Abstract
Schizandrin A (Sch A) exert anticancer and multidrug resistance-reversing effects in a variety of tumors, but its effect on 5-fluorouracil (5-Fu) in gastric cancer (GC) cells remains unclear. The aim of the present study was to examine the resistance-reversing effect of Schizandrin A and assess its mechanisms in 5-Fu-resistant GC cells.5-Fu-sensitive GC cells were treated with 5-Fu and 5-Fu-resistant GC cells AGS/5-Fu and SGC7901/5-Fu were were established. These cells were stimulated with Schizandrin A alone or co-treated with 5-Fu and their effect on tumor cell growth, proliferation, migration, invasion and ferroptosis-related metabolism were investigated both in vitro and in vivo. A number of additional experiments were conducted in an attempt to elucidate the molecular mechanism of increased ferroptosis. The results of our study suggest that Schizandrin A in combination with 5-Fu might be useful in treating GC by reverse drug resistance. It was shown that Schizandrin A coadministration suppressed metastasis and chemotherapy resistance in 5-Fu-resistant GC cells through facilitating the onset of ferroptosis, which is an iron-dependent form of cell death, which was further demonstrated in a xenograft nude mouse model. Mechanistically, Schizandrin A co-administration synergistically increased the expression of transferin receptor, thus iron accumulates within cells, leading to lipid peroxidation, which ultimately results in 5-Fu-resistant GC cells death. The results of this study have provided a novel strategy for increasing GC chemosensitivity, indicating Schizandrin A as a novel ferroptosis regulator. Mechanistically, ferroptosis is induced by Schizandrin A coadministration via increasing transferrin receptor expression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There has been an increase in the incidence of gastric cancer (GC) worldwide, a malignant tumor of the gastric mucosa. Because of its high lethality, GC ranks fourth in cancer-related deaths (Shi et al. 2018). Approximately 24,000 new cases of gastric cancer are diagnosed each year in the United States, and approximately 700,000 cases are diagnosed worldwide according to statistics from the American Cancer Society (Giaquinto et al. 2022). Although advances in diagnostic techniques, surgery, and neoadjuvant chemoradiotherapy have been made, however, due to the low penetration and compliance of gastroscopy screening, there are fewer GC patients diagnosed at an early stage, thus it is common for patients to be diagnosed with advanced GC at their first clinical appointmen, thereby missing the optimal therapeutic window. There are very few patients who survive 5 years after surgery resection, even when they undergo surgical resection (Giaquinto et al. 2022). Most patients with gastric cancer who undergo radical surgery often have recurrences and metastases after surgery, noth of these characteristics seriously affect the patient’s long-term survival. Chemoradiotherapy administered postoperatively has been shown to significantly improve overall survival and disease-free survival (Yildirim et al. 2023).
Postoperative treatment for GC consists mainly of chemotherapy, with 5-Fu and its derivatives being the cornerstones (Mahlberg et al. 2017). As the primary clinical therapeutic strategy to prolong survival and improve the quality of life for these patients, systemic 5-Fu chemotherapy remains the primary option (Kim et al. 2022). As a result, it can reduce the recurrence rate and improve the quality of life of patients with GC after operation. However, patients with GC tend to develop drug resistance after being treated with 5-Fu. Further research into GC molecular mechanisms and cellular resistance is thus needed to identify new therapeutic targets and develop individualized treatment strategies.
An iron-dependent form of cell death, known as ferroptosis, has received considerable research attention in the last few years. Ferroptosis is characterized by an accumulation of lipid reactive oxygen species, condensed mitochondrial membrane densities, reduced mitochondrial cristae and unlike other types of regulated cell death (Li et al. 2020). Ferroptosis plays an important role in regulating cell death, which is why it is thought to play a role in tumorigenesis (Stockwell et al. 2017). In fact, a number of studies have demonstrated that ferroptosis plays a crucial role in tumor development and drug resistance including GC (Zhang et al. 2020). Inducing ferroptosis seems to provide a novel strategy for managing chemotherapy-resistant GC.
It has been shown that novel therapeutic agents combined with chemotherapy prevents postoperative recurrences of GC better than modern medicine alone. There is growing evidence showing that natural bioactive products can have significant beneficial effects in attenuating the side effects of chemotherapeutic cancer treatment (Luo et al. 2019). A bioactive lignin compound isolated from Schisandra chinesnesis is Schizandrin A (Zhu et al. 2021). Schizandrin A has been shown to possess a number of biological properties, including neuroprotective, hepatoprotective, anti-inflammatory, antitumor, and antioxidation properties (Fu et al. 2022). In non-small cell lung cancer, Schizandrin A synergistic with gefitinib to enhances its efficacy (Xian et al. 2019). In the treatment of esophageal cancer, Schizandrin A synergistic strategies have been employed (Su et al. 2017). These results suggest that Schizandrin A may also have synergistic antitumor effects in GC and the potential mechanisms involved in this synergy remain unclear. The purpose of this study was to determine whether Schizandrin A could overcome the resistance to 5-Fu in GC using GC cells resistant to the 5-Fu treatment.
