Abstract
Nonalcoholic steatohepatitis (NASH) is the advanced form of nonalcoholic fatty liver disease, which is often accompanied by obese and/or type II diabetes mellitus. Approximately one-third of NASH patients develop hepatic fibrosis. Hepatic stellate cells are the major effector cells during liver fibrogenesis. Advanced liver fibrosis usually proceeds to cirrhosis and even hepatocellular carcinoma, leading to liver failure, portal hypertension and even death. Currently, there are no approved agents for treatment and prevention of liver fibrosis in human beings. Curcumin, the principal curcuminoid of turmeric, has been reported to show antitumor, antioxidant, and anti-inflammatory properties both in in vitro and in vivo systems. Accumulating data shows that curcumin plays a critical role in combating liver fibrogenesis. This review will discuss the inhibitory roles of curcumin and update the underlying mechanisms by which curcumin targets in inhibiting hepatic stellate cell activation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity and type II diabetes mellitus (T2DM) have been urgent public health concerns worldwide in recent years. Obese and/or type II diabetes mellitus patients are often coupled with nonalcoholic fatty liver disease (NAFLD). Nonalcoholic steatohepatitis (NASH) is the advanced form of NAFLD, featured by steatohepatitis. Approximately one-third of NASH patients develop hepatic fibrosis and even cirrhosis [1].
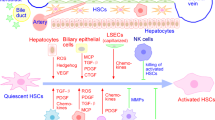
Liver fibrosis is featured by the excessive accumulation of extracellular matrix (ECM) proteins including collagen in the extracellular spaces. It might occur in most types of chronic liver diseases, including NASH. Advanced liver fibrosis results in cirrhosis and even hepatocellular carcinoma (HCC), leading to liver failure, portal hypertension and even death. The liver lobule consists of parenchymal cells (PCs) and non-parenchymal cells (NPCs). The latter includes endothelial cells, kupffer cells, natural killer cells, dendritic cells and hepatic stellate cells (HSCs) [2]. Activated HSCs are the major source of collagen products, which leads to the imbalance of formation and degradation of ECM in tissues. Additionally, portal fibroblasts, and myofibroblasts of bone marrow origin also contribute to collagen production in the injured liver [2]. These cells are activated by fibrogenic cytokines such as TGF-beta1, angiotensin II and leptin [3], and regulated by pro-inflammatory cytokines such as nuclear factor-kappaB (NF-kB), tumor necrosis factor alpha (TNF-α) and interleukin 6 (IL-6) [3–5]. Recent research demonstrated that advanced liver fibrosis in patients could be reversed, which has stimulated researchers to develop antifibrotic drugs [3, 6]. Potential antifibrotic therapies are aimed at inhibiting the activation of fibrogenic cells, inducing the apoptosis of activated HSCs and/or preventing the deposition of ECM proteins. Currently, no approved agents for treatment and prevention of liver fibrosis in human beings are available.
Curcumin is the principal curcuminoid of turmeric. The curcuminoids are natural phenols that are responsible for the yellow color of turmeric. Turmeric has been used historically as a component of Chinese traditional medicine for thousands of years. In the latter half of the 20th century curcumin was identified as the agent responsible for most of the biological activity of turmeric [7]. As of 2008, numerous clinical trials in humans focused on studying the effect of curcumin on various diseases, such as multiple myeloma, pancreatic cancer, myelodysplastic syndromes, colon cancer, psoriasis, and Alzheimer’s disease [8]. In both in vitro and animal studies, curcumin has shown antitumor [9–11], antioxidant [12], and anti-inflammatory properties [13]. This review will focus on the inhibitory roles played by curcumin and its underlying mechanisms in liver fibrogenesis in vivo and in vitro.
Curcumin Improves HSC Activation in CCl4-Induced Fibrotic Animal Models
HSCs are the main effectors during liver fibrogenesis. HSC activation initiates liver fibrosis regardless of etiology. The carbon tetrachloride (CCl4)-fibrotic animal model is commonly used to investigate the procedure of HSC activation in vivo. It is well documented that curcumin plays a critical role against CCl4 -induced liver fibrosis in mice and rat models, suggesting an inhibitory role of curcumin in targeting HSC activation in vivo, which is regarded as a direct and potential therapeutic approach. As shown in Table 1, CCl4-induced liver fibrosis models administered with curcumin for 4–8 weeks showed reduced liver damage and lowered α-SMA and procollagen expression in the livers. Curcumin takes action by targeting multiple sites in those models, such as tissue growth factor β (TGFβ) [14, 15], platelet-derived growth factor-β receptor (PDGF-βR) [16], toll-like receptors (TLRs) [17], matrix metalloproteinases (MMPs) [16, 18], peroxisome proliferator-activated receptors (PPARγ) [19], inflammatory cytokines [17, 19–21], apoptotic pathway [22, 23] and microRNAs [24]. Curcumin may synergistically combine with acupuncture [16], saikosaponin A [21] and its analogs [25]. Although the underlying mechanisms remain largely elusive, it is accepted that curcumin may target multiple pathways to stem hepatic stellate cell activation.
Curcumin Blocks Leptin Signaling Pathway in Hepatic Stellate Cells
Accumulating evidence has shown that leptin and its receptor play critical roles in the development of hepatic fibrosis that is triggered by hepatic stellate cell activation in animal models [26–28] and humans [29–31] with NASH. These observations collectively indicate the significance and essential nature of leptin and Ob-R in the activation of hepatic stellate cells.
An abnormally enhanced level of leptin activates Ob-R and its downstream signaling pathways in HSCs, which induce oxidative stress [32–36], cell proliferation [37], and overproduction of ECM [33, 34, 36, 38], leading to the activation of HSCs. An in vitro study shows that curcumin abrogates the stimulatory effects of leptin by interrupting leptin signaling via inhibiting the phosphorylation of Ob-R and suppressing Ob-R gene expression [36]. The latter is mediated by stimulating PPARγ activity and attenuating oxidative stress [36]. Also, curcumin eliminated stimulatory effects of leptin on HSC activation via increasing AMPK activity and regulating intracellular lipids in HSCs [39]. Moreover, curcumin prevented leptin from elevating levels of intracellular glucose in activated HSCs, leading to the inhibition of HSC activation [40]. The same group recently reported that curcumin contributes to the inhibition of HSC activation by eliminating the AGE-caused activation of leptin signaling in activated HSC [32]. Those observations provide novel insights into mechanisms of curcumin in inhibiting leptin-induced HSC activation in vitro. Further research needs to confirm the inhibitory roles of curcumin in leptin-induced HSC activation in in vivo systems. It apparently indicates that curcumin may exert as a therapeutic candidate for the treatment and prevention of liver fibrogenesis induced by hyperleptinemia which was commonly accompanied with NASH, obesity and/or T2DM (Fig. 1).
