Abstract
Background
Fully covered self-expandable metal stents (FCSEMS) are increasingly used for treatment of benign common bile duct (CBD) stricture or leakage, but dislodgement of FCSEMS is frequent.
Aims
To compare dislocation rate and clinical outcome of a standard fixed cell structure FCSEMS (S-FCSEMS) to a novel FCSEMS with an unfixed cell structure (N-FCSEMS).
Methods
We performed a retrospective analysis of all patients with FCSEMS insertion for benign biliary disease at our Hospital from 03/2008 to 03/2014. Both stent types N-FCSEMS and S-FCSEMS were applied as available unrelated to the indication.
Results
Twenty-nine patients (S-FCSEMS: 18, N-FCSEMS: 11) were included. Stent placement was technically successful in 28/29 (96.6 %) patients; stent removal was successful in 26/27 (96.2 %). Two patients with N-FCSEMS were excluded due to unsuccessful placement and withdrawal of consent for stent removal, respectively. Stent migration into the duodenum (distal migration) was observed in 9/18 (50 %) in the S-FCSEMS group compared to 0/9 in the N-FCSEMS (p < 0.005). FCSEMS migration into the CBD (proximal migration) was found in 2/18 (11 %, S-FCSEMS) versus 2/9 (22 %, N-FCSEMS, p = 0.514). A foreshortening of the N-FCSEMS occurred in 3/9 patients (33 %) compared to 0/18 S-FCSEMS (p = 0.08). Clinical resolution of the treated CBD-disease was observed in 5/9 (56 %, N-FCSEMS) versus 12/18 (67 %, S-FCSEMS) at the time of stent removal (p = 0.604) and in 0/9 and 10/18 (56 %) cases during follow-up, respectively (p < 0.005).
Conclusion
An unfixed cell structure of FCSEMS seems to prevent distal migration, but proximal migration still occurs and foreshortening of the N-FCSEMS constrains clinical outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fully covered, removable self-expandable metal stents (FCSEMS) are increasingly used for treatment of benign common bile duct (CBD) stenosis as well as leakage, and FCSEMS are a promising alternative to the current mainstay of treatment, i.e., temporary insertion of multiple non-expandable plastic stents in selected patients [1]. Endoscopic insertion of multiple plastic stents has proven to be safe and effective in patients with stenosis of the choledocho-choledochal anastomosis after orthotopic liver transplantation (OLT), in postoperative strictures from iatrogenic bile duct injury from laparoscopic cholecystectomy, and in patients with distal CBD stenosis in advanced chronic pancreatitis (CP) [2, 3]. However, to avoid septic complications, plastic stents require to be replaced at certain time intervals and stent exchanges need repeated interventions by endoscopic retrograde cholangio-pancreatography (ERCP). Thereby, patients undergo a mean of four to five ERCPs [2, 4] until resolution of the stenosis. Repeated treatment sessions are burdensome to the patient and might compromise the compliance.
An alternative endoscopic treatment option that offers equal efficiency without the need to repeat ERCP is therefore desirable for patients with benign biliary disease. Preliminary data indicate that FCSEMS can be successfully applied in postoperative biliary strictures and in CBD strictures that result from CP and that the clinical outcome might be similar to inserting multiple plastic stents while fewer ERCP procedures are needed [5]. However, migration of FCSEMS is reported to occur in up to 10–37 % of cases, which severely hampers this therapeutic approach and leads to the development of anti-migration features such as flared ends or anchoring flaps [6–10].
In this present study, we therefore compared the migration rate and clinical outcome of a standard fixed cell structure FCSEMS (S-FCSEMS) to a novel FCSEMS with an unfixed cell structure and irregular cell size as well as flared ends as anti-migration features (N-FCSEMS), which so far has only been described for use in pancreatic strictures [11].
Patients and Methods
Patients and Study Design
All patients treated with FCSEMS for benign biliary disease at Frankfurt University Hospital between March 2009 and March 2014 were retrospectively analyzed. Inclusion criteria were benign biliary disease, i.e., benign CBD stricture, postoperative bile duct leakage, and treatment of severe post-sphincterotomy bleeding. All patients were at least 18 years old. Patients might have undergone previous treatment with plastic stents. Exclusion criteria were malignant disease, previous treatment with a FCSEMS, or hepatico-jejunostomy. Demographic data, etiology of the stricture or the leakage, previous plastic stent insertion, size of the FCSEMS, indwell time, positioning of the stent at removal, and any complications were noted. All patients provided written informed consent before the procedures.