Materials and methods
Cell culture
AGS and SGC7901 were purchased from American Type Culture Collection (Manassas, VA, USA). 5-Fu resistant AGS and SGC7901 cells were generated by continuous exposure to increasing concentrations of 5-Fu (from 5 to 30 μg/ml) with repeated subcultures as previously described (Jiang et al. 2019). A continuous exposure to 5-FU for one month resulted in the production of AGS/5-Fu and SGC7901/5-Fu cells that were resistant to the 5-Fu. Cells were cultured in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum (HyClone, Logan, UT, USA) and 100 IU/ml penicillin and streptomycin in a 5% CO2 incubator at 37 °C. All cell lines used were tested and confirmed to be negative for mycoplasma. We purchased Schizandrin A (purity 99%) from Wako Pure Chemical (Osaka, Japan). 10 μM Erastin (Era) and 3 mM N-acetylcysteine (NAC) purchased from Thermo Fisher Scientific were used to treat cell as previously described (Wang et al. 2020). Deferiprone (DFP, 10 μM) and 5-Fu were obtained from Sigma-Aldrich (Kuang et al. 2023).
Cell viability assay
Three thousand cells were seeded per well in 96-well plates. At indicated time points, cell viability was determined using CellTiter-Glo Luminescent Cell Viability Assay (Promega, Madison, WI, USA) and the absorbance was measured with a TECAN Infinite M200 plate reader (TECAN, Männedorf, Switzerland).
Transwell assays
Migration and invasion were analyzed using Transwell assay. Cells were seeded into the upper chamber of the 24-well transwell insert (8 μl pores; precoated with Matrigel for invasion) in 200 μl serum-free medium. The lower chamber was added with 600 μl complete medium. After cultured for 24 h, the bottom cells were fixed in methanol and stained with 0.1% crystal violet, followed by photograph unsing an inverted microscope (Olympus, Japan).
Colony formation assay
Cells in the logarithmic phase were grouped and treated, and digested with trypsin. 1000 cells was evenly planted in a 6-well plate for routine cultivation, and a new culture medium was replaced every 3 days. After seven days, the 6-well plate was removed, and the cells were fixed with 4% paraformaldehyde for 20 min. PBS was used to clean the cells three times. After staining with 0.5% crystal violet for eight minutes, the cells were washed with water three times again and photographed by an electronic scanner (Fuji Xerox, Tokyo, Japan).
Flow cytometry
After corresponding treatment, following washing, centrifugation, and resuspension in 1 ml of buffer, cell suspensions were collected. In accordance with the manufacturer’s instructions (Vazyme, Nanjing, China), Annexin V-FITC Apoptosis Detection kit was used to detect apoptotic cells.Using an FC500 flow cytometer (Beckman-Coulter, Fullerton, CA, USA), Annexin-V FITC and PI fluorescence were monitored.
Lipid peroxidation and iron examination
Cellular malondialdehyde (MDA) was measured to determine lipid peroxidation. An MDA testing kit (Sigma-Aldrich, Cat #: MAK085) was used to determine the MDA content in cells. Iron concentration was determined by flame atomic absorption spectroscopy, as previously described (Citelli et al. 2015). The levels of intracellular Fe2 + were determined using FerroOrange (Dojindo, Japan) according to the manufacturers protocols.
Electron microscopy
Electron microscope was used to examine mitochondrial ultrastructure in treated cells as previously described (Sun et al. 2021). The collected cells were wash twice with pre-cooled PBS, and transfer them to a 1.5 mL centrifuge tube, centrifuge at 4 °C and 1000 r/min for 15 min. Discard the supernatant and add 500 first μ L glutaraldehyde (2.5%) for prefixed, and then fixed with 1% citric acid. After fixation, gradient ethanol dehydration was used, epoxy resin Epon812 was embedded, and semi thin sections were performed using LKB2 III ultrathin sectioning machine. After positioning, ultra thin sections were performed, and double staining was performed on the samples using uranium acetate dioxide and lead citrate. Finally, the JEM1200EX transmission electron microscope was used to observe, photograph, and record the ultrastructure of cells.
