Abstract
Background
It is unclear whether the quality of bowel preparation affects colonoscopic detection of non-polypoid colorectal neoplasms (NP-CRNs).
Aim
To evaluate the impact of bowel-cleansing quality on detection of NP-CRNs.
Methods
We performed a retrospective analysis of asymptomatic screening colonoscopy cases after standardized bowel preparation at an academic teaching hospital between June 2011 and May 2013. Primary outcome was a comparison of the adenoma detection rate (ADR) of non-polypoid morphology according to quality of bowel preparation. Secondary outcomes included detection prevalence of non-polypoid adenomas.
Results
Of the enrolled 6097 screening examinations, the preparation quality was rated as adequate (excellent or good) in 5224 (85.7 %), fair in 615 (10.1 %), and poor in 258 (4.2 %) patients. The prevalence of NP-CRNs was 40.5 % (1962/4847) of all CRNs. The overall ADR of non-polypoid morphology was 12.3 % (747/6097) of all colonoscopies, but it significantly differed among participating endoscopists (all P < 0.05). The ADR of non-polypoid morphology was significantly lower with fair- or poor-quality preparation, versus adequate-quality preparation (adjusted odds ratio [aOR] 0.55, 95 % confidence interval [CI] 0.41–0.75; aOR 0.49, 95 % CI 0.30–0.79, respectively). Poor-quality preparation was also associated with impaired detection of polypoid, proximal colon, and sub-centimeter adenomas (all P < 0.05).
Conclusions
Suboptimal (fair or poor) bowel preparation significantly impairs colonoscopic detection of NP-CRNs. Given that the prevalence of NP-CRNs is substantial in our average-risk screening cohort, ongoing efforts to improve the preparation quality are practically valuable in increasing the detection of NP-CRNs, thereby improving the efficacy of screening colonoscopies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colonoscopy is the most effective screening modality for colorectal cancer (CRC) and is also the best cancer prevention test, because of its potential to remove precancerous adenomas, which could otherwise progress to CRC [1–3]. However, the effectiveness of colonoscopy in detecting precancerous lesions depends on good visualization of the colonic mucosa, which is affected by the quality of bowel preparation. Indeed, inadequate bowel preparation has been reported in as many as 25 % of all colonoscopic examinations [4–6], suggesting that it can be a potential cause of ineffective colonoscopy screening because of missed lesions and subsequent interval CRCs [5–7]. In this regard, there is little doubt that optimal bowel preparation is an important indicator of high-quality colonoscopy [8].
Detection of non-polypoid (flat or depressed) colorectal neoplasms (NP-CRNs) is becoming an issue in colonoscopic screening. Current evidence indicates that NP-CRNs are widely prevalent worldwide, but they are more difficult to detect visually, and are more likely to harbor more advanced pathology than polypoid neoplasm [9–11]. In a study evaluating 1819 elective colonoscopies, the overall prevalence of NP-CRNs was reported to 9.35 and 5.84 % in the subpopulation of screening colonoscopies [9]. In the cited work, NP-CRNs were significantly associated with carcinomas, versus polypoid lesions (odds ratio [OR] 9.78, 95 % confidence interval [CI] 3.93–24.4) [9]. Moreover, serrated lesions, especially lesions in the proximal colon, as an important detection target for CRC screening, are likely to have non-polypoid flat (0-IIa type) morphology [12–14]. Given their subtle morphological characteristics of NP-CRNs, it seems reasonable that suboptimal bowel preparation may hinder endoscopic detection of NP-CRNs and consequently may be a potential cause of reduced CRC screening efficacy. Not surprisingly, a recent population-based study, by le Clercq et al. [7], reported that CRCs following colonoscopy were more likely to have a non-polypoid flat macroscopic appearance than prevalent CRCs (OR 1.70, 95 % CI 1.18–2.43; P = 0.004).
Several studies have reported an impact of bowel preparation quality on the detection of CRNs [5, 6]. However, there are few available data regarding whether the quality of bowel preparation may affect the detection of CRNs with respect to their morphological characteristics. Specifically, it is unclear whether suboptimal bowel preparation decreases the detection of NP-CRNs. Answering this question will be of clinical relevance in terms of a practical strategy to shorten surveillance intervals in patients with suboptimal bowel preparation. Additionally, earlier studies regarding the prevalence of NP-CRNs showed a high rate of variation by individual studies: 7–43 % of all adenomas detected and 6–24 % of all patients [9, 15–24]. Possible reasons for this high variation include inconsistent definitions of NP-CRN, heterogeneous populations, including patients at high risk for CRNs, and various combinations of examination techniques [25]. Also, data regarding the true prevalence of NP-CRNs in average-risk populations are scarce.
