Abstract
Background and Aims
Multiple clinical trials have demonstrated the efficacy and safety of tenofovir disoproxil fumarate (TDF) in chronic hepatitis B (CHB). However, long-term efficacy and safety data for TDF in real-life clinical practice are limited.
Methods
Prospective German field practice study in CHB-mono-infected patients. Patients were TDF-naïve but could have been treated previously with other HBV antivirals.
Results
Efficacy analysis included 400 patients; 301 (75 %) completed 36 months of TDF treatment. Both treatment-naïve and treatment-experienced patients showed a rapid decline in HBV DNA within 3 months of TDF initiation. After 36 months, HBV DNA < 69 IU/mL was achieved by 91 % of treatment-naïve patients (90 and 92 % in hepatitis B “e” antigen [HBeAg]-positive and [HBeAg]-negative, respectively) and 96 % of treatment-experienced patients (93 and 97 %, respectively). Three patients experienced virologic breakthrough, all with reported non-compliance. Overall, 5.7 % HBeAg-positive and 2.2 % HBeAg-negative patients lost hepatitis B surface antigen. Safety data were consistent with the known TDF safety profile; the most commonly reported adverse events possibly related to TDF were fatigue (2.0 %) and headache (2.0 %). Few patients (1.3 %) experienced renal-related adverse reactions. Creatinine clearance remained relatively stable over time; patients responded favorably where TDF was dose adjusted per label for decreased creatinine clearance.
Conclusions
TDF showed a favorable tolerability profile and induced rapid and sustained suppression of HBV DNA in patients with CHB treated for up to 3 years in routine clinical practice, irrespective of treatment history. Efficacy and safety in this heterogeneous patient population were consistent with data from clinical trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The principal treatment goal in patients with chronic hepatitis B infection (CHB) is to prevent disease progression and therefore disease-related death. The nucleotide analog tenofovir disoproxil fumarate (TDF), a potent inhibitor of HBV polymerase shown to suppress HBV DNA levels effectively, is a first-line option for the treatment of CHB in adult and adolescent patients. In Phase 3 clinical trials, TDF had superior antiviral efficacy to adefovir (ADV) in both hepatitis B “e” antigen positive (HBeAg+) and negative (HBeAg−) patients [1]. In these patients, long-term TDF therapy continued to suppress HBV, leading to regression of fibrosis and cirrhosis [2], without viral resistance being detected [3]. TDF has also been shown to be efficacious with a positive risk/benefit profile in patients with compensated liver disease and prior treatment failure to lamivudine (LAM) [4], ADV [5], ADV + LAM [6], and entecavir (ETV) [7]. TDF has also been shown to be effective in the treatment of patients with decompensated liver disease [8].
Studies describing the effectiveness and safety of TDF in real-life settings or routine practice in varied patient populations are few and predominantly retrospective [6, 9–14]. These studies are required to confirm the efficacy and safety established in controlled clinical trials [15].
We conducted a prospective field practice study to evaluate the safety and effectiveness of TDF in real-life clinical settings in Germany.
Methods
Study Design
GEMINIS is a prospective, multicenter field practice study. Patients were enrolled at 33 sites across Germany (private practice 80 %; hospital sites 20 %). Recruitment started in February 2009 and the last patient completed the study in June 2013. The study protocol was reviewed and approved by the respective ethics committees and was conducted according to the Declaration of Helsinki principles. All patients provided written informed consent.
Study Population
The study enrolled treatment-naïve or treatment-experienced patients ≥18 years of age who were mono-infected with HBeAg+ or HBeAg− CHB. TDF was administered at the treating physician’s discretion. Key exclusion criteria included prior treatment with TDF, evidence of hepatocellular carcinoma (HCC), or coinfection with human immunodeficiency virus (HIV), hepatitis C virus (HCV), or hepatitis D virus (HDV).
Data Collection and Study Assessments
Data were collected prospectively via an electronic case report form every 3–6 months for up to 36 months. Data were obtained from medical records and included the following if available: demographic and disease characteristics; laboratory values including transaminases and serum creatinine; HBV serology; HBV DNA levels; reverse transcriptase domain of HBV polymerase (pol/RT) mutations; results of liver examinations (e.g., liver biopsy, noninvasive markers of fibrosis, hepatic ultrasound, endoscopy); adverse events (AEs) considered to be at least possibly related to TDF; pregnancy; and relevant concomitant treatments. Study-specific laboratory tests, resistance monitoring, or prespecified visits were not required.
