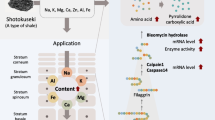
Abstract
Corneocytes and intercellular lipids form the stratum corneum. The content and composition of intercellular lipids in the stratum corneum significantly affect skin barrier function. The purpose of this study was to demonstrate the effect of Shotokuseki extract (SE) on intercellular lipid production and metabolism in human three-dimensional cultured human epidermis. SE or ion mixtures containing five common ions were applied to three-dimensional cultured human epidermis for 2–8 days for each assay. The mRNA expression levels of epidermal differentiation markers and lipid metabolism genes were quantified by real-time PCR. After extraction of lipids from the epidermis, ceramide, sphingosine, free fatty acids, and cholesterol were quantified by LC-MS/MS, GC-MS, or HPLC. The results showed that the application of SE increased the gene expression levels of epidermal differentiation markers keratin10 and transglutaminase. Elongation of very long-chain fatty acids protein 3, serine palmitoyl transferase, ceramide synthase 3, and acid ceramidase mRNA expression levels increased and fatty acid synthase mRNA expression decreased. The content of each lipid, [EOS] ceramide decreased and total sphingosine content increased on day 4. On day 8 of application, ceramide [NDS], [NP], and [EODS] increased and total free fatty acid content decreased. These results show that SE alters the lipid composition of the epidermis, increasing ceramides and decreasing free fatty acids in the epidermis. The composition of the ions in the SE may be responsible for the changes in lipid composition. These behaviors were different from those observed when the ion mixture was applied.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The skin is an important organ involved in the homeostasis of the body. It has a barrier function to prevent foreign substances from entering the body and a function to prevent evaporation of water from the body. The epidermis is divided into four layers based on the differentiation process of keratinocytes. Epidermal keratinocytes produced in the basal layer are directed toward the skin surface and change morphologically and characteristically physiological properties. Finally, all cell organelles are lost and the stratum corneum is formed. The cornified envelope (CE) is an insoluble membrane that surrounds stratum corneum cells, binds to intercellular lipids in the intercellular spaces, and plays an important role in skin barrier and moisturizing functions.
Intercellular lipids in the stratum corneum are composed mainly of ceramide (Cer), cholesterol (Chol), and free fatty acids (FFA). They are present in approximately equimolar amounts, and they form a regular, periodic structure (Swartzendruber et al. 1987; Bouwstra et al. 1991). In particular, Cer is the most important component for the barrier function of the stratum corneum, accounting for approximately 40–50% of the intercellular lipid components (Grubauer et al. 1989; Imokawa et al. 1991). The combination of fatty acids and sphingoids results in the presence of multiple Cer in the epidermis (Masukawa et al. 2008; Kono et al. 2006. Non-hydroxy fatty acids [N], α-hydroxy fatty acids [A], and esterified ω-hydroxy fatty acids [EO] are known as fatty acids, and dihydrosphingosine [DS], sphingosine [S], phytosphingosine [P], and 6-hydroxysphingosine [H] as sphingoids. For example, if [EO] is bound to a fatty acid and [S] to a sphingoid, it is represented as Cer [EOS]. The pathways for Cer formation in the skin can be broadly classified into the de novo pathway (Menaldino et al. 2003), the sphingomyelinase (SMase) pathway (Hannun and Obeid 2008; Wu et al. 2010), and the salvage pathway (Kitatani et al. 2008). The de novo pathway uses serine and palmitoyl CoA as starting materials and Cer is synthesized via condensation and acylation by the rate-limiting enzyme serine palmitoyl transferase (SPT) and ceramide synthase (CerS) (Menaldino et al. 2003). In the SMase pathway, Cer and phosphocholine are generated from the hydrolysis of sphingomyelin by SMase (Rabionet et al. 2014; Taniguchi and Okazaki 2014). Most of the Cer produced in the granular layer is metabolized by glucosylceramide synthase to glucosylceramide and by sphingomyelin synthase to sphingomyelin, some of which is transported to and accumulated in the granular layer of keratinocytes (Holleran et al. 2006). Glucosylceramide and sphingomyelin in lamellar granules are hydrolyzed by SMase and β-glucocerebrosidase present in lamellar granules as required (Hannun and Obeid 2008; Wu et al. 2010). In the salvage pathway, Cer recycles sphingosine (Sph), the smallest unit of sphingolipids, which is produced by CerS (Kitatani et al. 2008). Finally, CDase degrades Cer into FFA and sphingolipid (Kitatani et al. 2008; Kono et al. 2006). Cholesterol in skin is also biosynthesized via HMG-CoA reductase (Feingold 2007). Sterol regulatory element binding proteins (SREBPs) are transcriptional regulators of cholesterol synthesis and bind to SREBP cleavage activating proteins (SCAPs) to form SREBP/SCAP complexes when intracellular cholesterol levels are reduced. These also regulate the cholesterol content in the skin (Brown et al. 2018). FFA metabolism is mediated by acyl CoA and shares some energy-producing pathways with glucose metabolism (Houten et al. 2016). Fatty acids are also components of cell membranes and are included in Cer, phospholipids, and triacylglycerols. Fatty acids of various chain lengths are produced by the degradation of complex lipids or by the biosynthesis of palmitic acid by fatty acid synthase (FASN) (Jakobsson et al. 2006). Palmitic acid undergoes an elongation reaction by the extra-long-chain fatty acid elongation protein (ELOVL) to produce extra-long-chain fatty acid species characteristic of intercellular lipids (Kyselová et al. 2022).