Thus, the aim of the present study was to assess the impact of bowel preparation quality on the colonoscopic detection of NP-CRNs. We also assessed the detection prevalence of adenomas classified as NP-CRNs in a large asymptomatic, average-risk screening population.
Methods
Study Design and Population
This study was a retrospective analysis of a prospectively collected database of colonoscopy cases at an academic teaching hospital between June 2011 and May 2013. The colonoscopy database had been updated daily using a standardized reporting system. It included patient demographic data, procedure-related characteristics (indication, timing of colonoscopy [morning or afternoon], quality of bowel cleansing, procedure times, and procedure-related adverse events), polyp-related characteristics (as described below), and other colonoscopy quality indicators, such as cecal intubation.
Inclusion criteria were asymptomatic average-risk patients who underwent a first-time screening colonoscopy. Exclusion criteria included the following: patients with a familial history of CRC in a first-degree relative, polyposis syndrome, or hereditary non-polyposis colon cancer; and patients with a history of colon resection or inflammatory bowel disease. Duplicate or incomplete data, including those containing polyps without documented pathologic results, were also excluded.
Ethics
The study was approved by the institutional review board of Kyung Hee University Hospital, Seoul, Korea, approval no. KMC-IRB 1419-06. Informed consent was waived due to the retrospective nature of the study. The study was reported according to the STROBE guidelines.
Method of Bowel Preparation and Grading of Preparation Quality
During the study period, all patients were prepared with a split-dose regimen of 4-L polyethylene glycol (PEG-3350) solution for morning colonoscopies or a same-day regimen of 4-L PEG-3350 in cases of afternoon examinations. Endoscopists were recommended to rate bowel preparation quality after clearing efforts to remove fecal debris and retained fluid by forceful irrigation and suction. Then, the grade of bowel preparation was rated on a four-point scale based on the Aronchick scale: excellent, good, fair, and poor [26]. The Aronchick scale assesses the preparation quality of the entire colon as excellent (a small volume of clear liquid or greater than 95 % of the surface seen), good (a large volume of clear liquid covering 5–25 % of the surface but greater than 90 % of the surface was seen), fair (some semisolid stool that could be suctioned or washed away, but greater than 90 % of the surface was seen), or poor (semisolid stool that could not be suctioned or washed away and less than 90 % of the surface was seen) [26]. The detailed descriptions of preparation scale were included in the colonoscopy report form. For statistical analysis, excellent or good preparation was defined as “adequate” preparation, and we assessed specific differences among three groups (adequate, fair, and poor). For the best inter-observer agreement, regular educational programs regarding the rating of bowel preparation were provided to all participating endoscopists.
Evaluation of Colonic Polyps and Definition of NP-CRN
All colonoscopy was performed using high-definition colonoscopes (CF-H260AI, CF-H260AL, Olympus, Tokyo, Japan) by one of seven board-certified attending gastroenterologists (all having performed >10,000 colonoscopies). During the study period, a total of 18 fellows who were in first year training were involved in any part of the colonoscopy, but mostly in the insertion of the colonoscope under supervision of attendings. As is our standard practice, in cases with fellow involvement, the rest of the procedure including colonoscope withdrawal and assessment of any lesions was completed by one of the seven attending gastroenterologists.
All detected polyps were photographed, and their endoscopic characteristics were documented immediately after the colonoscopy, including size measured with open biopsy forceps or a snare and anatomical location. The morphology of all lesions detected was classified using the Paris classification of superficial GI lesions [27]. Neoplasms that protruded less than 2.5 mm into the colon lumen were defined as NP-CRNs, which included slightly elevated (0-IIa), flat (0-IIb), and slightly depressed (0-IIc) lesions. Each polyp underwent a biopsy or was resected and sent for histopathological diagnosis, based on the World Health Organization (WHO) criteria [28]. Serrated polyps (SPs) were classified as hyperplastic polyps, sessile SPs, and traditional serrated adenomas using the WHO criteria [28]. We defined advanced neoplasms as tubular adenomas ≥10 mm in size, villous adenomas, adenomas with high-grade dysplasia, SPs with high risk (traditional serrated adenoma, large [≥ 10 mm] sessile SPs, sessile SPs with dysplasia), and carcinomas. The colon was divided into four segments (cecum/ascending colon, transverse colon, descending/sigmoid colon, and rectum), and the proximal portion to the splenic flexure was defined as the proximal colon.