Virologic breakthrough was defined as a confirmed consecutive increase in HBV DNA level of more than 1 log10 IU/mL compared with the nadir (lowest value) HBV DNA level on therapy.
Statistics
The analysis population consisted of all patients who received at least one dose of TDF (All Treated). Mean, standard deviation (SD), were calculated for continuous variables, together with the total number of observations and the number of non-missing and missing values. For categorical variables, number and percent of patients were reported. Missing data were excluded from the efficacy analysis. HBV DNA levels were recorded as <lower limit of quantification (LLOQ) on the laboratory reports, but classified as <69 IU/mL in this manuscript. Data from one center with LLOQ = 100 IU/mL have been counted as below <69 IU/mL. The cumulative probability of hepatitis B surface antigen (HBsAg) loss was determined by Kaplan–Meier methodology. Fisher’s exact test was performed to determine whether baseline factors of body mass index, HBeAg status, or gamma glutamyl transferase (GGT) levels could predict elevated alanine (ALT) levels. Statistical analysis was performed using SAS® (Version 9.2 or higher).
Results
Study Population
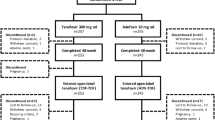
In total, 403 patients were prospectively enrolled. The safety analysis included data from all patients. Three patients were excluded from the efficacy analysis based on specified exclusion criteria so the efficacy population included 400 patients. The majority of patients were male, Caucasian (Table 1), and around two-thirds were HBeAg− (Table 2). At baseline, 35 patients (9 %) were aged ≥65 years; HBeAg− patients were older overall compared with HBeAg+ patients (mean 47 vs. 39 years, respectively).
Around half of the study population was of European descent (Table 1). Mode of HBV infection was known in 39 % of patients: vertical transmission or infection during childhood (15 %), family exposure (5 %), and infection via sexual exposure, professional exposure, nosocomial exposure, or drug (≤2.5 %). Viral genotype was only available in 5 % of patients so was reported for only around 5 % of the patients and was therefore not included in the analysis.
At baseline, HBeAg− and treatment-experienced patients had lower HBV DNA values compared with HBeAg+ and treatment-naïve patients (Fig. 1; Table 2). Of the 43 patients reported to have cirrhosis, clinical signs (ascites, splenomegaly and/or esophageal varices) were reported in 25. This was based on patient records of pretreatment liver biopsy (35 patients) or noninvasive liver fibrosis assessment by transient elastography (Table 1). Comorbidities were reported for 167 (42 %) patients. The most commonly reported were diabetes and hypertension (Table 1), and seven patients (2 %) had undergone an organ transplant prior to entering the study (four kidney, two liver, and one bone marrow).
Viral dynamics over time. Distribution of patient populations by HBV DNA levels during treatment. Bubble diameters are relative to the proportion of patients with HBV DNA at that specified level. a Treatment-naïve patients; *n = 1 (non-compliant); **n = 1 (TDF paused for >1 month); ***n = 6 (2 non-compliance and 4 = LLQ 200 IU/mL). b Treatment-experienced patients; *n = 1 (TDF paused for >7 days); **n = 2 (non-compliance); ***n = 2 (1 non-compliance and 1 = LLQ). c HBeAg+ patients; *n = 1 (non-compliant); **n = 2 (non-compliance); ***n = 1 (TDF paused for >7 days); ****n = 1 (=LLQ). d HBeAg−; *n = 1 (non-compliance); **n = 1 (TDF paused ≥1 month); ***n = 7 (3 non-compliance, 4 = LLQ). HBeAg hepatitis B “e” antigen, LLQ lower limit of quantification, TDF tenofovir disoproxil fumarate
Just over half of all patients were treatment-experienced, with approximately 60 % previously treated with LAM monotherapy or LAM + ADV (Table 2). Persistent viremia, viral relapse, and documented resistance mutations to other antivirals were the most common reasons for initiating treatment with TDF in treatment-experienced patients (Table 2). Approximately 9 % of treatment-naïve patients had undetectable viral load (defined as HBV DNA < 69 IU/mL) compared with 44 % of treatment-experienced patients (Table 2). Reasons for starting TDF therapy in treatment-experienced patients with non-detectable viral load at baseline were persistent viremia (43.2 %), adverse events at least possibly related to previous treatment (12.6 %), viral relapse (11.6 %), documented resistance (9.5 %), non-adherence (7.4 %), and other reasons (15.8 %).