Xenograft mouse models
About 5 × 105 active SGC7901/5-Fu cells were suspended in 200 μl PBS and subcutaneously injected into a group of seven nude mice, respectively. The volume of tumor was detected every 5 days. The 5-Fu and/or Schizandrin A treatments were administered during the experiment (5-Fu, 1 mg/kg/day, i.p.; Schizandrin A, 1 mg/kg/day, i.p.). A therapeutic period is defined as one day following the last dose and starting on the date of the first dose. After 15 days, the mice were sacrificed and xenografts were excised, weighed and took photos.
Western blot
The cell lysate was extracted by RIPA buffer containing Complete tablets (Roche, Basel, Switzerland) and phosphatase inhibitor (Sangon Biotech, Shanghai, China) on a shaker overnight. The PVDF membranes (Invitrogen) were incubated in the 5% BSA (Sangon Biotech) with the primary antibody overnight. The dilution of primary antibodies for blots were 1:1000. Protein bands were visualized using enhanced chemiluminescence detection kit (Thermo Scientific) and ChemiDoc Touch imaging system (Bio-Rad, Hercules, CA, USA). The raw data were processed by Image Lab software.
Immunohistochemistry
The xenografts were cut in small pieces to ensure fix entirely. After the sections were rehydrated as above mentioned, heat-induced antigen retrieval was performed in sodium citrate for 15 min in 95 °C water bath, followed by 3% hydrogen peroxide and 5% BSA to block endogenous peroxidase activity and non-specific antigens. Next, the sections were incubated with primary antibody Tfrc (1:1000) at 4 °C overnight. The next day, the sections were incubated in HRP conjugated secondary antibodies. Subsequently, sections were visualized using DAB and co-stained with hematoxylin (Sangon Biotech), mounted with neutral gum and took photos by a bright field microscope.
qRT-PCR
Lentiviral shRNA plasmids and lentiviruses were generated as previously described. The three targeting shRNAs were designed from Invitrogen or Sigma website. The empty FUGW-H1 vector was used as the control. Knockdown of Tfrc at transcript levels were determined by RT-qPCR. We chose the most efficient Tfrc-shRNA3 to experiment in vitro. shRNAs used in our study are listed below: Tfrc-shRNA1, GAACCGACATGGGTAGCAATT; Tfrc-shRNA2, ACATGACCATGCCCGCATAAA; Tfrc-shRNA3, CCAAGTCTGTATTCAGGATGG. Total RNAs were isolated through TRIzol reagent (Invitrogen) and transcribed into cDNA with a PrimeScript RT reagent Kit (Takara, China). Then qPCR was performed using SYBR Premix EX Taq™ II (Takara, Japan). The mRNA expressions were normalized to GAPDH. The following primers were used: Tfrc, forward: 5’-TTCTTGCGACATCAGCCCAT-3’ and reverse: 5′-AAAGAACGGAGCAGCCTCTC-3′; GAPDH, forward: 5′-GGAGCGAGATCCCTCCAAAAT-3′ and reverse: 5′- GGCTGTTGTCATACTTCTCATGG-3′.
Statistical analysis
GraphPad Prism 7.0 (GraphPad, CA, USA) was used to analyze results and perform the figures. Data were presented as means ± standard deviation (SD). Significant differences were analyzed using one-way ANOVA. P < 0.05 was considered a significant difference.
Results
Schizandrin A improves sensitivity of gastric cancer cells to 5-Fu and ferroptosis is implicated
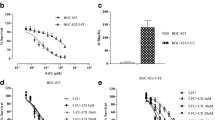
The viability of 5-Fu resistant GC cells (AGS/5-Fu and SGC7901/5-Fu) cells was assessed by performing a cell viability assay with different concentrations of Schizandrin A. It was found that AGS/5-Fu and SGC7901/5-Fu cells exposed to low concentrations (0.1–5 μM) of Schizandrin A had no effect on the viability of their cells (Fig. 1A, B). Whether Schizandrin A could enhance 5-Fu resistant GC cells sensitivity to 5-Fu were further explored according to these data. A combined treatment with non-toxic Schizandrin A (5 μM) can effectively increase 5-Fu cytotoxicity in 5-Fu resistant GC cells. In the following experiments, ferroptosis modulators were evaluated to determine whether ferroptosis play a vital role in increases AGS/5-Fu and SGC7901/5-Fu cell sensitivity to Schizandrin A plus 5-Fu-triggered cell death. While Erasin (an activator of ferroptosis) further enhanced Schizandrin A plus 5-Fu-triggered cell death, Deferiprone (DFP) and N-acetylcysteine (NAC) (two inhibitor of ferroptosis) block the cell death caused by them (Fig. 1C, D). According to the results, Schizandrin A plus 5-Fu induces iron-dependent cell death in AGS/5-Fu and SGC7901/5-Fu cells.