Multiple targets by curcumin in activated hepatic stellate cells. Curcumin inhibits HSC activation via 1 blocking leptin signaling pathway, 2 fighting against oxidative stress, 3 inducing PPARγ gene expression and its transactivity, 4 suppressing GLUT4 translocation from cytosol to membrane and hence lowering glucose uptake, and 5 promoting intracellular lipid accumulation
Curcumin Regulates Intracellular Glucose and Its Derivatives in Hepatic Stellate Cells
Approximately 15–40 % of NASH patients develop hepatic fibrosis [41]. T2DM- and NASH-associated hepatic fibrosis currently is the subject of significant scientific and clinical interest, and will remain so in the future. The correlation of hyperglycemia with the presence of liver fibrosis in NASH patients has been clinically described [42]. T2DM could be a predictor of worsening hepatic fibrosis. Hyperglycemia is suggested as a harmful prognostic factor in the evolution of NASH towards fibrosis. However, little attention has been paid to impacts of hyperglycemia on HSC activation and on NASH-associated hepatic fibrogenesis.
Glucose transport across the plasma membranes of mammalian cells is carried out by two distinct processes: facilitative transport, mediated by a family of facilitative glucose transporters (GLUTs); and sodium-dependent transport, mediated by the Na+/glucose co-transporters (SGLT). GLUTs are important for maintaining glucose metabolism homeostasis and are molecular targets of anti-diabetic drugs [43]. In the liver, glucose transporter-2 (GLUT2) and glucose transporter-4 (GLUT4) are the major GLUTs responsible for glucose transportation into hepatocytes [44, 45]. Due to its low affinity and high capacity, GLUT2 transports glucose in a large range of physiological concentrations of glucose, whereas GLUT4 action is extensively regulated by insulin-activated phosphoinositide 3-kinases (PI3K) [44, 45]. In the liver, GLUT2 is translocated from the cytoplasm to the plasma membrane in response to high levels of plasma glucose and is the primary carrier to transport plasma glucose into hepatocytes [44, 45]. An abnormally high level of intracellular glucose could be deleterious to cellular functions in some types of cells [46].
Published data showed that hyperglycemia stimulated activation of hepatic stellate cells and curcumin removed this action in vitro [47]. Extensive studies suggested that curcumin decreased intracellular glucose level of HSCs by suppressing membrane translocation and gene expression of glucose transporter-2 [47]. The same group also reported that curcumin blocked translocation of glucose transporter-4 by interrupting the insulin receptor substrates (IRS)/PI3K/AKT signaling pathway, a crosslink between leptin and insulin pathway, and increased glucokinase activity by increasing AMP-activated protein kinase (AMPK) activity and suppressing PKA activity, leading to increased conversion of glucose to G-6-P and lowered glucose levels in HSCs [40]. The latter was mediated by activating PPARγ and attenuating oxidative stress.
Hyperglycemia is a high-risk factor for the development of nonalcoholic steatohepatitis (NASH) [48], which is a poorly studied complication of T2DM. Hyperglycemia facilitates the non-enzymatic formation of advanced glycation end-products (AGEs), which are a heterogeneous group of molecules formed by non-oxidative and oxidative reactions of sugars with proteins and/or lipids [49]. AGEs accumulate in tissues and circulation during aging, as well as diabetic, chronic renal failure and liver fibrogenesis [50], leading to inflammation and pathogenesis [51]. One of the mechanisms by which glycated proteins are converted into AGEs is involved in oxidation reaction. One study shows that the formation of AGEs from Amadori products occurs partly because of oxidation [52]. Therefore agents with anti-oxidation properties that can prevent further oxidation of Amadori products may halt the accumulation of AGEs [53]. Khan et al. [54] reported that curcumin had the properties of anti-glycation. In vitro studies imply that AGEs play a critical role in HSC activation, which can be diminished by curcumin [32, 55, 56]. Effects of AGEs are mediated by their receptor system, which could be generally divided into two categories, such as receptor for AGEs (RAGE) and AGE receptors (AGE-Rs, also called OST-48).
On the one hand, RAGE, a member of the immunoglobulin superfamily of cell surface molecules, mediates effects of AGEs and involves oxidative stress, cell growth and inflammation [57]. The interaction of AGEs and RAGE may stimulate the activation of a diverse array of signaling cascades, including MAPKs, JAK/STAT, and PI3K [58]. It has been reported that RAGE is up-regulated in cultured HSCs [59] and AGEs induce cell proliferation of HSCs [60]. AGEs stimulated the activation of HSCs in vitro by inducing cell proliferation and stimulating expression of genes relevant to HSC activation [55]. The phytochemical curcumin eliminated the stimulating effects of AGEs and inhibited the gene expression of RAGE by attenuating oxidative stress and stimulating the activity of PPARγ in HSCs [56].
On the other hand, AGE-Rs, e.g., AGE-R1, are responsible for detoxification and clearance of AGEs [61]. In contrast to a dramatic increase in expression of RAGE in diabetes with high levels of AGEs [62], the abundance of AGE-R1 is significantly reduced in diabetic organs, e.g., kidney [63], suggesting a possible inverse relationship between AGEs-mediated cell injury and low expression of AGE-R1. In addition to its participation in AGE removal, AGE-R1 negatively regulates AGE pro-inflammatory signal processing [61]. Studies demonstrated that curcumin inhibited AGEs stimulated HSC activation at least partially by inducing AGE-R1 gene expression. This process was likely mediated by inhibiting extracellular signal-regulated kinases (ERKs) activity, inducing gene expression of PPARγ and stimulating its transactivity [55]. Furthermore, curcumin eliminated the effects of AGEs on the divergent regulation of gene expression of RAGE and AGE-R1 in HSCs by interrupting the AGE-caused activation of leptin signaling, leading to the inhibition of HSC activation [32].