Outcome Measurements and Statistical Analysis
The primary outcome was a comparison of the ADR of non-polypoid morphology according to quality of bowel preparation. We also compared ADRs by anatomical location and size of adenomas detected. Secondary outcomes included detection prevalence of adenomas of non-polypoid morphology (percentage of all patients) in average-risk screening colonoscopies. The detection rates of non-polypoid adenomas by individual endoscopists were also evaluated.
Data and variables are presented as means (SD) and categorical variables as absolute values and percentages. Continuous variables were tested using one-way ANOVA with post hoc multiple comparisons. Categorical variables were tested using the χ 2 test or Fisher’s exact test, as appropriate. To investigate the impact of preparation quality on adenoma detection with respect to morphology, location, and size, logistic regression analysis was performed after adjusting for age, gender, timing of colonoscopy (morning vs. afternoon), fellow participation, and individual endoscopists. For each variable, the adjusted ORs (aORs) and their 95 % CIs are reported. All data were analyzed using the SPSS software (version 18.0K for Windows, SPSS Korea, Seoul, Korea). P values <0.05 were considered statistically significant.
Results
Baseline Characteristics of Study Population
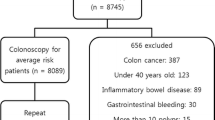
Of 13,323 colonoscopies performed during the study period, 6831 screening examinations were considered eligible for this study. Of these, 734 patients were excluded because of high-risk persons (n = 327), a history of colon resection (n = 21), inflammatory bowel disease (n = 35), polyps without documented pathology (n = 272), or incomplete or duplicated data (n = 79). After these exclusions, we analyzed 6097 asymptomatic, average-risk screening colonoscopies (Fig. 1).
Baseline characteristics of patients are summarized in Table 1. The mean (SD) age was 51.3 (±11.64) years, and 53.3 % (3247/6097) of the patients were men. Bowel preparation quality was rated as adequate (excellent or good) in 5224 (85.7 %), fair in 615 (10.1 %), and poor in 258 (4.2 %) patients. Mean times for colonoscope insertion, total procedure, and withdrawal were 5.6, 15.7, and 8.2 min, respectively. The failure rate of cecal intubation differed significantly among the three groups (0.5 % in adequate vs. 0.3 % in fair vs. 2.7 % in poor-quality preparation; P < 0.001).
Characteristics of Colorectal Neoplasms
In total, 5946 polyps were detected in the study population. Of these, 4847 lesions were histologically confirmed as neoplastic: adenomas (4104/4847, 84.7 %) and SPs (743/4847, 15.3 %). The proportion of NP-CRNs in overall neoplastic lesions was 40.5 % (1962/4847). Almost all NP-CRNs were flat (0-IIa or 0-IIb) lesions, while the proportion of depressed lesions (0-IIc) was very low (4/4847). More than half (53.4 %, 2590/4847) were located in the proximal colon, and 94.3 % (4570/4847) were of sub-centimeter size. The proportion of NP-CRNs classified as advanced neoplasms was 3.5 % (68/1962). Table 2 shows the detailed characteristics of all the neoplastic lesions detected.