Schizandrin A enhances the sensitivity of gastric cancer cells to 5-FU by promoting ferroptosis. A, B Effects of different concentrations of Schizandrin A on AGS/5-Fu and SGC7901/5-Fu cell viability. C, D Effects of addition of ferroptosis modulators on AGS/5-Fu and SGC7901/5-Fu cell sensitivity to Schizandrin A plus 5-Fu-triggered cell death.***P < 0.001; **P < 0.01; *P < 0.05
The effect of Schizandrin A combined with 5-Fu on the cell migration, invasion and proliferation of 5-Fu-resistant gastirc cancer cells
Scratch and Transwell assays were applied to evaluate Schizandrin A’s effects on migration and invasion suppression of 5-Fu-resistant GC cells in response to 5-Fu. In line with the previous results, we found that while Schizandrin A plus 5-Fu combination could effectively inhibit migration of AGS/5-Fu and SGC7901/5-Fu, and the addition of DFP block the synergistic effect of Schizandrin A/5-Fu treatment (Fig. 2A). The similar results were observed in tumor invasion test (Fig. 2B). To further identify effects of Schizandrin A on 5-Fu-induced growth suppression of 5-Fu-resistant GC cells, colony formation assays were conducted with AGS/5-Fu and SGC7901/5-Fu cells. Schizandrin A combined with 5-Fu could also significantly inhibit colony formation of AGS/5-Fu and SGC7901/5-Fu than Schizandrin A or 5-Fu alone did, however, addition of a ferroptosis blocker DFP significantly reverted this effect (Fig. 2C). It is thereby proposed that the ferroptosis is a possible mechanism for Schizandrin A/5-Fu synergistic effects.
Schizandrin A inhibited cell migration, invasion and proliferation in 5-Fu-resistant GC cells treated with DDP. A, B A transwell assay was performed to detect Schizandrin A’s effects on cell migration and invasion in AGS/5-Fu and SGC7901/5-Fu cells. Representative images are shown. C The sensitizing effects of Schizandrin A on AGS/5-Fu and SGC7901/5-Fu cells following 5-Fu treatment are demonstrated in a colony formation assay.***P < 0.001; **P < 0.01
Schizandrin A worked synergistically with 5-Fu to promote ferroptosis in 5-FU-resistant GC cells
Our flow cytometry analysis revealed that Schizandrin A synergistically with 5-Fu could significantly increased cell death in AGS/5-Fu and SGC7901/5-Fu cells, whereas iron chelator DFP inhibited it (Fig. 3A). In order to further understand the mechanism and role of Schizandrin A/5-Fu in ferroptosis, iron accumulation and lipid peroxidation were analyzed. Interestingly, the Schizandrin A synergistically with 5-Fu increased the levels of intracellular lipid peroxidation levels (Fig. 3B). The results atomic absorption spectroscopy and FerroOrange immunofluorescence which reacts specifically with Fe2+ both showed that the levels of iron content are increased upon Schizandrin A synergistically with 5-Fu. The above mention effects on Schizandrin A plus 5-Fu could also be reversed by DFP (Fig. 3C, D). Electron microscopy was used to analyze mitochondrial morphology qualitatively in SGC7901/5-Fu group cells. In the SGC7901/5-Fu group cells, TEM revealed typical ferroptosis ultrastructural changes, including smaller mitochondria, diminished or vanished mitochondria crista, and condensed mitochondrial membrane densities. In contrast, the addition of DFP alleviated these abnormalities of mitochondrial morphology (Fig. 3E).