FCSEMS removal was scheduled at 4–8 months after stent placement depending on the clinical indication. The patients were followed in our outpatient clinic every 3 months or when clinically indicated until 1 year later. This study was approved by the institutional review board of the Johann Wolfgang Goethe-University Hospital.
Material and Technique
The ERCP was performed by one of the six experienced endoscopists. We used duodenoscopes TJF-160 VR or TJF-Q 180V (both Olympus, Tokyo, Japan) with an outer diameter of 11.3 mm and a working channel of 4.2 mm. For placing the FCSEMS, a guidewire was introduced through the stricture or over the leakage. In all patients, endoscopic sphincterotomy preceded FCSEMS placement, and sphincterotomy was performed directly before FCSEMS insertion in case it had not been performed previously. The distal part of the FCSEMS was placed outside the duodenal papilla. When patients still had their gall bladder in situ, the proximal end of the FCSEMS was placed distal to the cystic duct orifice. Removal of the FCSEMS was carried out via a rat-tooth forceps or by use of a standard polypectomy snare by dragging the removal strings or grabbing the stent at its distal part.
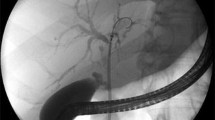
A FCSEMS with anti-migration features (Niti-S™ biliary stent, bumpy™ type, Taewoong Medical, Seoul, South Korea; N-FCSEMS) was compared to a standard FCSEMS (Taewoong Niti-S™ biliary stent, Taewoong Medical, Seoul, South Korea; S-FCSEMS). Both FCSEMS were fully covered and removable. The N-FCSEMS provides flared ends at both sides of the stent, an unfixed cell structure, and an irregular cell size resulting in different segmental radial forces that are expected to lessen the migration rate (Figs. 1, 2). The N-FCSEMS is fully covered with a polytetrafluoroethylene membrane at the body and a silicone membrane at both ends. At the distal end, there is a removal string. This stent was compared to an S-FCSEMS that features a silicone covering and a fixed cell structure and provides a retrieval sling at the distal end, but provides no anti-migration feature. N-FCSEMS and S-FCSEMS were applied as available for both stenotic and non-stenotic benign biliary diseases.
Follow-Up and Definitions
The placement of all FCSEMS was evaluated for (1) technical success, which was defined as insertion covering the leakage or stricture without any procedure-related complications and appropriate fluoroscopic positioning, (2) functional success as determined by successful bile drainage, (3) safety, e.g., procedure-related morbidity, (4) successful stent removal, (5) position of the FCSEMS at time of scheduled or early endoscopic removal, (6) complications that lead to early removal before the scheduled date, and (7) clinical resolution of the underlying disease.
The primary outcome measure was the position of the stent at removal; secondary outcome measures were removal success, resolution of the stricture or leakage, FCSEMS-related complications, and clinical outcome.
Distal migration was defined as dislocation of the FCSEMS into the duodenum with loss of intended function or the absence of the FCSEMS at the time of scheduled removal. Proximal migration was defined as hepatopetal dislocation of the FCSEMS into the CBD with loss of intended function and the absence of endoscopic visibility with the duodenoscope at a duodenal position.
Statistical Analysis
The present study is a retrospective cohort study. Data were analyzed by using BiAS (version 10.04, BiAS for Windows; Epsilon Verlag, Frankfurt, Germany). p values were two-tailed, and p below 0.05 was considered statistically significant.
Results
Patients
Twenty-nine patients were included in this study: 11 patients were treated with N-FCSEMS (diameter of 10 mm, length 6–8 cm), and 18 with S-FCSEMS (diameter of 10 mm, length of 5–10 cm). Etiology of benign biliary disease was anastomotic choledochal stricture after OLT (n = 8, 30 %), CBD stricture from CP (n = 6, 22 %), other stenotic disease such as postsurgical or inflammatory stricture (n = 5, 19 %), and non-stenotic biliary disease, e.g., post-interventional bleeding, bile duct leakage, or intraductal adenoma (n = 10, 37 %) (Table 1).