Prevalence of Colorectal Neoplasms per Colonoscopy
The overall polyp detection rate (PDR) was 46.4 % (2826/6097), and the PDRs differed significantly among the three groups (46.6 % in adequate vs. 47.3 % in fair vs. 38.8 % in poor-quality preparation, P = 0.042; Table 3). The overall detection rates of adenomas, SPs, and advanced neoplasms were 36.3 % (2213/6097), 7.5 % (458/6097), and 3.9 % (240/6097), respectively. The detection rates of adenomas and advanced neoplasms differed significantly among the three groups (36.7 % in adequate vs. 36.6 % in fair vs. 27.1 % in poor-quality preparation, P = 0.007; 4.1 % in adequate vs. 3.4 % in fair vs. 1.2 % in poor-quality preparation, P = 0.045, respectively), but not that of SPs. We also compared ADRs by morphology, location, and size (Table 4). The ADRs of polypoid and non-polypoid morphology differed among the three groups (29.5 % in adequate vs. 29.1 % in fair vs. 21.7 % in poor-quality preparation, and 12.9 % in adequate vs. 8.5 % in fair vs. 7.4 % in poor-quality preparation; all P < 0.05, respectively). Additionally, ADRs of proximal colon, diminutive (≤5 mm), or small adenoma (6–9 mm) differed significantly among the three groups (all P < 0.05).
Detection Rates of Overall Adenoma and Adenoma of NP-CRN by Individual Endoscopists
Among the seven participating gastroenterologists, the ADRs of overall adenoma and NP-CRN ranged from 28.0 to 40.0 % and from 23.5 to 35.1 %, respectively; their mean ADRs of overall adenoma and NP-CRN were 36.3 and 28.8 %, respectively. When compared with the highest level detector, the odds ratios for detection of NP-CRN of the six other gastroenterologists ranged from 0.56 (95 % CI 0.40–0.80) to 0.79 (95 % CI 0.64–0.98): The differences were statistically significant (all P < 0.05) (Table 5).
Logistic Regression Models for Adenoma Detection According to Quality of Bowel Preparation
In multiple logistic regression analysis, adjusting for potential confounding factors, poor-quality preparation was significantly associated with impaired detection of overall polyps, adenomas, and advanced neoplasms, compared with adequate-quality preparation (all P < 0.05) (Table 6). Poor quality of preparation was also associated with impaired detection of both polypoid and non-polypoid adenomas, compared with adequate-quality preparation (aOR 0.58, 95 % CI 0.42–0.79; aOR 0.49, 95 % CI 0.30–0.79, respectively; all P < 0.05). Moreover, fair-quality preparation significantly decreased the detection of adenoma with non-polypoid morphology, compared with adequate-quality preparation (aOR 0.55, 95 % CI 0.41–0.75, P < 0.001). With respect to anatomical location and size, detection in the proximal colon and of sub-centimeter adenomas were significantly lower with poor-quality preparation than adequate-quality preparation (all P < 0.05).
Discussion
NP-CRNs are likely to have more aggressive biological behavior, while endoscopic detection of NP-CRNs is often difficult in cases of suboptimal bowel preparation [9–11]. Thus, the suboptimal status of bowel preparation during colonoscopy is potentially associated with a risk of interval colorectal cancers, thereby causing the efficacy of colonoscopy screening to decline [5–7]. The strength of our study is to investigate the impact of bowel preparation quality on colonoscopic detection of NP-CRN in a large series. We found that the detection of NP-CRN was significantly associated with the quality of bowel preparation. Detection of non-polypoid adenomas was significantly lower with poor-quality preparation, as well as that of polypoid adenomas. Interestingly, fair-quality bowel preparation significantly impaired detection of adenomas with non-polypoid morphology by ~45 % versus adequate-quality preparation. Our results clearly indicate that suboptimal bowel preparation has a substantial negative impact on the detection of NP-CRNs in screening colonoscopy (the estimated risk: 45–51 %). Given the substantial prevalence of NP-CRNs in this study (40.5 % of all neoplastic lesions, 12.3 % [ADR of non-polypoid morphology] of all patients), our findings also suggest that detection of NP-CRN is vital for the efficacy of CRC screening and a potential target for training and quality improvement.