Taken together, AGEs might be regarded as one of the mechanisms by which HSCs are activated in high glucose conditions, which probably can, at least partially, explain why liver fibrosis is highly associated with type II diabetes mellitus. Curcumin has a potential to fight against AGE involved HSC activation. However, no evidence is available to show the role of curcumin in regulating gene expression of RAGE and AGE-R1 in vivo.
Curcumin Modulates Lipid Metabolism in Hepatic Stellate Cells
HSCs were previously called fat storing cells [64]. During hepatic injury, quiescent HSCs undergo profound phenotypic changes, including enhanced cell proliferation, loss of lipid droplets, de novo expression of α-smooth muscle actin, and excessive production of extracellular matrix. This process is called HSC activation. Freshly isolated HSCs in culture gradually and spontaneously become fully activated [65], mimicking the process seen in vivo, which provides a good model for elucidating underlying mechanisms of HSC activation and studying potential therapeutic intervention of the process [66, 67]. Accumulating evidence supports the proposal that recovering the accumulation of lipids could inhibit HSC activation [68, 69].
Lipid homeostasis is tightly controlled, via biosynthesis and cellular uptake, by a group of proteins. Several transcription factors, notably sterol regulatory element-binding protein-1c (SREBP-1c), PPARγ, and CCAAT/enhancer-binding protein-α (C/EBP α) have emerged as master regulators in lipogenesis as well as in lipid uptake and metabolism [70]. Interaction, cooperation, and cross talk have been observed among those regulators [71, 72]. It has been proposed that the process of HSC activation may be similar to that of adipocyte dedifferentiation, causally associated with transcriptional regulation of genes relevant to lipid accumulation [68, 69]. In vitro research demonstrated that curcumin could increase intracellular lipid accumulation in HSC via inducing expression of lipogenesis related genes, such as SREBP-1c, PPARγ, and C/EBP α, leading to an inhibition of HSC activation [39]. Similarly, the effect of curcumin on lipid metabolism has been also observed in HepG2 cells [73]. Despite the observation that curcumin paradoxically promotes lipid accumulation and inhibits HSC activation in vitro, curcumin and its water-soluble derivative displays an effective improvement in the lipid metabolism and delays the progression of hepatic fibrosis in rats and mice with steatohepatitis [74–76].
AMPK is a sensor of cellular energy homeostasis [77]. It is activated by rising AMP and falling ATP by a complex mechanism that results in an ultrasensitive response. The activation of AMPK by pharmacological agents presents a unique challenge, given the complexity of the biology, but holds a considerable potential to reverse the metabolic abnormalities [78]. In skeletal muscles, AMPK stimulates glucose transport and fatty acid (FA) oxidation. In the liver, it decreases glucose output, leading to lowered blood glucose levels in hyperglycemic individuals [79]. AMPK may play a key role in regulating the activation of SREBP-1 and lipogenesis [80]. The process of HSC activation is accompanied by depletion of intracellular lipid droplets, loss of lipid storage capacity, and suppression of expression of transcription factors, including SREBP-1, PPARγ, and C/EBPα [66, 68]. In vitro experiments demonstrated that curcumin inhibited HSC activation by activating AMPK activity, leading to the induction of the expression of genes relevant to lipid accumulation and to the elevation of the levels of intracellular fatty acids (FAs) and triglycerides (TGs) [39]. Interestingly, activation of AMPK by curcumin shows different functions in other cell types, such as hepatoma cells [81], HT-29 colon cancer cells [82], and 3T3-L1 adipocytes [75]. These observations collectively suggested that curcumin might show distinct effects on regulating gene expression and on lipid accumulation depending on cell types. Therefore, additional experiments are necessary to elucidate the mechanisms by which curcumin activates AMPK and shows distinct effects in different cell types.
Kang et al. [83] reported that curcumin inhibited low-density lipoprotein (LDL)-induced activation of HSCs by suppressing expression of LDL receptor, removed the role of oxidized LDL in activating HSCs by lowering gene expression of lectin-like oxidized LDL receptor-1 (LOX-1) [84], and attenuated expression of SREBP-2 by reducing the activity of specific protein-1, resulting in inhibition of HSC activation [85]. Recently, Kuo and colleagues reported that curcumin protects hepatocytes from high free fatty acid (HFFA)-induced lipoapoptosis and mitochondrial dysfunction, which partially occurs through the regulation of mitochondrial biogenesis [86].
Curcumin Balances Formation and Degradation of ECM Via Regulating TIMPs and MMPs
Fibrosis results due to increased deposition of ECM proteins which are predominantly synthesized by activated HSCs. The accumulation of these proteins is controlled by the rate of their synthesis and degradation. Physiologically, balance of ECM in liver is sustained by a group of enzymes called MMPs and their specific inhibitors, tissue inhibitors of metalloproteinases (TIMPs). Once secreted, MMP activity is regulated by the binding of TIMPs [87]. Overall, all MMPs are inhibited by at least one of the specific endogenous TIMPs once they are activated [87]. During chronic liver injury, HSCs are activated and differentiate into a fibroblast-like phenotype. The balance between MMPs and TIMPs is broken, excessive ECM accumulates in the extracellular spaces and finally fibrosis occurs.
The family of MMPs consists of 23 different members [88], but only a few are expressed in liver tissue and associated with activation of HSCs. They are MMP-1 [88], MMP-2 [89–95], MMP-3 [89, 96–99], MMP-9 [100, 101], and MMP-13 [89, 100, 102, 103]. Upregulation of the above MMPs may facilitate activation of hepatic stellate cells, leading to liver fibrosis.
In the TIMP family, four subtypes have been identified: TIMP-1, TIMP-2, TIMP-3, and TIMP-4. TIMP-1 and TIMP-2 are mainly produced by HSCs [104, 105]. TIMP-1 is also produced by Kupffer cells and hepatocytes especially in early stages of liver injury [102, 106–108], plays a putative role in tissue fibrosis [104, 109], and is also capable of inhibiting programmed cell death of HSCs, mediated via inhibition of pro-MMP activation and MMP activity [110, 111]. TIMP-2 is essential for MMP-2 activation in mice [112].