Stent Placement and Removal
In 28/29 patients (96.6 %), FCSEMS placement was technically successful and evacuation of bile through the SEMS was confirmed by fluoroscopical demonstration of flow of contrast medium. There were no procedure-related complications. In one patient, the N-FCSEMS did not bridge the stenosis and plastic stents were inserted after FCSEMS removal. This patient was excluded from further analysis as well as another patient who withdrew consent for FCSEMS removal. FCSEMS extraction was successful in 26/27 (96.2 %): One S-FCSEMS could not be removed at the first ERCP session due to proximal migration of the stent, but extraction was successfully performed at a second ERCP. S-FCSEMS were removed after a mean of 95-day indwell time (SD 69, range 5–239), and N-FCSEMS after a mean of 89 days (SD 88, range 4–215, p = 0.428).
Outcome of FCSEMS Treatment
Migration
There was a distal migration rate of 9/18 (50 %) in the S-FCSEMS group compared to nil in the N-FCSEMS group (p < 0.005). Distal migration occurred in both stenotic (n = 5) and non-stenotic diseases (n = 4). There was hepatopetal migration into the CBD observed in two of eighteen (11 %) patients in the N-FCSEMS and in two of nine (22 %) of the N-FCSEMS group (p = 0.514). Indication of stent placement in these patients was stenotic (N-FCSEMS: N = 2; S-FCSEMS: n = 1) and non-stenotic disease (S-FCSEMS: n = 1), respectively. In 3/9 N-FCSEMS (33 %, indications: stenosis: 1, non-stenotic disease: 2), a foreshortening of the stent led to malfunction. Thereby, the N-FCSEMS demonstrated shrinkage in length at the time of reapplication of ERCP in comparison with the initial procedure when the FCSEMS was inserted. Thus, the underlying disease was insufficiently treated. There was no foreshortening in the S-FCSEMS group (p = 0.08). There was no significant difference between S-FCSEMS (7/18, 39 %) and N-FCSEMS (4/9, 44 %) found being in situ at the time of removal (p = 0.797) (Table 2).
Non-Scheduled Removal of FCSEMS
Removal of FCSEMS before the scheduled date was observed in ten patients (N-FCSEMS: n = 5, S-FCSEMS: n = 5). Early removal was due to stent dysfunction in three patients with N-FCSEMS and four patients with S-FCSEMS. Two N-FCSEMS (22 %) and one S-FCSEMS (6 %) were removed early due to abdominal pain without any sign of stent dysfunction (p = 0.313). FCSEMS-related complications included cholestasis (N-FCSEMS: n = 1; S-FCSEMS: n = 2), cholangitis (N-FCSEMS: n = 1, S-FCSEMS: n = 1) leakage (N-FCSEMS: n = 1, S-FCSEMS: none), and bleeding (N-FCSEMS: none, S-FCSEMS: n = 1).
Clinical Outcome
Resolution of the treated disease was observed in 5/9 patients with N-FCSEMS (55 %) versus 12/18 S-FCSEMS (67 %) at the time of stent removal (p = 0.604) and in 0/9 versus 10/18 (56 %) patients during follow-up (p < 0.005), respectively.
Of five N-FCSEMS that had been removed before scheduled date, two patients (stenotic disease: 1, non-stenotic disease: 1) had stent dysfunction and were treated with plastic stents in the same session. The other three patients (stenotic disease: 1, non-stenotic disease: 2) had resolution of disease at the ERCP, but there was recurrence in follow-up, so plastic stents (n = 2) or another FCSEMS (n = 1) were applied later on. Of four patients with N-FCSEMS being removed on schedule, one still had a stenosis and plastic stents were inserted. Two patients had resolution of stricture at scheduled ERCP but recurrent stenosis later on. Of these, one is still treated with plastic stents whereas the other had resolution of stricture after another FCSEMS. Another patient with removal on schedule was found to have an ongoing bilioenteric fistula and went to surgery for hepatico-jejunostomy. Conclusively, there was no patient treated with the N-FCSEMS who had persistent clinical response at the time of follow-up after a mean time period of 24.9 months (SD 13, range 3–50).
Of the five patients with S-FCSEMS and early removal, two had an ongoing stricture and were immediately treated with plastic stents, two had no recurrence of disease, and one patient died due to a septic shock not related to the stent during follow-up. Thirteen stents in the S-FCSEMS group were removed on schedule; of these patients, three still had a stenosis at removal, two had a recurrent stenosis, and eight had no recurrence of disease. So overall, 8/13 patients (62 %) of the S-FCSEMS group with stent removal on schedule had persistent clinical resolution (Fig. 3).