Our data in line with previous studies reports that the diagnostic efficacy of colonoscopy was strongly related to the quality of bowel preparation. Early studies focused on the impact of preparation quality on polyp or adenoma detection with respect to the size of lesions [5, 6]. In an analysis of data from a national endoscopic database involving 93,004 colonoscopies, Harewood et al. [5] reported that inadequate preparation hindered detection of only polyps smaller than 9 mm. Froehlich et al. [6] reported that the detection of polyps of any size was significantly associated with the quality of bowel preparation. However, these two studies had some limitations, such as standardization of preparation methods and/or rating of preparation quality, which might limit the generalizability of the results [5, 6]. Recent studies have focused on the category of “fair” preparation. Sherer et al. [29] reported that fair-quality preparation did not decrease the detection rate of adenomas of any size or for advanced histology, but poor-quality preparation quality decreased the ADRs of diminutive and advanced histology. In a study by Anderson et al. [30], there were no significant differences in the overall, proximal ADR, or SP detection rate between fair and adequate-quality preparation quality, but proximal ADR was lowered statistically in poorly prepared versus adequately prepared colons (aOR 0.45, 95 % CI 0.24–0.84) [30]. Based on these results, the investigators suggested that “fair” preparation might be considered adequate [29, 30]. However, these previous studies did not determine whether the quality of bowel preparation affected the detection of CRN with respect to their morphology [5, 6, 29, 30].
Our study not only confirmed data regarding the negative impact of poor bowel preparation on the detection of CRNs but also provided the novel finding that even fair-quality preparation is significantly associated with impaired detection of NP-CRNs. Although the overall ADR was similar between adequate and fair-quality preparation (36.7 vs. 36.6 %), the ADR of non-polypoid morphology was significantly lower with fair-quality preparation than adequate-quality preparation (8.5 vs. 12.9 %, P = 0.002). The current results suggest that shortening of surveillance intervals should be considered for patients with fair and poor-quality preparation. Data from recent studies support our suggestion [31, 32]. In the study by Lebwohl et al. [31], the investigators evaluated the impact of suboptimal bowel preparation on adenoma miss rates using early repeat colonoscopy. The adenoma miss rate for patients with fair-quality preparation on the index colonoscopy was not significantly different from that of patients with poor-quality preparation on the index colonoscopy (38 vs. 49 %, P = 0.19) [31]. Menees et al. [32] also reported a 28 % adenoma miss rate on follow-up examination in patients with fair-quality preparation during the index colonoscopy. Given the retrospective nature of the current evidence, including our findings, and their conflicting results in the literature, however, there is a need for further prospective studies to address this issue [29–32]. In this regard, intraprocedural cleansing to enhance preparation quality could be a practical solution for patients with inadequate bowel preparation; thereby, it could reduce the need for early repeat colonoscopy. Regardless of the method of bowel preparation used, intraprocedural cleansing techniques such as water exchange colonoscopy and cleansing by simply using the water jet and suction have been proven to provide effective salvage cleansing and increase ADR in patients with inadequate bowel preparation [33–35]. Given the high costs of early repeat colonoscopy, colonoscopists need to focus more attention on alternatives of early repeat colonoscopy. We believe that intraprocedural cleansing should be considered as a part of work of colonoscopy [33].
We found that the proportion of NP-CRNs was 40.5 % (1962/4847) of all CRNs detected, and the prevalence of adenoma with non-polypoid morphology was 12.3 % (747/6097) in an asymptomatic average-risk screening population. Marked variations in the prevalence of NP-CRNs have been reported in many previous studies, in which the proportion of NP-CRNs ranged from 7 to 43 % of all adenomas detected and their detection prevalence ranged from 6 to 24 % of all patients [9, 15–24]. The variability in these results, as mentioned earlier, may be explained by several factors, such as inconsistent definitions of NP-CRN, heterogeneous target populations, combinations of imaging technologies, and the recent evolution of examination and bowel preparation techniques [10, 25]. To overcome these limitations, we included an exclusively average-risk screening population and used standardized protocols for the macroscopic classification of CRNs (the Paris classification), bowel preparation, and rating of bowel-cleansing quality. Our protocols for bowel preparation (split-dose or same-day regimen) are currently recommended by professional GI societies because of their established efficacy of bowel cleansing [36–40]. The percentage of adequate bowel preparation (85.7 %) of this study met the recently recommended performance target of bowel preparation quality (≥85 %) [8]; however, the results were much less than expected. This finding suggests that the perfect bowel preparation to be undertaken by the patients is still elusive despite the use of highly recommended approach [36–40]. Colonoscopists need to consider some form of intraprocedural salvage cleansing to ensure the colon is adequately cleaned for examination during withdrawal.