Shotokuseki extract (SE) is a water extract of Shotokuseki. Shotokuseki is a type of shale formed by the uplift of sedimentary layers of plankton and seaweed precipitated on the seafloor. We previously found Na, K, Mg, Ca, Zn, Al, and Fe in the SE (Tsukui et al. 2022a, b). We also reported that the addition of SE to epidermal keratinocytes increased the expression of epidermal differentiation marker genes and increased intracellular calcium concentrations compared with the application of the same concentration of calcium (Tsukui et al. 2022a, b). Furthermore, the contents of amino acids and pyrrolidone carboxylic acids were shown to increase after application of SE to human three-dimensional (3D) cultured epidermis (Tsukui et al. 2022a, b). Ionic components derived from natural products, including hot spring water and deep sea water, have been used to promote health and beauty. In fact, studies have reported that treatment with hot spring water or deep sea water containing various ions prevents inflammation and improves the barrier function in atopic dermatitis and psoriasis (Hodak et al. 2003; Proksch et al. 2005; Chun et al. 2017; Lee et al. 2018). SE is included as a cosmetic ingredient in various cosmetic products, such as lotions and creams. However, not enough is known about the effects of SE on the skin and skin cells. The aim of this study was to determine the effect of SE on the production and metabolism of stratum corneum intercellular lipids in 3D cultured human epidermis.
Method and materials
Materials
SE was provided by IONA International Corporation (Tokyo, Japan), a member of the Zeria Group (Tokyo, Japan). LabCyte EPI-MODEL 6D (3D human epidermis models) and culture medium were purchased from Japan Tissue Engineering (Gamagori, Aichi, Japan). N-Omega-CD3-octadecanoyl-D-erythro-sphingosine (Cer [NS] d3-18:0), N-(30-linoleoyloxy-triacontanoyl)-sphingosine (Cer [EOS] 30:0), N-tetracosanoyl-phytosphingosine (Cer [NP] 24:0), N®alpha-hydroxytetracosanoyl-phytosphingosine (Cer [AP] 24:0), and N-(30-linoleoyloxy-triacontanoyl)-phytosphingosine (Cer [EOP] 30:0) were purchased from Matreya, LLC (Pleasant Gap, PA, USA). N-Palmitoyl-D-erythro-sphingosine (Cer [NS] 16:0), N-stearoyl-D-erythro-sphingosine (Cer [NS] 18:0), N-behenoyl-D-erythro-sphingosine (Cer [NS] 22:0), N-lignoceroyl-D-erythro-sphingosine (Cer [NS] 24:0), N-(2’-(S)-hydroxypalmitoyl)-D-erythro-sphingosine (Cer [AS] 16:0), N-(2′-(S)-hydroxybehenoyl)-D-erythro-sphingosine (Cer [AS] 22:0), N-(2′-(S)-hydroxylignoceroyl)-D-erythro-sphingosine (Cer [AS] 24:0), N-(2′-(R)-hydroxypalmitoyl (d9)) 6-(R)-hydroxysphingosine (Cer [AH] d9-16:0), N-palmitoyl (d9) 6-(R)-hydroxysphingosine (Cer [NH] d9-16:0), N-palmitoyl (d9) dihydrosphingosine (Cer [NDS] d9-C16:0), N-(2′-(R)-hydroxypalmitoyl (d9)) D-erythro-sphinganine (Cer [ADS] d9-C16:0), D-erythro-sphingosine (C17 base) (Sph d17:1), and D-erythro-sphingosine (C20 base) (Sph d20:1) were purchased from Avanti Polar Lipids (Alabaster, AL, USA). Bovine serum albumin and cholesterol were purchased from Sigma-Aldrich (St. Louis, MO, USA). Primers were purchased from Thermo Fisher Scientific (Waltham, MA, USA). RNAiso Plus, PrimeScript™ RT Reagent Kit, and TB Green™ Premix ExTaq were purchased from TaKaRa Bio Inc. (Kusatsu, Shiga, Japan). Methyl ester fatty acid (GLC-68 A) was purchased by Nu-Chek Prep, Inc. (Elysian, MN, USA). o-Phthalaldehyde was purchased from Nacalai Tesque (Kyoto, Japan). All other chemicals and solvents were purchased from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan).