Published data suggested that curcumin targets the above two protein families that are responsible for fibrogenesis and fibrolysis, respectively, for anti-fibrotic therapeutic interventions: upregulation of MMP activity or downregulation of TIMP activity (Table 2). Curcumin downregulates TIMP-1 and TIMP-2 in vivo [18, 113–115] and in vitro [116], and upregulates MMP-2 [115], MMP-7 [114], MMP-9 [115] and MMP-13 [18, 114], resulting in the degradation of fibrillar collagens, the main components in ECM, leading to inhibition of HSC activation. It is also reported that curcumin has no effect on PDGF-induced TIMP-1 and TIMP-2 expression in rats [117].
Curcumin Activates PPAR-γ Signaling Pathway in Activated Hepatic Stellate Cells
PPARs belong to the superfamily of nuclear receptors [118]. PPARs form heterodimers with retinoid X receptors (RXRs) and these heterodimers regulate transcription of various genes. Three isoforms of PPARs are identified: PPAR-α, PPAR-δ, and PPAR-γ, of which PPAR-γ is the most well documented [119]. PPAR-γ is involved in fatty acid storage and glucose metabolism. The genes activated by PPARγ stimulate lipid uptake and adipogenesis by fat cells. PPARγ knockout mice fail to generate adipose tissue when fed a high-fat diet [120].
PPARγ is highly expressed in quiescent HSCs with a large amount of lipid droplets in the normal livers [25, 68, 121]. However, expression of PPAR-γ and its activity are dramatically reduced with HSC activation in vitro and in vivo [25, 68, 121], which is accompanied by an increase in cell growth and proliferation, loss of lipid droplet and vitamin A-storing capability, expression of α-SMA and type I collagen-alpha 1 and deposition of excessive ECM in extracellular space. Extensive data indicate that induction of PPARγ activity by its agonists reduces HSC proliferation and α1 (I) collagen production [68, 122]. Moreover, forced expression of PPARγ via adenoviral vector-mediated system draws the morphology of activated HSC back to the quiescent phenotype [123]. Additionally, PPAR-γ ligands inhibit cell proliferation and collagen-1(I) expression in primary HSCs [68]. The dramatic reduction in the level of PPAR-γ is accompanied by the process of HSC activation [68, 25, 121]. It is, therefore, implied that targeting PPAR-γ signaling is a potential therapeutic strategy in prevention and treatment of liver fibrosis.
Increasing data, including ours and others, shown in Table 3, demonstrate the role of curcumin in inhibition of liver fibrosis through dramatically inducing the expression of PPAR-γ at levels of transcription and translation as well as revived PPAR-γ trans-activating activity in activated HSC. Furthermore, activation of PPAR-γ by curcumin resulted in inhibition of transcription factor NF-kB trans-activating activity. On the other hand, blocking PPAR-γ activation by a specific PPAR-γ antagonist caused a marked reduction in inhibition of activated HSC proliferation. Together, our results have indicated that PPAR-γ activation by curcumin plays critical and significant roles in inhibition of activated HSC.
Conclusions
Liver fibrosis is triggered by activation of hepatic stellate cells, the major source of collagen products, resulting in an imbalance of formation and degradation of ECM in tissues. So far, no approved agents for treatment and prevention of liver fibrosis in human beings are available. Antifibrogenic agents that are involved in inhibiting HSC activation is of high priority and is urgently needed. Increasing evidence has shown that curcumin has antitumor, antioxidant, and anti-inflammatory properties. Our results and others, both in vitro and in vivo, demonstrate that curcumin plays a role in inhibiting HSC activation by blocking leptin signaling, regulating intracellular glucose and its derivatives and modulating lipid metabolism, as well as balancing formation and degradation of ECM. These results provide novel insights into therapeutic mechanisms of curcumin in inhibiting HSC activation and intervening liver fibrogenesis associated with NAFLD and/or NASH.
Abbreviations
- AGEs:
-
Advanced glycation end-products
- AGE-Rs:
-
AGE receptors
- AMPK:
-
AMP-activated protein kinase
- CCL4:
-
Carbon tetrachloride
- C/EBP-α:
-
CCAAT/enhancer-binding protein-α
- ECM:
-
Extracellular matrix
- ERK:
-
Extracellular signal-regulated kinases
- FA:
-
Fatty acid
- GLUTs:
-
Glucose transporters
- HCC:
-
Hepatocellular carcinoma
- HSCs:
-
Hepatic stellate cells
- IL-6:
-
Interleukin 6
- IRS:
-
Insulin receptor substrates
- JAK:
-
Janus kinase
- LOX-1:
-
LDL receptor-1
- MAPK:
-
Mitogen-activated protein kinase
- MMPs:
-
Matrix metalloproteinases
- NAFLD:
-
Nonalcoholic fatty liver disease
- NASH:
-
Nonalcoholic steatohepatitis
- NF-kB:
-
Nuclear factor-kappaB
- Ob-R:
-
Leptin receptor
- PDGF-βR:
-
Platelet-derived growth factor-β receptor
- PI3K:
-
Phosphoinositide 3-kinases
- PPARs:
-
Peroxisome proliferator-activated receptors
- RAGE:
-
Receptor for AGEs
- RXRs:
-
Retinoid X receptors
- SREBP-1c:
-
Sterol regulatory element-binding protein-1c
- STAT-3:
-
Signal transducer and activator of transcription
- T2DM:
-
Type II diabetes mellitus
- TGs:
-
Triglycerides
- TGFβ:
-
Tissue growth factor β
- TIMPs:
-
Metalloproteinases
- TLRs:
-
Toll-like receptors
- TNF-α:
-
Tumor necrosis factor alpha
References
Clark JM. The epidemiology of nonalcoholic fatty liver disease in adults. J Clin Gastroenterol. 2006;40:S5–S10.
Senoo H, Yoshikawa K, Morii M, Miura M, Imai K, Mezaki Y. Hepatic stellate cell (vitamin A-storing cell) and its relative–past, present and future. Cell Biol Int. 2010;34:1247–1272.
Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218.
Luedde T, Schwabe RF. NF-κB in the liver–linking injury, fibrosis and hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2011;8:108–118.
Connolly MK, Bedrosian AS, Mallen-St Clair J, et al. In liver fibrosis, dendritic cells govern hepatic inflammation in mice via TNF-α. J Clin Invest. 2009;119:3213–3225.
Ramachandran P, Iredale JP. Reversibility of liver fibrosis. Ann Hepatol. 2009;8:283–291.
Aggarwal BB1, Sundaram C, Malani N, Ichikawa H. Curcumin: the Indian solid gold. Adv Exp Med Biol. 2007;595:1–75.
Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci. 2008;65:1631–1652.
Ströfer M, Jelkmann W, Depping R. Curcumin decreases survival of Hep3B liver and MCF-7 breast cancer Cells. Strahlenther und Onkol. 2011;187:393–400.
Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol. 2006;71:1397–1421.
Choi H, Chun YS, Kim SW, Kim MS, Park JW. Curcumin inhibits hypoxia-inducible factor-1 by degrading aryl hydrocarbon receptor nuclear translocator: a mechanism of tumor growth inhibition. Mol Pharmacol. 2006;70:1664–1671.
Shukla PK, Khanna VK, Ali MM, Khan MY, Srimal RC. Anti-ischemic effect of curcumin in rat brain. Neurochem Res. 2008;33:1036–1043.
Stix G. Spice healer. Sci Am. 2007;296:54–67.
Yao QY, Xu BL, Wang JY, Liu HC, Zhang SC, Tu CT. Inhibition by curcumin of multiple sites of the transforming growth factor-beta1 signalling pathway ameliorates the progression of liver fibrosis induced by carbon tetrachloride in rats. BMC Complement Altern Med. 2012;12:156.
Reyes-Gordillo K, Segovia J, Shibayama M, et al. Curcumin prevents and reverses cirrhosis induced by bile duct obstruction or CCl4 in rats: role of TGF-beta modulation and oxidative stress. Fundam Clin Pharmacol. 2008;22:417–427.
Zhang XP, Zhang F, Zhang ZL, et al. Acupuncture combined with curcumin disrupts platelet-derived growth factor β receptor/extracellular signal-regulated kinase signalling and stimulates extracellular matrix degradation in carbon tetrachloride-induced hepatic fibrosis in rats. Acupunct Med. 2012;30:324–330.
Tu CT, Yao QY, Xu BL, Wang JY, Zhou CH, Zhang SC. Protective effects of curcumin against hepatic fibrosis induced by carbon tetrachloride: modulation of high-mobility group box 1, Toll-like receptor 4 and 2 expression. Food Chem Toxicol. 2012;50:3343–3351.
Morsy MA, Abdalla AM, Mahmoud AM, Abdelwahab SA, Mahmoud ME. Protective effects of curcumin, α-lipoic acid, and N-acetylcysteine against carbon tetrachloride-induced liver fibrosis in rats. J Physiol Biochem. 2012;68:29–35.
Fu Y, Zheng S, Lin J, Ryerse J, Chen A. Curcumin protects the rat liver from CCl4-caused injury and fibrogenesis by attenuating oxidative stress and suppressing inflammation. Mol Pharmacol. 2008;73:399–409.
Bassiouny AR, Zaky A, Kandeel KM. Alteration of AP-endonuclease1 expression in curcumin-treated fibrotic rats. Ann Hepatol. 2011;10:516–5130.
Wu SJ, Tam KW, Tsai YH, Chang CC, Chao JC. Curcumin and saikosaponin a inhibit chemical-induced liver inflammation and fibrosis in rats. Am J Chin Med. 2010;38:99–111.
Shu JC, He YJ, Lv X, Ye GR, Wang LX. Curcumin prevents liver fibrosis by inducing apoptosis and suppressing activation of hepatic stellate cells. J Nat Med. 2009;63:415–420.
Priya S, Sudhakaran PR. Curcumin-induced recovery from hepatic injury involves induction of apoptosis of activated hepatic stellate cells. Indian J Biochem Biophys. 2008;45:317–325.
Hassan ZK, Al-Olayan EM. Curcumin reorganizes miRNA expression in a mouse model of liver fibrosis. Asian Pac J Cancer Prev. 2012;13:5405–5408.
Marra F, Efsen E, Romanelli RG, et al. Ligands of peroxisome proliferator-activated receptor gamma modulate profibrogenic and proinflammatory actions in hepatic stellate cells. Gastroenterology. 2000;119:466–478.
Leclercq IA, Farrell GC, Schriemer R, Robertson GR. Leptin is essential for the hepatic fibrogenic response to chronic liver injury. J Hepatol. 2002;37:206–213.
Friedman JM. Modern science versus the stigma of obesity. Nat Med. 2004;10:563–569.
Sakaida I, Jinhua S, Uchida K, Terai S, Okita K. Leptin receptor-deficient Zucker (fa/fa) rat retards the development of pig serum-induced liver fibrosis with Kupffer cell dysfunction. Life Sci. 2003;73:2491–2501.
García-Suárez C, Crespo J, Fernández-Gil PL, et al. Plasma leptin levels in patients with primary biliary cirrhosis and their relationship with degree of fibrosis. Gastroenterol Hepatol. 2004;27:47–50.
Henriksen JH, Holst JJ, Møller S, Brinch K, Bendtsen F. Increased circulating leptin in alcoholic cirrhosis: relation to release and disposal. Hepatology. 1999;29:1818–1824.
Cayón A, Crespo J, Mayorga M, Guerra A, Pons-Romero F. Increased expression of Ob-Rb and its relationship with the overexpression of TGF-β1 and the stage of fibrosis in patients with nonalcoholic steatohepatitis. Liver Int. 2006;26:1065–1071.
Tang Y, Chen A. Curcumin eliminates the effect of advanced glycation end-products (AGEs) on the divergent regulation of gene expression of receptors of AGEs by interrupting leptin signaling. Lab Invest. 2014;94:503–516.
Cao Q, Mak KM, Lieber CS. Leptin enhances alpha1(I) collagen gene expression in LX-2 human hepatic stellate cells through JAK-mediated H2O2-dependent MAPK pathways. J Cell Biochem. 2006;97:188–197.
Cao Q, Mak KM, Ren C, Lieber CS. Leptin stimulates tissue inhibitor of metalloproteinase-1 in human hepatic stellate cells: respective roles of the JAK/STAT and JAK-mediated H2O2-dependant MAPK pathways. J Biol Chem. 2004;279:4292–4304.
Abu-Tair L, Doron S, Mahamid M, Amer J, Safadi R. Leptin modulates lymphocytes’ adherence to hepatic stellate cells is associated with oxidative status alterations. Mitochondrion. 2013;13:473–480.
Tang Y, Zheng S, Chen A. Curcumin eliminates leptin’s effects on hepatic stellate cell activation via interrupting leptin signaling. Endocrinology. 2009;150:3011–3020.