TDF Treatment
All treatment-naïve patients and most treatment-experienced patients started TDF treatment as monotherapy. Where TDF was given as an initial combination therapy (n = 35), it was most frequently combined with ETV (19/35), LAM (8/35), or telbivudine (4/35). Five patients received an oral antiviral agent in addition to TDF during the study. The first patient received add-on treatment due to suboptimal virologic response, the second patient still had a high viral load after 3 months of treatment, and the third patient received prophylactic add-on therapy due to having a lymphoma. The reasons for additional oral treatment were not documented for the remaining patients. Overall, 301 patients (75 %) remained in the study at month 36. TDF was discontinued in 51 patients; known reasons included: unwilling to continue (n = 21); AEs considered possibly related to TDF (n = 11); family planning (n = 4); non-compliance (n = 4); suboptimal response (persistent viremia) (n = 2); and HBsAg seroconversion (n = 2).
Virologic Response
In both treatment-naïve and treatment-experienced patients, there was a rapid decline in HBV DNA within the first 3 months after initiating treatment (Fig. 1). Similar trends were observed in HBeAg+ and HBeAg− patients.
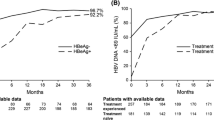
After 12 months, 120/150 (80 %) treatment-naïve (HBeAg+ 59 %; HBeAg− 89 %) and 159/181 (88 %) treatment-experienced patients (HBeAg+ 81 %; HBeAg− 92 %) achieved HBV DNA < 69 IU/mL, rising to 111/122 (91 %) treatment-naïve (HBeAg+ 90 %; HBeAg− 92 %) and 153/160 (96 %) (HBeAg+ 89 %; HBeAg− 99 %) treatment-experienced patients at 36 months (Fig. 2a). Similar results were seen in treatment-experienced patients, with 90 % of patients previously treated with LAM, ETV, or ADV-containing regimens achieving HBV DNA < 69 IU/mL at month 36 (Fig. 2b), including patients who were switched to TDF from ADV for safety or efficacy reasons (Fig. 2c). Baseline ALT levels did not affect efficacy; at month 36, 71/76 (93 %) patients with ALT ≥ 1.5 × ULN and 186/197 (94 %) with ALT < 1.5 × ULN achieved HBV DNA < 69 IU/mL (Fig. 2d). Lastly, similar efficacy was seen in patients ≥65 years of age and in patients <65 years of age (HBV DNA < 69 IU/mL at 36 months: 95 vs. 88 %, respectively).
HBV DNA suppression over time. The proportion of patients with HBV DNA <69 IU/mL based on: a HBeAg status and if treatment naïve or treatment experienced; b prior treatment; c reason for ADV discontinuation (efficacy or safety); and d baseline ALT values. ADV adefovir, ALT alanine aminotransferase, BL baseline, HBeAg hepatitis B “e” antigen
Virologic failure on TDF was rare. Three patients experienced virologic breakthrough (defined as two confirmed consecutive increases in HBV DNA of more than 1 log10 IU/mL from nadir). Two patients discontinued TDF due to persistent viremia (one patient was switched to ETV 0.5 mg/day but continued to have persistent viremia and no further data are available for the second patient). Non-compliance was reported in all patients with virologic breakthrough.
Biochemical Response
Mean (SD) ALT improved from 85 (160.81) U/L at baseline to 35 U/L (20.53) at month 36. The proportion of patients with normal ALT at month 36 was 216/288 (75 %) versus 174/383 (45 %) at baseline. Similar findings were seen irrespective of HBeAg status and treatment history. The only baseline factor predictive of elevated ALT > ULN at month 36, was elevated GGT (p < 0.001).