Discussion
Benign biliary strictures are amenable to minimally invasive ERCP with excellent treatment results in most cases [9]. Until lately, treatment of benign biliary disease was based on plastic stent placement [3]. Recently, FCSEMS insertion has yielded encouraging results with similar efficacy as non-expandable plastic stents. FCSEMS offer a longer indwell patency, and patients do not need to undergo repeated interventions for scheduled stent exchanges as they do for plastic stents. The main drawback of using FCSEMS in benign biliary disease might be a migration rate which is reported to be 10–40 % [7, 9, 12–14].
Recent developments of FCSEMS include anti-migration modifications such as anchoring flaps and flared ends to decrease the stent migration rate of FCSEMS. A large retrospective study reported a low migration rate of 10.5 % for FCSEMS with flared ends [8], and a migration rate of 3.3 % was shown by a recent prospective study for FCSEMS with anchoring flaps [10]. A comparative pilot study of the two anti-migration features observed a significantly lower rate of migration in FCSEMS with an anchoring flap in comparison with FCSEMS with flared ends (0 vs. 33 %, p = 0.04) [15]. However, anchoring flaps come along with an increased rate of duodenal or intraductal ulcerations in 43–67 %, and significant bleeding at removal has been reported [16, 17].
In the present study, we retrospectively analyzed the migration rate of a stent with flared ends that features an unfixed irregular cell structure to avoid migration in comparison with a standard FCSEMS that is without these features. To our knowledge, this is the first report on this type of stent in the treatment of benign biliary disease. We observed no case of distal migration of the N-FCSEMS compared to 50 % in the S-FCSEMS group, and we found a successful removal rate of 100 % without any removal-related complications in the N-FCSEMS group. These results suggest that the design of the N-FCSEMS contributes to anchoring the stent in the CBD without causing any mucosal injuries, which might also be attributed to the high conformability of the stent. On the other hand, the unfixed cell structure does not prevent the stent from hepatopetal migration as we observed a proximal migration rate of 22 versus 11 % in the S-FCSEMS group. Furthermore, in three patients we found the stent to be shortened and thus not covering the leakage or stenosis any more. The foreshortening was due to a secondary contraction of the unfixed cell structure which led to distal migration of the proximal end of the stent. The distal end remained in place within the CBD. This type of stent dysfunction has not been described so far for other FCSEMS with a fixed cell structure and can be attributed to the new design. In two patients, we had to remove the stent earlier than scheduled due to abdominal pain. One patient presented 7 days after stent placement and asked for removal because of new onset of abdominal discomfort. Another patient presented 47 days after stent placement with abdominal pain and a slightly elevated C-reactive protein, so the stent was removed as well. In both cases, the stent was in situ and there were no signs of cholangitis. Due to the small patient number of our study and the fact that both patients were known to have a low threshold of pain sensitivity, it is difficult to say whether the unfixed cell structure is indeed correlated to a higher rate of abdominal pain. However, the high rate did not meet our initial expectation that the conformability of the stent would reduce symptoms such as abdominal pain or discomfort.
We observed no long-term clinical resolution in the five patients with removal on schedule in the group with the unfixed cell structure. Reasons for these results can be manifold, but our hypothesis is that there might be reduced tension of the stent due to the unfixed cell structure and conformability. This could have lead to a decreased pressure on the CBD mucosa, resulting in a higher rate of recurrent stenosis. On the other hand, this might be an advantage for non-stenotic benign biliary disease such as biliary leaks where the reduced pressure can be rather protective for mucosal microvascularization. Apart from our results, good clinical outcome was reported for the N-FCSEMS in pancreatic strictures, so eventually more data are needed to describe long-term outcome in benign biliary disease sufficiently.
Limitations of our study are a retrospective, single-center design with small cohorts and different etiologies of benign biliary disease.
In conclusion, the novel FCSEMS with flared ends and an unfixed cell structure prevents distal migration and causes no mucosal injuries but is still associated with proximal migration. Furthermore, foreshortening can lead to dysfunction. These findings support further development of the stent design and the initiation of a prospective study to compare effectiveness and safety of both stent types.