The detection prevalence of adenomas classified as NP-CRNs (12.3 %) in the current study is very similar to data from a recent population-based study by Reinhart et al. [41] (12.4 %, 2207/17,771 in an asymptomatic screening population). In their study, the investigators also used the Paris classification for definition of “flat” adenomas (Paris classification 0-IIa, 0-IIb, 0-IIc), the same definition used here. In two other studies involving screening colonoscopies, the prevalence of adenomas classified as NP-CRNs ranged from 4.3 to 5.84 % [9, 42]. Given that our findings are one of the highest values published, we believe that they may represent the real prevalence of adenomas classified as NP-CRNs in an average-risk screening population, thereby suggesting a specific detection target for adenomas classified as NP-CRNs in screening colonoscopies (at least 1 of 10 screening colonoscopies). Moreover, the detection of NP-CRN was highly dependent upon the participating endoscopists in our study (Table 5). The training of endoscopists in the detection of NP-CRN can be a way to potentially improve the efficacy of colonoscopy screening [43].
Generally, most SPs, especially proximal SPs, assume shape-like NP-CRNs. Thus, we considered that the detected rate of SPs would be lower with inadequate preparation than adequate-quality preparation. However, we found no significant difference among the three groups (Table 3). We presently have no explanation for this finding, but two recent studies reported similar results. de Wijkerslooth et al. reported that of 1354 patients, 12 % had one or more proximal SPs and there was no significant difference between proximal SP detection and the quality of bowel preparation [44]. Anderson et al. [30] also reported that there was no significant difference in overall or proximal SP detection rate between suboptimal preparation and adequate-quality preparation (fair-quality preparation: OR 0.82, 95 % CI 0.58–1.15; poor-quality preparation: OR 0.75, 95 % CI 0.31–1.80). We believe that additional studies should focus on the relationship between quality of bowel preparation and the detection of serrated lesions.
Our study had several limitations. First, the grading method of bowel preparation used in our study (a four-point scale corresponding to the Aronchick scale) has been used in many clinical trials and routine practice, but it has not been adequately validated. We had consistently tried to minimize this problem in several ways, including a regular educational program, an inclusion of detailed description in the colonoscopy report, and a rating of preparation quality after clearing efforts to removed fecal debris or residual fluid; however, inter-observer variability may exist. Therefore, it would be ideal to use more validated grading system of preparation quality such as the Boston bowel preparation scale in future studies [45, 46]. Additionally, our study was a retrospective analysis of single-center colonoscopy database. We could not explore the results of follow-up colonoscopies in patients rated as inadequate (fair or poor) preparation. Prospective comparative trials are also required to verify the clinical relevance of bowel preparation on the detection of NP-CRNs.
In conclusion, suboptimal (fair or poor) bowel preparation significantly impairs the detection of NP-CRNs. poor-quality preparation is also critically associated with impaired detection of overall adenomas, advanced neoplasms, adenomas in the proximal colon, and those with sub-centimeter size. Given that the prevalence of adenoma with non-polypoid morphology was as high as 12.3 % in our cohort of asymptomatic average-risk screening colonoscopy, efforts to improve preparation quality can be a practical solution to increase detection rate of NP-CRNs, thus improving the efficacy of screening colonoscopy.
References
Baxter NN, Warren JL, Barrett MJ, Stukel TA, Doria-Rose VP. Association between colonoscopy and colorectal cancer mortality in a US cohort according to site of cancer and colonoscopist specialty. J Clin Oncol. 2012;30:2664–2669.
Brenner H, Chang-Claude J, Seiler CM, Rickert A, Hoffmeister M. Protection from colorectal cancer after colonoscopy: a population-based, case-control study. Ann Intern Med. 2011;154:22–30.
Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369:1095–1105.
Ness RM, Manam R, Hoen H, Chalasani N. Predictors of inadequate bowel preparation for colonoscopy. Am J Gastroenterol. 2001;96:1797–1802.
Harewood GC, Sharma VK, de Garmo P. Impact of colonoscopy preparation quality on detection of suspected colonic neoplasia. Gastrointest Endosc. 2003;58:76–79.
Froehlich F, Wietlisbach V, Gonvers JJ, Burnand B, Vader JP. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: the European Panel of Appropriateness of Gastrointestinal Endoscopy European multicenter study. Gastrointest Endosc. 2005;61:378–384.
le Clercq CM, Bouwens MW, Rondagh EJ, et al. Postcolonoscopy colorectal cancers are preventable: a population-based study. Gut. 2014;63:957–963.
Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. Am J Gastroenterol. 2015;110:72–90.
Soetikno RM, Kaltenbach T, Rouse RV, et al. Prevalence of nonpolypoid (flat and depressed) colorectal neoplasms in asymptomatic and symptomatic adults. JAMA. 2008;299:1027–1035.
Ignjatovic A, Saunders BP. Non-polypoid colorectal neoplasms are relatively common worldwide. Gastrointest Endosc Clin N Am. 2010;20:417–429.
Kim HN, Raju GS. Bowel preparation and colonoscopy technique to detect non-polypoid colorectal neoplasms. Gastrointest Endosc Clin N Am. 2010;20:437–448.
Rondagh EJ, Bouwens MW, Riedl RG, et al. Endoscopic appearance of proximal colorectal neoplasms and potential implications for colonoscopy in cancer prevention. Gastrointest Endosc. 2012;75:1218–1225.
Tadepalli US, Feihel D, Miller KM, et al. A morphologic analysis of sessile serrated polyps observed during routine colonoscopy (with video). Gastrointest Endosc. 2011;74:1360–1368.
Rex DK, Hewett DG, Snover DC. Editorial: detection targets for colonoscopy: from variable detection to validation. Am J Gastroenterol. 2010;105:2665–2669.
Kudo SE, Kashida H. Flat and depressed lesions of the colorectum. Clin Gastroenterol Hepatol. 2005;3:S33–S36.
Kubota O, Kino I, Kimura T, Harada Y. Nonpolypoid adenomas and adenocarcinomas found in background mucosa of surgically resected colons. Cancer. 1996;77:621–626.
Lee SK, Kim TI, Shin SK, Kim WH, Kim H, Kim NK. Comparison of the clinicopathologic features between flat and polypoid adenoma. Scand J Gastroenterol. 2008;43:1116–1121.
Park DH, Kim HS, Kim WH, et al. Clinicopathologic characteristics and malignant potential of colorectal flat neoplasia compared with that of polypoid neoplasia. Dis Colon Rectum. 2008;51:43–49.
Jaramillo E, Slezak P, Watanabe M, Rubio C. Endoscopic detection and complete removal of a micro-invasive carcinoma present in a flat colonic adenoma. Gastrointest Endosc. 1994;40:369–371.
Fujii T, Rembacken BJ, Dixon MF, Yoshida S, Axon AT. Flat adenomas in the United Kingdom: are treatable cancers being missed? Endoscopy. 1998;30:437–443.
Rembacken BJ, Fujii T, Cairns A, et al. Flat and depressed colonic neoplasms: a prospective study of 1000 colonoscopies in the UK. Lancet. 2000;355:1211–1214.
Tsuda S, Veress B, Toth E, Fork FT. Flat and depressed colorectal tumours in a southern Swedish population: a prospective chromoendoscopic and histopathological study. Gut. 2002;51:550–555.
Lanspa SJ, Rouse J, Smyrk T, Watson P, Jenkins JX, Lynch HT. Epidemiologic characteristics of the flat adenoma of Muto. A prospective study. Dis Colon Rectum. 1992;35:543–546.
Saitoh Y, Waxman I, West AB, et al. Prevalence and distinctive biologic features of flat colorectal adenomas in a North American population. Gastroenterology. 2001;120:1657–1665.
Kahi CJ, Hewett DG, Rex DK. Relationship of non-polypoid colorectal neoplasms to quality of colonoscopy. Gastrointest Endosc Clin N Am. 2010;20:407–415.
Aronchick CA, Lipshutz WH, Wright SH, Dufrayne F, Bergman G. A novel tableted purgative for colonoscopic preparation: efficacy and safety comparisons with Colyte and Fleet Phospho-Soda. Gastrointest Endosc. 2000;52:346–352.
The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58:S3–S43.
Bosman FTCF, Hruban RH, Theise ND. WHO Classification of Tumours of the Digestive System. 4th ed. Berlin: Springer; 2010.
Sherer EA, Imler TD, Imperiale TF. The effect of colonoscopy preparation quality on adenoma detection rates. Gastrointest Endosc. 2012;75:545–553.