Serologic Response
Eleven patients lost HBsAg during the study, all of whom had received TDF monotherapy. The annual rate of HBsAg loss was 3.00 % in the first year, 5.23 % in year 2, and 6.43 % in year 3 for HBeAg+ patients and 0.88 % in year 1, 1.37 % in year 2, and 2.95 % in year 3 for HBeAg− patients. Among the 106 patients who were HBeAg+ at baseline and also had available HBsAg data, six patients (6/106, 5.7 %) lost HBsAg; the cumulative probability of HBsAg loss over the 36-month period was 6.43 % in these patients (Fig. 3). All six HBeAg+ patients who lost HBsAg also seroconverted to anti-HBs; however, one patient who seroconverted to anti-HBs after 19 months of treatment reverted after 7 months of continued treatment and was HBsAg+ at the end of the study. In this patient, HBV DNA was 10 IU/mL at the time of HBsAg loss and 12 IU/mL at the end of the study. This patient switched to ETV at the end of the study due to a renal event. One HBeAg+ patient discontinued TDF 26 months after HBsAg loss, and the response was durable at their last available time point. The other four patients who were HBeAg+ at baseline and lost HBsAg continued treatment and remained HBsAg− at their last available time point. Five patients (5/232, 2.2 %) who were HBeAg− at baseline (4/5 treatment experienced) lost HBsAg and were HBsAg− at their last available time point. None of the HBeAg− patients seroconverted to anti–HBs. Of all the 124 patients who were HBeAg+ at baseline, 25 (21 %) lost HBeAg during the study and remained HBeAg− at their last available time point.
Safety
An overall summary of AEs considered by the physicians to be at least possibly related to TDF is shown in Table 3. Twelve patients discontinued TDF due to an AE, 10 of which were considered to be at least possibly related to TDF, including four renal-related events (abnormal renal function tests, 2; renal failure, 2). The most frequently reported AEs considered by the physicians to be at least possibly related to TDF were consistent with the known safety profile of TDF and included fatigue, headache, and nausea, all reported by ≤2 % of patients. Serious adverse events (SAEs) were reported in 11 patients, only one of which (renal failure) was considered related to TDF. HCC was confirmed in one patient after 6 months of TDF treatment and suspected on the basis of clinician perception in a further patient after 3 months of treatment. Both patients continued on TDF, but were subsequently lost to follow-up after 18 and 12 months, respectively. Both patients were male, treatment-naïve, and HBeAg−, and both had cirrhosis with either varices or splenomegaly at baseline.
Five deaths were reported, none of which were considered related to TDF (one ileus/intestinal obstruction, one primary transplant failure after liver transplantation, one multi-organ failure after sepsis, one pneumonia, and one cause not reported).
Eight pregnancies were reported and TDF was administered during all trimesters in five of the eight pregnancies. TDF was well tolerated and all newborns were healthy and HBsAg− at birth.
Mean creatinine clearance, serum creatinine, and serum phosphorus remained relatively stable over 36 months of treatment (Table 4).
Renal endpoints were observed in approximately 5 % of patients: seven patients with a serum creatinine increase >0.5 μmol/L from baseline at any time point during the study and 18 patients with creatinine clearance <50 mL/min at any time point during the study. Of these patients, nine had renal insufficiency at baseline. Overall, the TDF dose was reduced in 8/18 patients with baseline or on treatment creatinine clearance <50 mL/min, most of whom (6/8) subsequently remained stable on TDF through month 36. Figure 4 shows representative creatinine clearance data for patients who underwent dose reductions at baseline or on treatment. Mean changes from baseline in creatinine clearance and serum creatinine were similar in patients aged ≥65 years and those aged <65 years (4.01 vs. 3.95 µmol/L at 12 months, 1.77 vs. 2.83 µmol/L at 24 months, and 1.33 vs. 2.54 µmol/L at 36 months, respectively).
Renal failure was reported as an AE (one serious) in three patients; all events were considered by the physician to be at least possibly related to TDF. Two of these patients had renal dysfunction at baseline and entered the study with creatinine clearance <50 mL/min, and both had been previously treated with LAM and subsequently ADV, and received a TDF dose in excess of the recommended dose in the Summary of Product Characteristics (SmPC). Creatinine clearance declined further on treatment, and the patients were switched to LAM or lost to follow-up.