Schizandrin A enhances the sensitivity of gastric cancer cells to 5-FU by promoting ferroptosis. A PI-Annexin V double staining followed by flow cytometry was used to assess AGS/5-Fu and SGC7901/5-Fu cell death. B MDA, a metabolite produced by lipid peroxidation, was used to measure lipid peroxidation on AGS/5-Fu and SGC7901/5-Fu cells. C Assays of cellular iron content in AGS/5-Fu and SGC7901/5-Fu cells were conducted by atomic absorption spectrometry. D Typical images of the fluorescent FerroOrange probe showing intracellular Fe2+ levels in AGS/5-Fu and SGC7901/5-Fu cells. Scale bar, 1 μm. E Electron microscopy was used to observe the ultrastructural changes of the mitochondria. Scale bar, 200 μm. ***P < 0.001; **P < 0.01; *P < 0.05
Schizandrin A worked synergistically with 5-Fu to enhances the anticancer activity in vivo
To further investigate the effect of Schizandrin A synergistically with 5-Fu in counteracting 5-Fu resistance in GC growth in vivo, we established the mouse model of GC induced by nude mice inoculation experiment with SGC7901/5-Fu cells and drug administration with Schizandrin A synergistically with 5-Fu or their alone for 15 days. Compared with nontreated-Con mice, Schizandrin A or 5-Fu administrated alone had little effect on tumor growth, however, the combination of these drugs inhibited tumor growth compared to each drug alone (Fig. 4A, B). Schizandrin A significantly enhanced the inhibitory effects of 5-Fu on SGC7901/5-Fu cell proliferation in nude mice, which was highly consistent with our in vitro results. As part of an investigation into lipid peroxidation, 4-HNE immunohistochemistry was conducted in the tumor tissues. Consistent with the results in vitro, the histological analysis indicated that combination treatment groups exhibited stronger immunoreactivity for 4-HNE (Fig. 4C). These results indicate that combination treatment significantly enhances ferroptosis and inhibits gastric carcinogenesis in vivo.
Schizandrin A enhances the sensitivity of gastric cancer cells to 5-FU by promoting ferroptosis in vivo. A A representative subcutaneous xenograft tumor 15 days after inoculation. B The tumor growth curves for all treatment groups are presented. C The expressions of 4-HNE were determined by immunohistochemical staining.Scale bar, 50 μm. ***P < 0.001
Combinative treatment of Schizandrin A and 5-Fu upregulates transferin receptor expression in 5-Fu-resistant GC cells
Based on the increases in the content of iron upon co-treatment, the expression of genes involved in iron absorption was examined to determine whether it was achieved normal-iron status through upregulation of iron absorption. Then, we collected Schizandrin A and/or 5-Fu treated AGS/5-Fu and SGC7901/5-Fu cells to detect the expression of transferin receptor by western blot and immunohistochemical staining, and the results showed that combinative treatment of Schizandrin A and 5-Fu enhance the expression of transferin receptor (Fig. 5A, B). Animal experiments also showed that the in vivo tumor transferin receptor expression was promoted by combinative treatment (Fig. 5C). Taken together, these findings strongly suggested that transferin receptor contributed to the ferroptosis-mediated growth inhibition in AGS/5-Fu and SGC7901/5-Fu cells.
Combinative treatment of Schizandrin A and 5-Fu promoting transferin receptor expression. A The protein levels of transferin receptor were detected in AGS/5-Fu and SGC7901/5-Fu cells combinative treatment of Schizandrin A and 5-Fu. B SGC7901/5-Fu cells were fluorescently immunostained for DAPI (blue) and transferin receptor (red). Scale bar, 20 μm. C The expressions of transferin receptor were determined by immunohistochemical staining in nude mice tumor tissue. Scale bar, 50 μm. ***P < 0.001
Schizandrin A sensitizes 5-Fu-resistant GC cells to ferroptosis by prompting transferin receptor expression.
We next investigate whether combinative treatment of Schizandrin A and 5-Fu mediated GC growth inhibition and susceptibility to 5-Fu through transferin receptor. To test this, we knocked down transferin receptor with shRNA-expressing lentivirus which stably expressing the control shRNA or Tfrc shRNA. An RT-qPCR assay was then used to determine the recombinant lentivirus’s expression efficiency. As shown in Fig. 6A, knockdown of transferin receptor with shRNA3 markedly decreased the levels of transferin receptor in AGS/5-Fu and SGC7901/5-Fu cells. Consistent with our expectation, the inhibition of cell growth, cell migration and cell invasion by the combination treatment was reversed by the application of the transferin receptor knockdown (Fig. 6B–D). In addition, ferroptosis indicators, the levels of intracellular ferrous iron and lipid peroxidation, were restored in the AGS/5-Fu and SGC7901/5-Fu cells upon transferin receptor suppression (Fig. 6E, F). Taken together, these data highlight that combinative treatment of Schizandrin Ais sensitive to 5-Fu-resistant GC cells by inducing ferroptosis and promoting transferin receptor expression.