Preparation of SE and ion mixture solution
To prepare SE, purified water was added to Shotokuseki (a type of shale), and after a certain period of time, the Shotokuseki was removed to prepare an extract (solution) equivalent to 2% Shotokuseki. Ion mixture solution (IM) was prepared by a previously described method (Tsukui et al. 2022a, b). The solution contained 0.03 mM Na, 0.01 mM K, 0.14 mM Mg, 0.21 mM Ca, and 0.004 mM Zn, equivalent to the concentrations of each ion in SE. IM contains five ions (Na, K, Ca, Mg and Zn). SE is Shotokuseki (natural products, a type of rock) extract and assumed to contain many ions. The difference in effect between IM and SE is suggested to be the effect of ions other than Na, K, Ca, Mg, and Ca contained in SE. The 5 ions are the major biological ions (Frieden 1985), and many functions in the epidermis have been reported (Sugawara et al. 2012; Sasaki et al. 2017; Nakagawa et al. 2004; Denda et al. 2007; Lee et al. 2010; Emri et al. 2015).
Cell culture
The 3D human epidermis model was pre-incubated for 3 h and then treated with SE or IM solution every day for 2–8 days from the stratum corneum side. The epidermis model was cultured in culture medium provided by the manufacturer. The culture medium was changed every day. The epidermis model was incubated in a humidified atmosphere of 5% CO2 at 37 °C.
RNA isolation and reverse-transcription quantitative PCR
Total RNA was isolated from the 3D cultured human epidermis model using RNAiso Plus, in accordance with the manufacturer’s instructions. RNA concentration and purity were determined by NanoDrop 1000 (Thermo Fisher Scientific). Reverse transcription was performed using PrimeScript™ RT Reagent Kit. RT-qPCR was performed on a Step One Plus Real-Time PCR system (Applied Biosystems) with TB Green™ Premix ExTaq. Amplification was started at 95 °C for 30 s as the first step, followed by 40 successive cycles of PCR involving 95 °C for 5 s and 60 °C for 30 s. Relative expression of target genes was calculated using the ΔΔCt method. The primer sequences are available in Supplementary Table 1. The mRNA level of the untreated epidermis model (control) on day 2 or 4 of incubation was expressed as 1.0 and the others were expressed as its relative values.
Lipid extraction from 3D cultured human epidermis model
Lipid extraction was performed by a modified version of a method reported previously (Otsuka et al. 2021; Murakami et al. 2022). The 3D cultured human epidermis model was immersed in chloroform/methanol (2/1, v/v) and sonicated by an ultrasonic homogenizer (SONIFIER 250 Advanced; BRANSON, Danbury, CT, USA). The sample was then centrifuged at 12,000 × g for 10 min at 4 °C, after which the supernatant was recovered and dried under a flowing nitrogen gas. The lipids were stored at − 30 °C until each analysis. The residues were used for protein quantification.
Protein quantification
Protein was quantified using a modified version of the method described by Roth (Roth 1971). The residue was added to 8 mol/L KOH and incubated at 50 °C for 18 h. After incubation, the sample was neutralized with 5 mol/L HCl, centrifuged, and the supernatant was aliquoted onto a microplate. Each well was supplemented with OPA reagent solution [0.5 mg/mL OPA, 5% ethanol, and 0.25% 2-mercaptoethanol prepared in 100 mM borate buffer (pH10)]. After 5 min, fluorescence intensity was measured at 340/460 nm using a plate reader (SpectraMax iD5; Molecular Devices, Sunnyvale, CA, USA). Quantitation was performed using a calibration curve based on BSA.