Discussion
This field practice study of 400 patients demonstrated that TDF is an effective treatment for CHB in both treatment-naïve and treatment-experienced patients in clinical practice.
The patients in this study represent a heterogeneous population of HBeAg + and HBeAg− patients from 18 to 82 years of age. They were either treatment naïve or treatment experienced, from diverse geographic regions, and with a range of comorbidities, including mild-to-severe renal impairment. Additionally, patients were enrolled who had previously undergone transplantation or had become pregnant while taking TDF. The treatment-experienced patients included in this study had a broad range of prior treatment regimens (i.e., LAM, ADV, ETV, interferon, or combinations thereof) as well as a range of reasons for prior treatment discontinuation (i.e., persistent viremia, relapse, resistance, AEs, non-adherence). Since these patients are typical in routine clinical practice, it is important to demonstrate the antiviral efficacy of TDF among these diverse groups. This analysis presents data from the prospectively planned 36-month study period, and patients from the GEMINIS cohort will be followed for a further 3 years in the roll-over “VIR-Life” study.
Approximately 10 % of patients were reported to have cirrhosis at baseline, and just over half of these patients had symptoms of advanced disease and/or decompensation. There was no report of a history of advanced liver disease for the majority, and few patients had liver biopsy or transient elastography data available. Interestingly, over 70 % of the patients enrolled in this study were unaware of how they acquired the disease, suggesting that educational initiatives are needed to increase understanding of the disease as well as increased screening.
Based on the German National Guidelines [16] and the current European Association for the Study of the Liver (EASL) clinical practice guidelines [17], treatment appeared to be indicated in the majority of the treatment-naïve patients started on TDF based on baseline HBV DNA and ALT values. The majority of treatment-naïve patients had HBV DNA >2000 IU/mL, and 42 and 68 % of HBeAg− and HBeAg+ patients, respectively, had ALT > 1.5 × ULN. Since virologic suppression has been shown to be associated with a reduced probability of disease progression [18–21], treatment initiation becomes paramount to managing the disease and preventing progression to advanced liver disease and its associated complications.
TDF produced potent suppression of HBV DNA, irrespective of the patient population. HBeAg+ and HBeAg− treatment-naïve and treatment-experienced populations had a very robust response to TDF with 89–99 % of patients achieving HBV DNA <69 IU/mL at 3 years. In all treatment-experienced groups, irrespective of previous therapy, TDF caused rapid and durable suppression of HBV DNA. These data are consistent with the literature showing efficacy across a broad range of populations reported in clinical trials and real-life studies and in retrospective analyses [2, 5, 8, 10, 22]. Consistent with previously reported clinical trial results for TDF, there was an increased proportion of patients with normal ALT following 36 months of treatment [22].
Among HBeAg+ patients, the cumulative probability of HBsAg loss over 3 years was 6.4 %. Five HBeAg− patients in the current study lost HBsAg over the 3 year period; this is higher than the rate seen in HBeAg- patients in pivotal clinical trials, where only one HBeAg− patient with HBsAg loss was reported over 5 years of treatment [1, 2, 22]. Although these are encouraging results, patient numbers are small and only one patient was treatment-naïve prior to initiating TDF treatment. The lower rate of HBeAg loss reported for this study compared with the published 3-year clinical trial data (34 % HBeAg loss and 26 % HBeAg seroconversion [22] ) is most likely a reflection of a high proportion of patients with missing HBeAg serology data at end of study in the GEMINIS cohort; for example, only 30 % of patients had HBeAg serology data at 36 months.