Lang T, Ikejima K, Yoshikawa M, et al. Leptin facilitates proliferation of hepatic stellate cells through up-regulation of platelet-derived growth factor receptor. Biochem Biophys Res Commun. 2004;323:1091–1095.
García-Ruiz I, Gómez-Izquierdo E, Díaz-Sanjuán T, et al. Sp1 and Sp3 transcription factors mediate leptin-induced collagen α1(I) gene expression in primary culture of male rat hepatic stellate cells. Endocrinology. 2012;153:5845–5856.
Tang Y, Chen A. Curcumin protects hepatic stellate cells against leptin-induced activation in vitro by accumulating intracellular lipids. Endocrinology. 2010;151:4168–4177.
Tang Y, Chen A. Curcumin block translocation of glucose transporter-4 by interrupting the IRS/PI3K/AKT signalling pathway and increase glucokinase activity by increasing AMPK activity and suppressing PKA activity, leading to increased conversion of glucose to G-6-P. Br J Pharmacol. 2010;161:1137–1149.
Younossi ZM. Review article: current management of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Aliment Pharmacol Ther. 2008;28:2–12.
Gholam PM, Flancbaum L, Machan JT, Charney DA, Kotler DP. Nonalcoholic fatty liver disease in severely obese subjects. Am J Gastroenterol. 2007;102:399–408.
Asano T, Ogihara T, Katagiri H, et al. Glucose transporter and Na+/glucose cotransporter as molecular targets of anti-diabetic drugs. Curr Med Chem. 2004;11:2717–2724.
Leturque A, Brot-Laroche E, Le Gall M, Stolarczyk E, Tobin V. The role of GLUT2 in dietary sugar handling. J Physiol Biochem. 2005;61:529–537.
Zhao FQ, Keating AF. Functional properties and genomics of glucose transporters. Curr Genomics. 2007;8:113–128.
Jellinger PS. Metabolic consequences of hyperglycemia and insulin resistance. Clin Cornerstone. 2007;8:S30–S42.
Lin J, Chen A. Curcumin decrease intracellular glucose level of HSCs by cells by suppressing membrane translocation and gene expression of glucose transporter-2. Mol Cell Endocrinol. 2011;333:160–171.
Tsochatzis E, Papatheodoridis GV, Manesis EK, Kafiri G, Tiniakos DG, Archimandritis AJ. Metabolic syndrome is associated with severe fibrosis in chronic viral hepatitis and nonalcoholic steatohepatitis. Aliment Pharmacol Ther. 2008;27:80–89.
Bierhaus A, Humpert PM, Morcos M, et al. Understanding RAGE, the receptor for advanced glycation end products. J Mol Med. 2005;83:876–886.
Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625.
Libby P, Plutzky J. Diabetic macrovascular disease: the glucose paradox? Circulation. 2002;106:2760–2763.
Rashbar S, Figaroia JL. Novel inhibitors of advanced glycation end products. Arch Biochem Biophys. 2003;419:63–79.
Bonnefont RD. Glucose and reactive oxygen species. Curr Opin Clin Nutr Metab Care. 2002;5:561–568.
Khan I, Ahmad H, Ahmad B. Anti-glycation and anti-oxidation properties of Capsicum frutescens and Curcuma longa fruits: possible role in prevention of diabetic complication. Pak J Pharm Sci. 2014;27:1359–1362.
Lin J, Tang Y, Kang Q, Chen A. Curcumin eliminates the inhibitory effect of advanced glycation end-products (AGEs) on gene expression of AGE receptor-1 in hepatic stellate cells in vitro. Lab Invest. 2012;92:827–841.
Lin J, Tang Y, Kang Q, Feng Y, Chen A. Curcumin inhibits gene expression of receptor for advanced glycation end-products (RAGE) in hepatic stellate cells in vitro by elevating PPARγ activity and attenuating oxidative stress. Br J Pharmacol. 2012;166:2212–2227.
Schmidt AM, Yan SD, Yan SF, Stern DM. The biology of the receptor for advanced glycation end products and its ligands. Biochim Biophys Acta. 2000;1498:99–111.
Ramasamy R, Yan SF, Schmidt AM. RAGE: therapeutic target and biomarker of the inflammatory response–the evidence mounts. J Leukoc Biol. 2009;86:505–512.
Fehrenbach H, Weiskirchen R, Kasper M, Gressner AM. Up-regulated expression of the receptor for advanced glycation end products in cultured rat hepatic stellate cells during transdifferentiation to myofibroblasts. Hepatology. 2001;34:943–952.
Iwamoto K, Kanno K, Hyogo H, et al. Advanced glycation end products enhance the proliferation and activation of hepatic stellate cells. J Gastroenterol. 2008;43:298–304.
Lu C, He JC, Cai W, Liu H, Zhu L, Vlassara H. Advanced glycation endproduct (AGE) receptor 1 is a negative regulator of the inflammatory response to AGE in mesangial cells. Proc Natl Acad Sci USA. 2004;101:11767–11772.
Brett J, Schmidt AM, Yan SD, et al. Survey of the distribution of a newly characterized receptor for advanced glycation end products in tissues. Am J Pathol. 1993;143:1699–1712.
Li YM, Mitsuhashi T, Wojciechowicz D, et al. Molecular identity and cellular distribution of advanced glycation endproduct receptors: relationship of p60 to OST-48 and p90 to 80 K-H membrane proteins. Proc Natl Acad Sci USA. 1996;93:11047–11052.
Ito T, Nemoto M. Kupfer’s cells and fat storing cells in the capillary wall of human liver. Okajimas Folia Anat Jpn. 1952;24:243–258.
Friedman SL, Rockey DC, McGuire RF, Maher JJ, Boyles JK, Yamasaki G. Isolated hepatic lipocytes and Kupffer cells from normalhumanliver: morphological and functional characteristics in primary culture. Hepatology. 1992;15:234–243.
Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669.
Kisseleva T, Brenner DA. Hepatic stellate cells and the reversal of fibrosis. J Gastroenterol Hepatol. 2006;21:S84–S87.
Miyahara T, Schrum L, Rippe R, et al. Peroxisome proliferator-activated receptors and hepatic stellate cell activation. J Biol Chem. 2000;275:35715–35722.
Tsukamoto H, She H, Hazra S, Cheng J, Miyahara T. Antiadipogenic regulation underlies hepatic stellate cell transdifferentiation. J Gastroenterol Hepatol. 2006;21:S102–S105.