References
Dumonceau J, Tringali A, Blero D, et al. Biliary stenting: indications, choice of stents and results: European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Endoscopy. 2012;44:277–298.
Albert JG, Filmann N, Elsner J, et al. Long-term follow-up of endoscopic therapy in stenosis of the bilio-biliary anastomosis associated with orthotopic liver transplantation. Liver Transpl. 2013;19:586–593.
Costamagna G, Tringali A, Mutignani M, et al. Endotherapy of postoperative biliary strictures with multiple stents: results after more than 10 years of follow-up. Gastrointest Endosc. 2010;72:551–557.
Poley JW, Lekkerkerker MN, Metselaar HJ, Kuipers EJ, Bruno MJ. Clinical outcome of progressive stenting in patients with anastomotic strictures after orthotopic liver transplantation. Endoscopy. 2013;45:567–570.
Poley J, Cahen DL, Metselaar HJ, et al. A prospective group sequential study evaluating a new type of fully covered self-expandable metal stent for the treatment of benign biliary strictures (with video). Gastrointest Endosc. 2012;75:783–789.
Traina M, Tarantino I, Barresi L, et al. Efficacy and safety of fully covered self-expandable metallic stents in biliary complications after liver transplantation: a preliminary study. Liver Transpl. 2009;15:1493–1498.
Cahen DL, Rauws EA, Gouma DJ, Fockens P, Bruno MJ. Removable fully covered self-expandable metal stents in the treatment of common bile duct strictures due to chronic pancreatitis: a case series. Endoscopy. 2008;40:697–700.
Kahaleh M, Brijbassie A, Sethi A, et al. Multicenter trial evaluating the use of covered self-expanding metal stents in benign biliary strictures: time to revisit our therapeutic options? J Clin Gastroenterol. 2013;47:695–699.
Deviere J, Nageshwar Reddy D, et al. Successful management of benign biliary strictures with fully covered self-expanding metal stents. Gastroenterology. 2014;147:385–395.
Mangiavillano B, Manes G, Baron TH, et al. The use of double lasso, fully covered self-expandable metal stents with new “anchoring flap” system in the treatment of benign biliary diseases. Dig Dis Sci. 2014;59:2308–2313.
Moon SH, Kim MH, Park do H, et al. Modified fully covered self-expandable metal stents with antimigration features for benign pancreatic-duct strictures in advanced chronic pancreatitis, with a focus on the safety profile and reducing migration. Gastrointest Endosc. 2010;72:86–91.
Wagh MS, Chavalitdhamrong D, Moezardalan K, et al. Effectiveness and safety of endoscopic treatment of benign biliary strictures using a new fully covered self expandable metal stent. Diagn Ther Endosc. 2013;2013:183513.
Perri V, Boskoski I, Tringali A, et al. Fully covered self-expandable metal stents in biliary strictures caused by chronic pancreatitis not responding to plastic stenting: a prospective study with 2 years of follow-up. Gastrointest Endosc. 2012;75:1271–1277.
Tarantino I, Mangiavillano B, Di Mitri R, et al. Fully covered self-expandable metallic stents in benign biliary strictures: a multicenter study on efficacy and safety. Endoscopy. 2012;44:923–927.
Park DH, Lee SS, Lee TH, et al. Anchoring flap versus flared end, fully covered self-expandable metal stents to prevent migration in patients with benign biliary strictures: a multicenter, prospective, comparative pilot study (with videos). Gastrointest Endosc. 2011;73:64–70.
Wang AY, Ellen K, Berg CL, Schmitt TM, Kahaleh M. Fully covered self-expandable metallic stents in the management of complex biliary leaks: preliminary data—a case series. Endoscopy.. 2009;41:781–786.
Mahajan A, Ho H, Sauer B, et al. Temporary placement of fully covered self-expandable metal stents in benign biliary strictures: midterm evaluation (with video). Gastrointest Endosc. 2009;70:303–309.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Walter, D., Sarrazin, C., Trojan, J. et al. No Distal Migration in Unfixed Versus Fixed Cell Structure Covered Self-Expanding Metal Stents for Treatment of Benign Biliary Disease. Dig Dis Sci 60, 2495–2501 (2015). https://doi.org/10.1007/s10620-015-3656-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-015-3656-2