The safety profile observed for TDF over the 3-year period in GEMINIS was consistent with the known safety profile for the drug [1]. TDF was well tolerated in all patient populations with only 3 % of patients discontinuing due to AEs considered by the treating physician to be at least possibly related to TDF. Renal function was generally stable over time, and when patients were managed according to the SmPC, with appropriate dose adjustments for creatinine clearance estimates, patients responded favorably. When the dose of TDF was not adjusted, particularly in patients with known renal insufficiency, renal function deteriorated. These data emphasize the importance of following the SmPC dose modification guidance. Although serum creatinine levels are commonly used markers of renal function, other urinary and serum proteins may be earlier, more accurate markers of renal toxicity [23]. However, testing of such biomarkers is not routinely used in clinical practice and creatinine clearance was therefore used in this real-life study to monitor renal function. Lastly, TDF was shown to be well tolerated in patients who became pregnant during the study with no apparent effects on the fetus, even when administered during the first trimester of pregnancy. TDF has been assigned pregnancy category B by the US Food and Drug Administration (FDA) and has been shown to be well tolerated in pregnancy in a number of studies, reducing maternal HBV DNA levels and as a consequence, vertical transmission [24, 25].
This study was limited by the variable amount of missing data throughout the study resulting in some limitations to the analysis and the evaluation of an “as treated” population. In addition, sites used local laboratories for testing so data were heterogeneous. Nevertheless, our data represent a well-documented and prospective real-life clinical dataset of CHB treatment in routine practice in Germany.
In conclusion, data from this real-world cohort demonstrate that TDF is a potent first-line therapy option for CHB management. This study confirms that long-term treatment with TDF in clinical practice is effective and well tolerated across a broad spectrum of populations, which is consistent with findings in the clinical registration trials and other studies.
References
Marcellin P, Heathcote EJ, Buti M, et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med. 2008;359:2442–2455.
Marcellin P, Gane E, Buti M, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468–475.
Kitrinos KM, Corsa A, Liu Y, et al. No detectable resistance to tenofovir disoproxil fumarate after 6 years of therapy in patients with chronic hepatitis B. Hepatology. 2014;59:434–442.
Fung S, Kwan P, Fabri M, et al. Randomized comparison of tenofovir disoproxil fumarate vs emtricitabine and tenofovir disoproxil fumarate in patients with lamivudine-resistant chronic hepatitis B. Gastroenterology. 2014;146:980–988.
Berg T, Marcellin P, Zoulim F, et al. Tenofovir is effective alone or with emtricitabine in adefovir-treated patients with chronic-hepatitis B virus infection. Gastroenterology. 2010;139:1207–1217.
Patterson SJ, George J, Strasser SI, et al. Tenofovir disoproxil fumarate rescue therapy following failure of both lamivudine and adefovir dipivoxil in chronic hepatitis B. Gut. 2011;60:247–254.
Pan CQ, Hu KQ, Yu AS, et al. Response to tenofovir monotherapy in chronic hepatitis B patients with prior suboptimal response to entecavir. J Viral Hepatitis. 2012;19:213–219.
Liaw YF, Sheen IS, Lee CM, et al. Tenofovir disoproxil fumarate (TDF), emtricitabine/TDF, and entecavir in patients with decompensated chronic hepatitis B liver disease. Hepatology. 2011;53:62–72.
Lampertico P, Soffredini R, Yurdaydin C, et al. Four years of TDF for NUC naïve field practice European patients suppress HBV replication in most patients with a favorable renal safety profile but do not prevent HCC in patients with or without cirrhosis. Presented at AASLD, Washington DC, USA, November 1–5, 2013; poster 933.
van Bömmel F, deMan RA, Wedemeyer H, et al. Long-term efficacy of tenofovir monotherapy for hepatitis B virus-monoinfected patients after failure of nucleoside/nucleotide analogues. Hepatology. 2010;51:73–80.
van Bömmel F, de Man RA, Rutter R, et al. A European multicenter analysis of long term tenofovir (TDF) monotherapy for chronic hepatitis B in real life setting: efficacy, safety and HCC incidence. Presented at AASLD, Washington DC, USA, November 1–5, 2013; poster 941.
Levrero M, Cimino L, Lampertico P, et al. OptiB—A multicenter prospective open label study on Tenofovir (TDF) for chronic hepatitis B patients with suboptimal response to adefovir (ADV) or ADV/LAM treatment. Presented at EASL, Berlin, Germany, March 30–April 3, 2011; poster 732.