Knockdown of transferin receptor block the combined treatment-mediated sensitivity to ferroptosis induction. A We infected AGS/5-Fu and SGC7901/5-Fu cells with the three lentiviral shRNAs constructs and examined transferin receptor expression for validation of lentivirus knockdown efficiency. B Cell viability was measured by CCK8 of AGS/5-Fu and SGC7901/5-Fu cells treated with the indicated treatment for 72 h. C, D Using a transwell invasion assay, we determined how much cell migration and invasion occurred in AGS/5-Fu and SGC7901/5-Fu cells transfected with Tfrc-shRNA. E FerroOrange probe was used to detect intracellular Fe2+ levels. F MDA was used to measure lipid peroxidation on AGS/5-Fu and SGC7901/5-Fu cells. ***P < 0.001; **P < 0.01
Discussion
Despite the fact that 5-Fu-based chemotherapy significantly prolonged life expectancy for patients, resistance to 5-Fu is a major obstacle to treatment success. Because 5-Fu is an important first-line chemotherapeutic agent for GC, we selected it as the positive drug in our study. It is possible to improve the anti-tumor activity of 5-Fu and to reduce drug resistance by utilizing some optimizing strategies, including 5-Fu-based combination therapies. In the present study, we found that Schizandrin A plus 5-Fu inhibited the proliferation and increased apoptosis in 5-Fu-resistant GC cells, enhances the synthesis of transferrin receptors, revealing for the first time a mechanism of ferroptosis-mediated tumor drug resistance inhibition.
The 5-Fu resistant GC cells used in this study were constructed using the previously described method and the preliminary toxicity of Schizandrin A on these cells was tested (Jiang et al. 2019). The inhibition of gastric cancer cell growth by the use of non-toxic Schizandrin A alone and 5-Fu in combination is the first exciting discovery in this study. This is the first report on the role of Schizandrin A in regulating gastric cancer chemotherapy resistance. In order to verify the mechanism of ferroptosis in this synergistic inhibitory effect, we administered activators and inhibitors of ferroptosis after synergistic administration, and observed the impact of intervention on this synergistic effect from both positive and negative perspectives. As an activator of ferroptosis, Erastin has been found to induce ferroptosis in gastric cancer cells (Yang et al. 2020), and our experimental results replicate this conclusion. Among two chosen ferroptosis inhibitors, one is iron chelating agent and the other is redox regulatory compound. We found that both strategies can effectively resist tumor growth inhibition caused by synergistic effects, and iron chelating agent shows potential better effects. This is the basis for choosing DFP as an intervention drug in our subsequent experiments.
Our results show that synergistic drugs can effectively inhibit tumor growth, providing evidence for the feasibility of early clinical intervention. Tumor metastasis refers to the process of tumor cells separating from the primary site and ultimately colonizing distant organs, which is an important cause of cancer recurrence related death (Bakir et al. 2020). Liang et al. have shown that the migration and invasion ability are enhanced after GC cells develop 5-Fu resistance (Liang et al. 2022). The synergistic use of the two drugs significantly inhibits the migration and invasion of GC cells, which provides theoretical feasibility for hindering the metastasis of GC after surgery. The above effects can all be blocked by the iron chelating drug DFP has further sparked our interest in exploring the mechanisms related to iron metabolism. As a crucial trace element in the biological world, the stable concentration and balanced distribution of iron ensure that the intracellular iron content is nontoxic (Park and Chung 2019). When influenced by various factors such as abnormal lipid oxidation, intracellular iron becomes the key to inducing ferroptosis, which was first proposed by Dixon in 2012 (Dixon et al. 2012). Our results show that synergistic therapy increases intracellular iron content and lipid peroxidation, and causes ferroptosis characteristic changes in mitochondrial. These evidences further confirm the inducing effect of synergistic therapy on inducing ferroptosis in 5-Fu-resistant cells.
Recent researches had identified multiple ferroptosis-related molecular mechanisms involved in drug resistance in GC. Fu et al. found that upregulation of ATF3 significantly enhanced the inhibitory effect of Erastin and RSL3 on GC cells exposed to cisplatin, thus enhance the sensitivity of GC cells to cisplatin by inducing ferroptosis (Fu et al. 2021). The STAT3-ferroptosis negative regulatory axis could also suppresses tumor growth and alleviates chemoresistance in gastric cancer (Ouyang et al. 2022). It is also worth noting that Sorafenib-induced GC chemoresistance can be reversed by overexpressing GPX4 or activating Keap1/Nrf2 to induce ferroptosis (Cai et al. 2021). These studies all demonstrate that regulating ferroptosis may be an effective strategy for targeting drug-resistant GC cells, thereby enhancing their sensitivity to chemotherapy drugs. Our study found that the synergistic effect of Schizandrin A and 5-Fu increased the expression of transferrin receptor responsible for iron absorption, which is an unreported way to regulate ferroptosis in GC. These results suggest that different cancers and chemotherapy drugs may have their own potential ferroptosis-related molecular therapeutic targets. Future research on drug resistance mechanisms still needs to further clarify the potential regulatory roles of different ferroptosis molecules, in order to improve understanding of this field and provide a basis for the development of related adjuvant drugs.