Analysis of Cer and Sph by LC-MS/MS
Cers were analyzed using the protocols reported by Ohno et al. (Ohno et al. 2017), with some modifications. Liquid chromatography tandem mass spectrometry (LC-MS/MS) analysis was performed using an HPLC (Shimadzu, Kyoto, Japan) connected to a mass spectrometer (LCMS8045, Shimadzu). The chromatographic separation was achieved using a Shim-Pack GISS C18 (1.9 μm, 2.1 × 100 mm) (Shimadzu GLC, Tokyo, Japan) at 55 °C. The flow rate was 0.4 mL/min in the binary gradient system using mobile phase A [acetonitrile/water (3/2, v/v) containing 10 mM ammonium formate] and mobile phase B [isopropanol/acetonitrile (9/1, v/v) containing 10 mM ammonium formate]. The gradient steps were as follows: 0 min, 40% B; 0–18 min, linear gradient to 100% B; 18.1–25 min, 100% B; 25–25.1 min, linear gradient to 40% B; and 25.1–28 min, 40% B. The following settings were selected: nebulizer gas, 3.0 L/min; drying gas, 8.0 L/min; heating gas, 8.0 L/min; interface temperature, 100 °C; desolvation temperature, 150 °C; and heat block temperature, 250 °C. Each Cer was detected by multiple reaction monitoring (MRM) mode using the appropriate parameter, as described in Supplementary Table 2. Quantification of ceramide was performed by calculating the ratio of the peak area of each ceramide species. [EODS] and [EOH] ceramides are not commercially available, so the [EOS] ceramide standard with a common structure was used for quantification.
Sphs were analyzed using the protocols reported by Lan et al. (Lan et al. 2011), with some modifications. The chromatographic separation was achieved using a Shim-Pack GISS (1.9 μm, 2.1 × 100 mm) at 50 °C. The flow rate was 0.2 mL/min in the isocratic elution system using a mobile phase of methanol/water (95/5, v/v) containing 0.1% formic acid. The following settings were selected: nebulizer gas, 3.0 L/min; drying gas, 8.0 L/min; heating gas, 8.0 L/min; interface temperature, 100 °C; desolvation temperature, 150 °C; and heat block temperature, 250 °C. Each Sph was detected by MRM mode using the appropriate parameters, as described in Supplementary Table 3.
Analysis of Chol by HPLC
The quantification of cholesterol was based on a report by Paulazo and Sodero (Paulazo and Sodero 2020). The lipids were dissolved in the mobile phase (mentioned below) and transferred to HPLC vials. The measurements were performed using HPLC (Alliance 2695; Waters, Milford, MA, USA). A UV detector (Waters 2487; Waters) was set at a wavelength of 210 nm. The chromatographic separation was achieved using an InertSustain® C18 (5 μm, 4.6 × 150 mm; GL Science, Tokyo, Japan) with a mobile phase containing isopropanol/acetonitrile/water (6/3/1, v/v/v), at a flow rate of 1 mL/min at 40 °C.
Analysis of FFA composition by GC-MS
FFA levels in 3D cultured human epidermis were determined by a modified version of the method reported by Hashimoto et al. (Hashimoto et al. 1999). A mixture of lipids, augmented with 0.005% BHT/methanol containing tricosanoic acid (TCA) as an internal standard, and acetyl chloride, was incubated at 98 °C for 60 min with 0.5 mol/L sodium hydroxide/10% sodium chloride and octane. The mixture was shaken for 3 min at room temperature and centrifuged at 700 ×g for 15 min. The octane phase, containing the fatty acid methyl esters, was subjected to gas chromatography-mass spectrometry (GCMS-QP2010 Ultra; Shimadzu, Kyoto, Japan) and analysis by an automatic sampler (AOC-5000; Shimadzu). GC was performed using a capillary column (DB-WAX, 30 m × 0.25 mm × 0.25 μm; Agilent, Santa Clara, CA, USA) at 200 °C. For sample injection, the split method was used with a split ratio of 10.0; for the carrier gas, nitrogen gas was used. GC was set up as follows: 250 °C, 100 °C (held for 5 min), increased to 180 °C at 20 °C/min, increased to 200 °C at 4 °C/min, and to 250 °C at 2°C/min (held for 5 min). The following settings were selected: pressure, 73.0 kPa; total flow, 14.0 mL/min; column flow, 1.0 mL/min; and line velocity, 37.2 cm2/s. MS was undertaken in electron ionization (EI) mode with the following settings: ion source temperature, 200 °C; and interface temperature, 250 °C. Quantification of fatty acid methyl esters (FAME) was performed in selected ion monitoring (SIM) mode of the fragments (saturated, m/z = 74; monosaturated, m/z = 55; disaturated, m/z = 67; polysaturated, m/z = 79). Data analysis was performed with LabSolution software (Shimadzu). Quantification was based on external calibration with FAME mix standard (GLC-68 A).