Lefterova MI, Lazar MA. New developments in adipogenesis. Trends Endocrinol Metab. 2009;20:107–114.
Kast-Woelbern HR, Dana SL, Cesario RM, et al. Rosiglitazone induction of Insig-1 in white adipose tissue reveals a novel interplay of peroxisome proliferator-activated receptorγ and sterol regulatory element-binding protein in the regulation of adipogenesis. J Biol Chem. 2004;279:23908–23915.
Lefterova MI, Zhang Y, Steger DJ, et al. PPARγ and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes Dev. 2008;22:2941–2952.
Peschel D, Koerting R, Nass N. Curcumin induces changes in expression of genes involved in cholesterol homeostasis. J Nutr Biochem. 2007;18:113–119.
Zeng CH1, Zeng P, Deng YH, et al. The effects of curcumin derivative on experimental steatohepatitis. Zhonghua Gan Zang Bing Za Zhi. 2011;19:454–459.
Ejaz A, Wu D, Kwan P, Meydani M. Curcumin inhibits adipogenesis in 3T3-L1 adipocytes and angiogenesis and obesity in C57/BL mice. J Nutr. 2009;139:919–925.
Hasan ST, Zingg JM, Kwan P, Noble T1, Smith D, Meydani M. Curcumin modulation of high fat diet-induced atherosclerosis and steatohepatosis in LDL receptor deficient mice. Atherosclerosis. 2014;232:40–51.
Fisslthaler B, Fleming I. Activation and signaling by the AMP activated protein kinase in endothelial cells. Circ Res. 2009;105:114–127.
Zhang BB, Zhou G, Li C. AMPK: an emerging drug target for diabetes and the metabolic syndrome. Cell Metab. 2009;9:407–416.
Gruzman A, Babai G, Sasson S. Adenosine monophosphateactivatedprotein kinase (AMPK) as a new target for antidiabetic drugs: a review on metabolic, pharmacological and chemical considerations. Rev Diabet Stud. 2009;6:13–36.
You M, Matsumoto M, Pacold CM, Cho WK, Crabb DW. The role of AMP-activated protein kinase in the action of ethanol in the liver. Gastroenterology. 2004;127:1798–1808.
Kim T, Davis J, Zhang AJ, He X, Mathews ST. Curcumin activates AMPK and suppresses gluconeogenic gene expression in hepatoma cells. Biochem Biophys Res Commun. 2009;388:377–382.
Lee YK, Park SY, Kim YM, Park OJ. Regulatory effect of the AMPK-COX-2 signaling pathway in curcumin-induced apoptosis in HT-29 colon cancer cells. Ann NY Acad Sci. 2009;1171:489–494.
Kang Q, Chen A. Curcumin suppresses expression of low-density lipoprotein (LDL) receptor, leading to the inhibition of LDL-induced activation of hepatic stellate cells. Br J Pharmacol. 2009;157:1354–1367.
Kang Q, Chen A. Curcumin eliminates oxidized LDL roles in activating hepatic stellate cells by suppressing gene expression of lectin-like oxidized LDL receptor-1 (LOX-1). Lab Invest. 2009;89:1275–1290.
Kang Q, Chen A. Curcumin inhibits srebp-2 expression in activated hepatic stellate cells in vitro by reducing the activity of specificity protein-1. Endocrinology. 2009;150:5384–5394.
Kuo JJ, Chang HH, Tsai TH, Lee TY. Curcumin ameliorates mitochondrial dysfunction associated with inhibition of gluconeogenesis in free fatty acid-mediated hepatic lipoapoptosis. Int J Mol Med. 2012;30:643–649.
Hemmann S, Graf J, Roderfeld M, Roeb E. Expression of MMPs and TIMPs in liver fibrosis—a systematic review with special emphasis on anti-fibrotic strategies. J Hepatol. 2007;46:955–975.
Somerville RP, Oblander SA, Apte SS. Matrix metalloproteinases: old dogs with new tricks. Genome Biol. 2003;4:216.
Knittel T, Mehde M, Kobold D, Saile B, Dinter C, Ramadori G. Expression patterns of matrix metalloproteinases and their inhibitors in parenchymal and non-parenchymal cells of rat liver: regulation by TNF-alpha and TGF-beta1. J Hepatol. 1999;30:48–60.
Ikeda K, Wakahara T, Wang YQ, Kadoya H, Kawada N, Kaneda K. In vitro migratory potential of rat quiescent hepatic stellate cells and its augmentation by cell activation. Hepatology. 1999;29:1760–1767.
Strongin AY, Collier I, Bannikov G, Marmer BL, Grant GA, Goldberg GI. Mechanism of cell surface activation of 72-kDa type IV collagenase. Isolation of the activated form of the membrane metalloprotease. J Biol Chem. 1995;270:5331–5338.
Takahara T, Furui K, Funaki J, et al. Increased expression of matrix metalloproteinase-II in experimental liver fibrosis in rats. Hepatology. 1995;21:787–795.
Zhou X, Hovell CJ, Pawley S, et al. Expression of matrix metalloproteinase-2 and -14 persists during early resolution of experimental liver fibrosis and might contribute to fibrolysis. Liver Int. 2004;24:492–501.
Parsons CJ, Bradford BU, Pan CQ, et al. Antifibrotic effects of a tissue inhibitor of metalloproteinase-1 antibody on established liver fibrosis in rats. Hepatology. 2004;40:1106–1115.
Watanabe T, Niioka M, Ishikawa A, et al. Dynamic change of cells expressing MMP-2 mRNA and MT1-MMP mRNA in the recovery from liver fibrosis in the rat. J Hepatol. 2001;35:465–473.
Suzuki K, Enghild JJ, Morodomi T, Salvesen G, Nagase H. Mechanisms of activation of tissue procollagenase by matrix metalloproteinase 3 (stromelysin). Biochemistry. 1990;29:10261–10270.
Nagase H, Enghild JJ, Suzuki K, Salvesen G. Stepwise activation mechanisms of the precursor of matrix metalloproteinase 3 (stromelysin) by proteinases and (4-aminophenyl)mercuric acetate. Biochemistry. 1990;29:5783–5789.