In summary, through the above experiments, we have preliminarily identified the mechanism of the combination of two drugs to overcome 5-Fu resistance in GC. Schizandrin A combined with 5-Fu may induce upregulation of transferrin receptor expression, promote the excessive accumulation of intracellular iron content and lipid peroxidation levels, and ultimately induce ferroptosis in 5-Fu resistant GC cells, thereby overcoming 5-Fu resistance.
Data availability
The datasets used or analysed during the current study are available from the corresponding author on reasonable request.
References
Bakir B, Chiarella AM, Pitarresi JR, Rustgi AK (2020) EMT, MET, plasticity, and tumor metastasis. Trends Cell Biol 30:764–776. https://doi.org/10.1016/j.tcb.2020.07.003
Cai S, Fu S, Zhang W, Yuan X, Cheng Y, Fang J (2021) SIRT6 silencing overcomes resistance to sorafenib by promoting ferroptosis in gastric cancer. Biochem Biophys Res Commun 577:158–164. https://doi.org/10.1016/j.bbrc.2021.08.080
Citelli M, Fonte-Faria T, Nascimento-Silva V, Renovato-Martins M, Silva R, Luna AS, Silva SV, Barja-Fidalgo C (2015) Obesity promotes alterations in iron recycling. Nutrients 7:335–348. https://doi.org/10.3390/nu7010335
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B 3rd, Stockwell BR (2012) Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149:1060–1072. https://doi.org/10.1016/j.cell.2012.03.042
Fu D, Wang C, Yu L, Yu R (2021) Induction of ferroptosis by ATF3 elevation alleviates cisplatin resistance in gastric cancer by restraining Nrf2/Keap1/xCT signaling. Cell Mol Biol Lett 26:26. https://doi.org/10.1186/s11658-021-00271-y
Fu K, Zhou H, Wang C, Gong L, Ma C, Zhang Y, Li Y (2022) A review: pharmacology and pharmacokinetics of Schisandrin A. Phytother Res 36:2375–2393. https://doi.org/10.1002/ptr.7456
Giaquinto AN, Miller KD, Tossas KY, Winn RA, Jemal A, Siegel RL (2022) Cancer statistics for African American/Black people 2022. CA Cancer J Clin 72:202–229. https://doi.org/10.3322/caac.21718
Jiang S, Miao D, Wang M, Lv J, Wang Y, Tong J (2019) MiR-30-5p suppresses cell chemoresistance and stemness in colorectal cancer through USP22/Wnt/β-catenin signaling axis. J Cell Mol Med 23:630–640. https://doi.org/10.1111/jcmm.13968
Kim NH, Park JH, Koo DH, Jung YS, Yang JY, Lee HY (2022) A pilot study of peritumor administration of 5-FU for preventing bleeding in advanced gastric cancer. Korean J Gastroenterol 80:273–280. https://doi.org/10.4166/kjg.2022.099
Kuang H, Sun X, Liu Y, Tang M, Wei Y, Shi Y, Li R, Xiao G, Kang J, Wang F, Peng J, Xu H, Zhou F (2023) Palmitic acid-induced ferroptosis via CD36 activates ER stress to break calcium-iron balance in colon cancer cells. FEBS J. https://doi.org/10.1111/febs.16772
Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao N, Sun B, Wang G (2020) Ferroptosis: past, present and future. Cell Death Dis 11:88. https://doi.org/10.1038/s41419-020-2298-2
Liang S, Xiang T, Liu S, Xiang W (2022) Inhibition of NLRC5 attenuates the malignant growth and enhances the sensitivity of gastric cancer cells to 5-FU chemotherapy by blocking the carcinogenic effect of YY1. Exp Ther Med 24:601. https://doi.org/10.3892/etm.2022.11538
Luo H, Vong CT, Chen H, Gao Y, Lyu P, Qiu L, Zhao M, Liu Q, Cheng Z, Zou J, Yao P, Gao C, Wei J, Ung COL, Wang S, Zhong Z, Wang Y (2019) Naturally occurring anti-cancer compounds: shining from Chinese herbal medicine. Chin Med 14:48. https://doi.org/10.1186/s13020-019-0270-9
Mahlberg R, Lorenzen S, Thuss-Patience P, Heinemann V, Pfeiffer P, Möhler M (2017) New perspectives in the treatment of advanced gastric cancer: S-1 as a novel Oral 5-FU therapy in combination with cisplatin. Chemotherapy 62:62–70. https://doi.org/10.1159/000443984