Statistical analysis
All data are presented as mean ± standard deviation (S.D.). Statistical analysis among three or more groups was performed by Dunnett’s or Tukey’s post hoc test using JMP (version 15.0.0; SAS Institute Inc., Cary, NC, USA).
Result
To assess the effect of SE on epidermal differentiation in the 3D cultured human epidermis model, we measured the gene expression levels of K10 and TGase1 by RT-qPCR. We also measured the mRNA expression of the basal epidermal keratinocyte marker K5. Treatment with SE for 2, 4, 6, and 8 days changed the relative expression of the K10 gene, with changes to 0.5-, 1.5-, 1.9-, and 0.6-fold at 5% SE, respectively. The changes on days 2, 4, and 6 were statistically significant compared with control group at the same timepoint. The expression level of the TGase1 gene increased by 1.6-, 1.7-, 3.8-, and 5.8-fold at 5% SE, respectively. In particular, the increase after 8 days was statistically significant compared with control group at the same timepoint. The expression level of K5, a marker of basal epidermal keratinocytes, increased by 1.1-, 1.2-, 1.2-, and 1.0-fold when the epidermis model was treated with 5% SE, with the increase after 6 days being statistically significant compared with control group at the same timepoint (Fig. 1).
Effects of Shotokuseki extract on keratinocyte differentiation marker gene expression in 3D cultured human epidermis model. Changes in K5, K10, and TGase1 mRNA expression levels after application of Shotokuseki extract (SE) for 2–8 days. mRNA expression levels assessed by reverse-transcription quantitative PCR. Data are expressed as mean ± S.D. of four independent experiments. *p < 0.05, **p < 0.01, and ***p < 0.001 (compared with control group at the same timepoint). Statistical significance was evaluated using Dunnett’s test
We assessed the effect of lipid production and metabolism gene expression in the 3D cultured human epidermis model treatment with SE for 4 or 8 days. FASN is associated with FFA production, and its mRNA level was changed by 1.4- and 2.8-fold upon treatment with 5% SE for 4 and 8 days. However, after 8 days, the expression was significantly lower than in the control group. ELOVL3 is involved in the carbon chain elongation reaction of FFA, and its mRNA level was increased by 4.6- and 18.7-fold at 5% SE, both of which constituted significant increases. SPT1 and CerS3 are involved in Cer production in the epidermis. The mRNA expression of SPT1 genes was increased by 2.1- and 3.1-fold at 5% SE. The expression of CerS3 genes was increased by 2.8- and 0.3-fold at 5% SE. The expression levels of SPT1 and CerS3 were significantly increased after 4 days and significantly decreased after 8 days compared with the levels in the control at the same timepoint. aCDase, GCS, and SMS1 are involved in the metabolism of Cer to sphingolipids. aCDase mRNA level was increased by 4.4- and 25.6-fold at 5% SE. Notably, the increase after 4 days was statistically significant compared with control group at the same timepoint. The mRNA level of SMS1 was increased by 1.1- and 5.3-fold at 5% SE, with the increase after 8 days being statistically significant compared with control group at the same timepoint. In contrast, the mRNA level of the GCS gene was decreased to 1.0- and 0.5-fold at 5% SE. The decrease after 8 days was statistically significant compared with control group at the same timepoint (Fig. 2).