Anderson JC, Butterly LF, Robinson CM, Goodrich M, Weiss JE. Impact of fair bowel preparation quality on adenoma and serrated polyp detection: data from the New Hampshire Colonoscopy Registry by using a standardized preparation-quality rating. Gastrointest Endosc. 2014;80:463–470.
Lebwohl B, Kastrinos F, Glick M, Rosenbaum AJ, Wang T, Neugut AI. The impact of suboptimal bowel preparation on adenoma miss rates and the factors associated with early repeat colonoscopy. Gastrointest Endosc. 2011;73:1207–1214.
Menees SB, Kim HM, Elliott EE, Mickevicius JL, Graustein BB, Schoenfeld PS. The impact of fair colonoscopy preparation on colonoscopy use and adenoma miss rates in patients undergoing outpatient colonoscopy. Gastrointest Endosc. 2013;78:510–516.
MacPhail ME, Hardacker KA, Tiwari A, Vemulapalli KC, Rex DK. Intraprocedural cleansing work during colonoscopy and achievable rates of adequate preparation in an open-access endoscopy unit. Gastrointest Endosc. 2015;81:525–530.
Leung FW, Harker JO, Jackson G, et al. A proof-of-principle, prospective, randomized controlled trial (RCT) demonstrating improved outcomes in scheduled unsedated colonoscopy by the water method. Gastrointest Endosc. 2010;72:693–700.
Hsieh YH, Koo M, Leung FW. A patient-blinded randomized, controlled trial (RCT) comparing air insufflation (AI), water immersion (WI) and water exchange (WE) during minimally sedated colonoscopy. Am J Gastroenterol. 2014;109:1390–1400.
Bucci C, Rotondano G, Hassan C, et al. Optimal bowel cleansing for colonoscopy: split the dose! A series of meta-analyses of controlled studies. Gastrointest Endosc. 2014;80:566–576.
Matro R, Shnitser A, Spodik M, et al. Efficacy of morning-only compared with split-dose polyethylene glycol electrolyte solution for afternoon colonoscopy: a randomized controlled single-blind study. Am J Gastroenterol. 2010;105:1954–1961.
Wexner SD, Beck DE, Baron TH, et al. A consensus document on bowel preparation before colonoscopy: prepared by a task force from the American Society of Colon and Rectal Surgeons (ASCRS), the American Society for Gastrointestinal Endoscopy (ASGE), and the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES). Gastrointest Endosc. 2006;63:894–909.
Hassan C, Bretthauer M, Kaminski MF, et al. Bowel preparation for colonoscopy: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy.. 2013;45:142–150.
Johnson DA, Barkun AN, Cohen LB, et al. Optimizing adequacy of bowel cleansing for colonoscopy: recommendations from the US multi-society task force on colorectal cancer. Gastroenterology. 2014;147:903–924.
Reinhart K, Bannert C, Dunkler D, et al. Prevalence of flat lesions in a large screening population and their role in colonoscopy quality improvement. Endoscopy. 2013;45:350–356.
Chiu HM, Lin JT, Chen CC, et al. Prevalence and characteristics of nonpolypoid colorectal neoplasm in an asymptomatic and average-risk Chinese population. Clin Gastroenterol Hepatol. 2009;7:463–470.
Sanduleanu S, Rondagh EJ, Masclee AA. Development of expertise in the detection and classification of non-polypoid colorectal neoplasia: experience-based data at an academic GI unit. Gastrointest Endosc Clin N Am. 2010;20:449–460.
de Wijkerslooth TR, Stoop EM, Bossuyt PM, et al. Differences in proximal serrated polyp detection among endoscopists are associated with variability in withdrawal time. Gastrointest Endosc. 2013;77:617–623.
Lai EJ, Calderwood AH, Doros G, Fix OK, Jacobson BC. The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc. 2009;69:620–625.
Calderwood AH, Jacobson BC. Comprehensive validation of the Boston Bowel Preparation Scale. Gastrointest Endosc. 2010;72:686–692.
Conflict of interest
The authors disclosed no financial relationships relevant to this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oh, C.H., Lee, C.K., Kim, JW. et al. Suboptimal Bowel Preparation Significantly Impairs Colonoscopic Detection of Non-polypoid Colorectal Neoplasms. Dig Dis Sci 60, 2294–2303 (2015). https://doi.org/10.1007/s10620-015-3628-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-015-3628-6