Lim L, Patterson S, George J, et al. Tenofovir rescue therapy achieves long-term suppression of HBV replication in patients with multi-drug resistant HBV: 5 year follow-up of the TDF109 cohort. Presented at AASLD, Boston MA, USA, November 9–13, 2012; poster 361.
Peterson J, Ratziu V, Buti M, et al. Entecavir plus tenofovir combination as rescue therapy in pre-treated chronic hepatitis B patients: an international multicenter cohort study. J Hepatol. 2012;56:520–526.
Pol S, Lampertico P. First-line treatment of chronic hepatitis B with entecavir or tenofovir in “real-life” settings: from clinical trials to clinical practice. J Viral Hepatitis. 2012;19:377–386.
Cornberg M, Protzer U, Petersen J, et al. Aktualisierung der s3-leitlinie zur prophylaxe, diagnostik und therapie der hepatitis-b-virusinfektion. Z Gastroenterol. 2011;49:871–930.
EASL. EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167–185.
Iloeje UH, Yang HI, Su J, et al. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology. 2006;130:678–686.
Zoutendik R, Rejinders GP, Zoulim F, et al. Virological response to entecavir is associated with a better clinical outcome in chronic hepatitis B patients with cirrhosis. Gut. 2013;62:760–765.
Yuan HJ, Yuen MF, Ka-Ho WD, Sablon E, Lai CL. The relationship between HBV–DNA levels and cirrhosis-related complications in Chinese with chronic hepatitis B. J Viral Hepatol.. 2005;12:373–379.
Chen CJ, Iloeje UH, Yang HI. Serum hepatitis B virus DNA as a predictor of the development of cirrhosis and hepatocellular carcinoma. Curr Hepatol Rep. 2007;6:9–16.
Heathcote EJ, Marcellin P, Buti M, et al. Three year efficacy and safety of tenofovir disoproxil fumarate treatment for chronic hepatitis B. Gastroenterology. 2011;140:132–143.
Lock E. Sensitive and early markers of renal injury: where are we and what is the way forward? Toxicol Sci. 2010;116:1–4.
Tsai PJ, Chang A, Yamada S, Tsai N, Bartholomew ML. Use of tenofovir disoproxil fumarate in highly viremic, hepatitis B mono-infected pregnant women. Dig Dis Sci. 2014;59:2797–2803.
Greenup AJ, Tan PK, Nguyen V, et al. Efficacy and safety of tenofovir disoproxil fumarate in pregnancy to prevent perinatal transmission of hepatitis B virus. J Hepatol. 2014;61:502–507.
Funding
This study was supported by Gilead Sciences GmbH.
Author contributions
All authors were involved in data acquisition and analysis and interpretation of the data, contributed to drafting the manuscript with regards to important intellectual content and approved the manuscript prior to submission. Medical writing assistance was provided by Carol Lovegrove and Liesje Quine, Elements Communications Ltd, Westerham, UK and Jane Anderson, Chapel Hill, North Carolina, USA and funded by Gilead Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
JP is an investigator and speaker/consultant for Bristol-Myers Squibb, Novartis, Roche, AbbVie and a speaker/consultant for Abbott, Boehringer, Gilead, and GlaxoSmithKline. CE is a speaker/consultant for Bristol-Myers Squibb, Gilead, Janssen, Merck Sharp & Dohme and Roche. SM is a speaker/consultant for Gilead, Bristol-Myers Squibb, Roche and Merck Sharp & Dohme. DH is on the Speakers Bureau for Gilead, Janssen, Merck Sharp & Dohme, Roche, and has served on Advisory Boards for Gilead, Janssen and Roche. KB is a speaker/consultant for Bristol-Myers-Squibb, Gilead, Roche, Merck Sharp & Dohme, Novartis, Janssen-Cilag, and Gilead. SR and TW are employees of Gilead. JS, HH, CJ, and CT, have no conflicts of interest to declare.
Research involving human participants and/or animals
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Petersen, J., Heyne, R., Mauss, S. et al. Effectiveness and Safety of Tenofovir Disoproxil Fumarate in Chronic Hepatitis B: A 3-Year Prospective Field Practice Study in Germany. Dig Dis Sci 61, 3061–3071 (2016). https://doi.org/10.1007/s10620-015-3960-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-015-3960-x