Effects of Shotokuseki extract on intercellular lipid metabolism gene expression in 3D cultured human epidermis model. Changes in FASN, ELOVL3, SPT1, CerS3, aCDase, SMS1, and GCS mRNA expression levels after application of Shotokuseki extract (SE) for 4 or 8 days. mRNA expression levels were assessed by reverse-transcription quantitative PCR. Data are expressed as mean ± S.D. of four independent experiments. *p < 0.05, **p < 0.01, and ***p < 0.001 (compared with control group at the same timepoint). Statistical significance was evaluated using Dunnett’s test
To evaluate the effect of SE on the intercellular lipid content of the stratum corneum, we measured the Cer, FFA, Sph, and Chol contents in the 3D cultured human epidermis model after SE application for 4 or 8 days. Regarding the Cer content of the epidermis model after 4 days of incubation, Cer [EOS] decreased in the 5% SE group compared with that in the control group Fig. 3a. After 8 days, Cer [NDS], [NP], and [EODS] and total Cer content increased in the 5% SE group compared with the levels in the control group Fig. 3a, b. Additionally, the Cer [NP]/[NS] ratio increased upon treatment with 5% SE for 8 days Fig. 3c. The FFA content of the epidermis model after 4 days did not change in the SE group compared with that in the control group. After 8 days, total FFA content increased in the 5% SE group compared with that in the control group Fig. 4. Regarding Sph content of the epidermis model after 4 days, in the 5% SE and IM groups there were increases in Sph total content compared with that in the control group Fig. 4. After 8 days, changes in Sph content were observed, but were not statistically significant. Contents of Cer, FFA, and Sph at each FFA carbon chain length are shown in the supplementary figures. Changes in Chol content were observed after 4 and 8 days, but were not statistically significant Fig. 4.
Effects of Shotokuseki extract on ceramide content in 3D cultured human epidermis model. Changes in ceramide (Cer) levels for each sphingoid base a, total Cer levels b, and Cer [NP]/[NS] ratio c after application of Shotokuseki extract (SE) or ion mixture (IM) for 4 or 8 days. The amount of Cers was quantified by LC-MS/MS. Data are expressed as mean ± S.D. of three independent experiments. *p < 0.05, **p < 0.01, and ***p < 0.001. Statistical significance was evaluated using Tukey’s post hoc multiple comparison test
Effects of Shotokuseki extract on lipid content in 3D cultured human epidermis model. Changes in free fatty acid (FFA), sphingosine (Sph), and cholesterol (Chol) levels after application of Shotokuseki extract (SE) or ion mixture (IM) for 4 or 8 days. Amounts of FFA, Sph, and Chol were quantified by GC-MS, LC-MS/MS, or HPLC. Data are expressed as mean ± S.D. of three independent experiments. *p < 0.05, **p < 0.01, and ***p < 0.001. Statistical significance was evaluated using Tukey’s post hoc multiple comparison test
Discussion
The content of intercellular lipids and natural moisturizing factors in the stratum corneum is considered to be related to the skin barrier function. We previously reported that SE increases natural moisturizing factor content in a 3D cultured human epidermis. However, the effect on intercellular lipids in the stratum corneum was unknown. This study investigated the effect of SE on the production and metabolism of intercellular lipids in the stratum corneum.
First, the effects on epidermal differentiation markers were investigated. The epidermis is a layered structure formed by the differentiation of keratinocytes (Lechler and Fuchs 2005). The final differentiation of keratinocytes results in the formation of the stratum corneum, which is essential for the skin barrier. We previously reported that the application of SE to keratinocytes in monolayer culture upregulated the expression of markers of epidermal differentiation (Tsukui et al. 2022a, b). As reported in this paper, we examined a 3D cultured human epidermis model. The mRNA expression levels of the early differentiation marker K10 and late differentiation marker TGase1 were significantly increased. Although SE calcium concentrations were low, SE also affected cell differentiation in the 3D cultured human epidermis. It is assumed that the stratum corneum is more completely formed when epidermal differentiation is promoted. As such, we expected that the production of molecules involved in the epidermal barrier, such as intercellular lipids in the stratum corneum, would be enhanced, and thus we next examined the effects on the production and metabolism of lipids in the epidermis.