Ouyang S, Li H, Lou L, Huang Q, Zhang Z, Mo J, Li M, Lu J, Zhu K, Chu Y, Ding W, Zhu J, Lin Z, Zhong L, Wang J, Yue P, Turkson J, Liu P, Wang Y, Zhang X (2022) Inhibition of STAT3-ferroptosis negative regulatory axis suppresses tumor growth and alleviates chemoresistance in gastric cancer. Redox Biol 52:102317. https://doi.org/10.1016/j.redox.2022.102317
Park E, Chung SW (2019) ROS-mediated autophagy increases intracellular iron levels and ferroptosis by ferritin and transferrin receptor regulation. Cell Death Dis 10:822. https://doi.org/10.1038/s41419-019-2064-5
Shi P, Wan J, Song H, Ding X (2018) The emerging role of circular RNAs in gastric cancer. Am J Cancer Res 8:1919–1932
Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascón S, Hatzios SK, Kagan VE, Noel K, Jiang X, Linkermann A, Murphy ME, Overholtzer M, Oyagi A, Pagnussat GC, Park J, Ran Q, Rosenfeld CS, Salnikow K, Tang D, Torti FM, Torti SV, Toyokuni S, Woerpel KA, Zhang DD (2017) Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell 171:273–285. https://doi.org/10.1016/j.cell.2017.09.021
Su X, Gao C, Shi F, Feng X, Liu L, Qu D, Wang C (2017) A microemulsion co-loaded with Schizandrin A-docetaxel enhances esophageal carcinoma treatment through overcoming multidrug resistance. Drug Deliv 24:10–19. https://doi.org/10.1080/10717544.2016.1225854
Sun L, Dong H, Zhang W, Wang N, Ni N, Bai X, Liu N (2021) Lipid peroxidation, GSH depletion, and SLC7A11 Inhibition are common causes of EMT and ferroptosis in A549 cells, but different in specific mechanisms. DNA Cell Biol 40:172–183. https://doi.org/10.1089/dna.2020.5730
Wang L, Liu Y, Du T, Yang H, Lei L, Guo M, Ding HF, Zhang J, Wang H, Chen X, Yan C (2020) ATF3 promotes erastin-induced ferroptosis by suppressing system Xc. Cell Death Differ 27:662–675. https://doi.org/10.1038/s41418-019-0380-z
Xian H, Feng W, Zhang J (2019) Schizandrin A enhances the efficacy of gefitinib by suppressing IKKβ/NF-κB signaling in non-small cell lung cancer. Eur J Pharmacol 855:10–19. https://doi.org/10.1016/j.ejphar.2019.04.016
Yang Y, Luo M, Zhang K, Zhang J, Gao T, Connell DO, Yao F, Mu C, Cai B, Shang Y, Chen W (2020) Nedd4 ubiquitylates VDAC2/3 to suppress erastin-induced ferroptosis in melanoma. Nat Commun 11:433. https://doi.org/10.1038/s41467-020-14324-x
Yildirim HC, Guven DC, Akyildiz A, Yalcin S, Dizdar O (2023) A meta-analysis of the association between adjuvant chemoradiotherapy and disease-free survival in gastric cancer according to the histology. Ir J Med Sci. https://doi.org/10.1007/s11845-023-03297-7
Zhang H, Deng T, Liu R, Ning T, Yang H, Liu D, Zhang Q, Lin D, Ge S, Bai M, Wang X, Zhang L, Li H, Yang Y, Ji Z, Wang H, Ying G, Ba Y (2020) CAF secreted miR-522 suppresses ferroptosis and promotes acquired chemo-resistance in gastric cancer. Mol Cancer 19:43. https://doi.org/10.1186/s12943-020-01168-8
Zhu L, Wang Y, Lv W, Wu X, Sheng H, He C, Hu J (2021) Schizandrin A can inhibit non-small cell lung cancer cell proliferation by inducing cell cycle arrest, apoptosis and autophagy. Int J Mol Med 48:214. https://doi.org/10.3892/ijmm.2021.5047
Author information
Authors and Affiliations
Contributions
All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hu, L., Zhang, Z., Zhu, F. et al. Schizandrin A enhances the sensitivity of gastric cancer cells to 5-FU by promoting ferroptosis. Cytotechnology 76, 329–340 (2024). https://doi.org/10.1007/s10616-024-00623-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-024-00623-4