase in the protein expression of e-cadherin or other tight junction proteins.
Abbreviations
- EE:
-
Esophageal epithelia
- EFS:
-
Electrical field stimulation
- GERD:
-
Gastroesophageal reflux disease
- LES:
-
Lower esophageal sphincter
- L-NAME:
-
L-NG-nitroarginine methyl ester
- PD:
-
Transepithelial electrical potential difference
- R:
-
Transepithelial electrical resistance
- Isc:
-
Short-circuit current
References
Jacobson BC, Somers SC, Fuchs CS, Kelly CP, Camargo CA Jr. Body-mass index and symptoms of gastroesophageal reflux in women. N Engl J Med. 2006;354:2340–2348.
Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology. 2007;132:2087–2102.
El-Serag HB, Ergun GA, Pandolfino J, Fitzgerald S, Tran T, Kramer JR. Obesity increases oesophageal acid exposure. Gut. 2007;56:749–755.
Nilsson M, Lagergren J. The relation between body mass and gastro-oesophageal reflux. Best Pract Res Clin Gastroenterol. 2004;18:1117–1123.
Samsom M, Vermeijden JR, Smout A, et al. Prevalence of delayed gastric emptying in diabetic patients and relationship to dyspeptic symptoms: a prospective study in unselected diabetic patients. Diabetes Care. 2003;26:3116–3122.
De Block CE, De Leeuw IH, Pelckmans PA, Van Gaal LF. Current concepts in gastric motility in diabetes mellitus. Curr Diabetes Rev. 2006;2:113–130.
Feldman M, Schiller LR. Disorders of gastrointestinal motility associated with diabetes mellitus. Ann Intern Med. 1983;98:378–384.
Russell RO, Gannan R, Coatsworth J, et al. Relationship among esophageal dysfunction, diabetic gastroenteropathy and peripheral neuropathy. Dig Dis Sci. 1983;28:289–293.
Sun XM, Tan JC, Zhu Y, Lin L. Association between diabetes mellitus and gastroesophageal reflux disease: a meta-analysis. World J Gastroenterol. 2015;21:3085–3092.
Horowitz M, Samsom M. Gastrointestinal function in diabetes mellitus. Wiley, London. Ch 3. Oesophageal Function. Andre J. P. M. Smout. pp 97–116.
Lluch I, Ascaso JF, Mora F, et al. Gastroesophageal reflux in diabetes mellitus. Am J Gastroenterol. 1999;94:919–924.
Murray FE, Lombard MG, Ashe J, et al. Esophageal function in diabetes mellitus with special reference to acid studies and relationship to peripheral neuropathy. Am J Gastroenterol. 1987;82:840–843.
Stewart IM, Hosking DJ, Preston BJ, Atkinson M. Oesophageal motor changes in diabetes mellitus. Thorax. 1976;31:278–283.
Keshavarzian A, Iber FL, Nasrallah S. Radionuclide oesophageal emptying and manometric studies in diabetes mellitus. Am J Gastroenterol. 1987;82:625–631.
Rerup CC. Drugs producing diabetes through damage of the insulin secreting cells. Pharmacol Rev. 1970;22:485–518.
Tobey NA, Orlando RC. Mechanisms of acid injury to rabbit esophageal epithelium. Role of basolateral cell membrane acidification. Gastroenterology. 1991;101:1220–1228.
Tobey NA, Hosseini SS, Caymaz-Bor C, Wyatt HR, Orlando GS, Orlando RC. The role of pepsin in acid injury to esophageal epithelium. Am J Gastroenterol. 2001;96:3062–3070.
Park SY, Song HJ, Sohn UD. Participation of rho-associated kinase in electrical stimulated and acetylcholine-induced contraction of feline esophageal smooth muscle. Eur J Pharmacol. 2009;607:220–225.
Abdulnour-Nakhoul S, Nakhoul NL, Orlando R. Lumen-to-surface pH gradient in opossum and rabbit esophagi: role of submucosal glands. Am J Physiol Gastrointest Liver Physiol. 2000;278:G113–G120.
Horgan JH, Doyle JS. A comparative study of esophageal motility in diabetics with neuropathy. Chest. 1971;60:170–174.
Crist J, Gidda JS, Goyal RK. Intramural mechanism of esophageal peristalsis: roles of cholinergic and noncholinergic nerves. Proc Natl Acad Sci USA. 1984;81:3595–3599.
Wang L, Xue L, Yan H, Li J, Lu Y. Effects of ROCK inhibitor, Y-27632, on adhesion and mobility in esophageal squamous cell cancer cells. Mol Biol Rep. 2010;37:1971–1977.
Yang J, Zhao J, Liao D, Gregersen H. Biomechanical properties of the layered oesophagus and its remodelling in experimental type-1 diabetes. J Biomech. 2006;39:894–904.
Acknowledgments
This study has been supported by Ege University Scientific Research Council (project no. 2011-TIP-025).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Capanoglu, D., Coskunsever, D., Olukman, M. et al. Esophageal Epithelial Resistance and Lower Esophageal Sphincter Muscle Contraction Increase in a Chronic Diabetic Rabbit Model. Dig Dis Sci 61, 1879–1887 (2016). https://doi.org/10.1007/s10620-016-4111-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-016-4111-8