First, we quantified Cer, FFA, Sph, and Chol contents in 3D cultured human epidermis after SE application for 4 or 8 days. After 4 days of SE application, there was no change in each of the lipid contents (Figs. 3 and 4). Meanwhile, Cer [NDS], [NP], and [EODS] contents increased after 8 days of SE application, while FFA content also increased (Fig. 4). Mieremet et al. reported that promotion in Cer synthesis and a decrease in FFA supplemented with palmitic acid (Mieremet et al. 2019). Our results are also consistent with the present results. These are not many studies on epidermal lipid metabolism have measured on lipid composition. We believe it is significant that each lipid was quantified and an overall increase or decrease in lipids. The application of SE did not change the cholesterol content. Uchino et al. reported increased cholesterol sulfate in the skin of patients with dry skin and psoriasis (Uchino et al. 2020; Uchino et al. 2023). Studies have also reported that treatment with hot spring water or deep sea water containing various ions reduced inflammation and improved the barrier function in atopic dermatitis and psoriasis (Hodak et al. 2003; Proksch et al. 2005; Chun et al. 2017; Lee et al. 2018). The normalization of cholesterol content through the application of various ions may also be a factor in improving barrier function. Because the present results are not well supported, future studies should investigate how SE and various ions alter cholesterol content in patients with dry skin and psoriasis. To determine the reason for this variation in lipid content, we examined the expression levels of mRNAs related to lipogenesis and metabolism. There were increases in the mRNA expression levels of SPT1, the rate-limiting enzyme for Cer production; CerS3, which is involved in Cer production; and ELOVL3, which is involved in the chain length elongation reaction of FAA. Furthermore, mRNA expression of aCDase, which is involved in Cer degradation, was increased (Fig. 2). These results suggest that CerS may be responsible for the increased Cer content and decreased Sph and FFA contents in the epidermis after 8 days of SE application. CerS1–6 have been reported as CerS isozymes (Kihara 2016), and in epidermis in particular, CerS3 has also been reported to catalyze amide bond formation using a wide range of fatty acyl CoA from mid-chain fatty acids to long-chain fatty acids (C18–C26) as substrates (Kanoh et al. 2019). Here, CerS3 expression was increased on day 4 and decreased on day 8 by SE application. The increase in Cer content may be related to the increase in CerS3 on day 4. However, on day 8, the expression level may have decreased due to negative feedback in response to Cer synthesis. Yokose et al. reported that the Cer [NP]/[NS] ratio increases with keratinocyte differentiation (Yokose et al. 2020). Our results are also consistent with the present results because SE increases keratinocyte differentiation marker K10 and TGase mRNA expression (Fig. 1), and the Cer [NP]/[NS] ratio is also increased Fig. 3c. CDases are classified according to their optimum pH as acidic CDases (aCDases), neutral CDases, and alkaline CDases (alkCDases). aCDases and alkCDases are expressed in the epidermis (Lin et al. 2012). aCDases are lysosome-localized enzymes that are highly active at pH 4.5 (Houben et al. 2007). As SE is acidic, the increased expression of aCDase mRNA may be due to the pH change caused by the application of SE.
In Cer synthesis in the skin, [S] and [P] base Cer are synthesized from [DS] base Cer via the activities of dihydroceramide desalase (DES 1) and dihydroceramide hydroxylase (DES 2), respectively (Mizutani et al. 2009). In this study, there was a significant increase of Cer [NDS]. These results indicate that the application of SE may change the expression of DES 1 and DES 2 in the epidermis. Furthermore, it has been suggested that the expression of magnesium ion transporter (MgtE) is involved in the synthesis of acylceramides in the epidermis (Honda et al. 2018). MgtE is less selective for Mg2+ and to transport other divalent ions (Sahni and Scharenberg 2013). Because SE contains a variety of divalent ions, these divalent ions may have caused misrecognition and promoted the expression of MgtE, which promoted Cer synthesis. Furthermore, it is often reported that calcium/magnesium ratios recovery skin barrier function, and they might be due to enhanced lipid synthesis (Proksch et al. 2005; Lee et al. 2018; Denda et al. 1999). Therefore, the ion balance of SE may have contributed to changes in lipid composition.