Ogata Y, Enghild JJ, Nagase H. Matrix metalloproteinase 3 (stromelysin) activates the precursor for the human matrix metalloproteinase 9. J Biol Chem. 1992;267:3581–3584.
Knauper V, Lopez-Otin C, Smith B, Knight G, Murphy G. Biochemical characterization of human collagenase-3. J Biol Chem. 1996;271:1544–1550.
Han YP, Zhou L, Wang J, et al. Essential role of matrix metalloproteinases in interleukin-1-induced myofibroblastic activation of hepatic stellate cell in collagen. J Biol Chem. 2004;279:4820–4828.
Roderfeld M, Geier A, Dietrich CG, et al. Cytokine blockade inhibits hepatic tissue inhibitor of metalloproteinase-1 expression and up-regulates matrix metalloproteinase-9 in toxic liver injury. Liver Int. 2006;26:579–586.
Iredale JP, Benyon RC, Arthur MJ, et al. Tissue inhibitor of metalloproteinase-1 messenger RNA expression is enhanced relative to interstitial collagenase messenger RNA in experimental liver injury and fibrosis. Hepatology. 1996;24:176–184.
Yasui H, Andoh A, Bamba S, Inatomi O, Ishida H, Fujiyama Y. Role of fibroblast growth factor-2 in the expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in human intestinal myofibroblasts. Digestion. 2004;69:34–44.
Iredale JP. Tissue inhibitors of metalloproteinases in liver fibrosis. Int J Biochem Cell Biol. 1997;29:43–54.
Herbst H, Wege T, Milani S, et al. Tissue inhibitor of metalloproteinase-1 and -2 RNA expression in rat and human liver fibrosis. Am J Pathol. 1997;150:1647–1659.
Kossakowska AE, Edwards DR, Lee SS, et al. Altered balance between matrix metalloproteinases and their inhibitors in experimental biliary fibrosis. Am J Pathol. 1998;153:1895–1902.
Roeb E, Purucker E, Breuer B, et al. TIMP expression in toxic and cholestatic liver injury in rat. J Hepatol. 1997;27:535–544.
Bergheim I, Guo L, Davis MA, Duveau I, Arteel GE. Critical role of plasminogen activator inhibitor-1 in cholestatic liver injury and fibrosis. J Pharmacol Exp Ther. 2006;316:592–600.
Schuppan D, Ruehl M, Somasundaram R, Hahn EG. Matrix as a modulator of hepatic fibrogenesis. Semin Liver Dis. 2001;21:351–372.
Yoshiji H, Kuriyama S, Miyamoto Y, et al. Tissue inhibitor of metalloproteinases-1 promotes liver fibrosis development in a transgenic mouse model. Hepatology. 2000;32:1248–1254.
Murphy FR, Issa R, Zhou X, et al. Inhibition of apoptosis of activated hepatic stellate cells by tissue inhibitor of metalloproteinase-1 is mediated via effects on matrix metalloproteinase inhibition: implications for reversibility of liver fibrosis. J Biol Chem. 2002;277:11069–11076.
Wang Z, Juttermann R, Soloway PD. TIMP-2 is required for efficient activation of proMMP-2 in vivo. J Biol Chem. 2000;275:26411–26415.
Vizzutti F, Provenzano A, Galastri S, et al. Curcumin limits the fibrogenic evolution of experimental steatohepatitis. Lab Invest. 2010;90:104–115.
Pinlaor S, Prakobwong S, Hiraku Y, Pinlaor P, Laothong U, Yongvanit P. Reduction of periductal fibrosis in liver fluke-infected hamsters after long-term curcumin treatment. Eur J Pharmacol. 2010;638:134–141.
Rajagopalan R, Sridharana S, Menon VP. Hepatoprotective role of bis-demethoxy curcumin analog on the expression of matrix metalloproteinase induced by alcohol and polyunsaturated fatty acid in rats. Toxicol Mech Methods. 2010;20:252–259.
Jiang Y, Li ZS, Jiang FS, Deng X, Yao CS, Nie G. Effects of different ingredients of zedoary on gene expression of HSC-T6 cells. World J Gastroenterol. 2005;11:6780–6786.
Bruck R, Ashkenazi M, Weiss S, et al. Prevention of liver cirrhosis in rats by curcumin. Liver Int. 2007;27:373–383.
Green S, Wahli W. Peroxisome proliferator-activated receptors: finding the orphan a home. Mol Cell Endocrinol. 1994;100:149–153.
Auwerx J. PPARgamma, the ultimate thrifty gene. Diabetologia. 1999;42:1033–1049.
Jones JR, Barrick C, Kim KA, et al. Deletion of PPARγ in adipose tissues of mice protects against high fat diet-induced obesity and insulin resistance. Proc Natl Acad Sci USA. 2005;102:6207–6212.
Galli A, Crabb D, Price D, et al. Peroxisome proliferator-activated receptor gamma transcriptional regulation is involved in platelet-derived growth factor-induced proliferation of human hepatic stellate cells. Hepatology. 2000;31:101–108.
Galli A, Crabb DW, Ceni E, et al. Antidiabetic thiazolidinediones inhibit collagen synthesis and hepatic stellate cell activation in vivo and in vitro. Gastroenterology. 2002;122:1924–1940.
Hazra S, Xiong S, Wang J, et al. PPARgamma induces a phenotypic switch from activated to quiescent hepatic stellate cells. J Biol Chem. 2003;279:11392–11401.
Zheng S, Chen A. Activation of PPARgamma is required for curcumin to induce apoptosis and to inhibit the expression of extracellular matrix genes in hepatic stellate cells in vitro. Biochem J. 2004;384:149–157.
Xu J, Fu Y, Chen A. Activation of peroxisome proliferator-activated receptor-gamma contributes to the inhibitory effects of curcumin on rat hepatic stellate cell growth. Am J Physiol Gastrointest Liver Physiol. 2003;285:G20–G30.
Acknowledgments
Many thanks to Keith Blomenkamp, research assistant in Department of Pediatrics at Saint Louis University, for the English edition. This work is supported by NSFC (National Natural Science Foundation of China) granted to Dr. Youcai Tang (NSFC 31471330).
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tang, Y. Curcumin Targets Multiple Pathways to Halt Hepatic Stellate Cell Activation: Updated Mechanisms In Vitro and In Vivo. Dig Dis Sci 60, 1554–1564 (2015). https://doi.org/10.1007/s10620-014-3487-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-014-3487-6