This study investigated the effects of SE containing various ions on the differentiation and lipogenesis of 3D cultured human epidermis. These results indicate that differentiation is also promoted in the layered epidermis model, as we previously found in keratinocytes. Lipid content in epidermis increased for Cer and decreased for FFA and Sph. These findings suggest that the application of SE promoted the synthesis of Cer from FFA and sphingoid. Future studies are needed to determine which of the ions in SE are involved in Cer synthesis. The composition and structure of intercellular lipids in the stratum corneum may be altered by the increased Cer content and decreased FFA and Sph content. However, no change in cholesterol content was observed in the present work. Further studies are needed to understand the actual barrier function and compound permeability after the application of SE.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Bouwstra JA, Gooris GS, van der Spek JA, Bras W (1991) Structural investigations of human stratum corneum by small-angle X-ray scattering. J Invest Dermatol 97:1005–1012
Brown MS, Radhakrishnan A, Goldstein JL (2018) Retrospective on cholesterol homeostasis: the Central Role of Scap. Annu Rev Biochem 87:783–807. https://doi.org/10.1146/annurev-biochem-062917-011852
Chun SY, Lee KS, Nam KS (2017) Refined Deep-Sea Water suppresses inflammatory responses via the MAPK/AP-1 and NF-κB signaling pathway in LPS-Treated RAW 264.7 macrophage cells. Int J Mol Sci 18:2282. https://doi.org/10.3390/ijms18112282
Denda M, Katagiri C, Hirao T, Maruyama N, Takahashi M (1999) Some magnesium salts and a mixture of magnesium and calcium salts accelerate skin barrier recovery. Arch Dermatol 291:560–563
Denda M, Tsutsumi M, Inoue K, Crumrine D, Feingold KR, Elias PM (2007) Potassium channel openers accelerate epidermal barrier recovery. Br J Dermatol 157:888–893. https://doi.org/10.1111/j.1365-2133.2007.08198.x
Emri E, Miko E, Bai P, Boros G, Nagy G, Rózsa D, Juhász T, Hegedűs C, Horkay I, Remenyik É, Emri G (2015) Effects of non-toxic zinc exposure on human epidermal keratinocytes. Metallomics 7:499–507. https://doi.org/10.1039/c4mt00287c
Feingold KR (2007) Thematic review series: skin lipids. The role of epidermal lipids in cutaneous permeability barrier homeostasis. J Lipid Res 48:2531–2546. https://doi.org/10.1194/jlr.R700013-JLR200
Frieden E (1985) New perspectives on the essential Trace Elements. J Chem Edu 62:917. https://doi.org/10.1021/ED062P917
Grubauer G, Feingold KR, Harris RM, Elias PM (1989) Lipid content and lipid type as determinants of the epidermal permeability barrier. J Lipid Res 30:89–96
Hannun YA, Obeid LM (2008) Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol 9:139–150. https://doi.org/10.1038/nrm2329
Hashimoto M, Shinozuka K, Gamoh S, Tanabe Y, Hossain MS, Kwon YM, Hata N, Misawa Y, Kunitomo M, Masumura S (1999) The hypotensive effect of docosahexaenoic acid is associated with the enhanced release of ATP from the caudal artery of aged rats. J Nutr 129:70–76. https://doi.org/10.1093/jn/129.1.70
He X, Okino N, Dhami R, Dagan A, Gatt S, Schulze H, Sandhoff K, Schuchman EH (2003) Purification and characterization of recombinant, human acid ceramidase. Catalytic reactions and interactions with acid sphingomyelinase. J Biol Chem 278:32978–32986. https://doi.org/10.1074/jbc.M301936200
Hodak E, Gottlieb AB, Segal T, Politi Y, Maron L, Sulkes J, David M (2003) Climatotherapy at the Dead Sea is a remittive therapy for psoriasis: combined effects on epidermal and immunologic activation. J Am Acad Dermatol 49:451–457. https://doi.org/10.1067/s0190-9622(03)00916-2
Holleran WM, Takagi Y, Uchida Y (2006) Epidermal sphingolipids: metabolism, function, and roles in skin disorders. FEBS Lett 580:5456–5466. https://doi.org/10.1016/j.febslet.2006.08.039
Honda Y, Kitamura T, Naganuma T, Abe T, Ohno Y, Sassa T, Kihara A (2018) Decreased skin barrier lipid acylceramide and differentiation-dependent gene expression in Ichthyosis Gene Nipal4-Knockout mice. J Invest Dermatol 138:741–749. https://doi.org/10.1016/j.jid.2017.11.008
Houben E, Uchida Y, Nieuwenhuizen WF, De Paepe K, Vanhaecke T, Holleran WM, Rogiers V (2007) Kinetic characteristics of acidic and alkaline ceramidase in human epidermis. Skin Pharmacol Physiol 20:187–194. https://doi.org/10.1159/000101388
Houten SM, Violante S, Ventura FV, Wanders RJ (2016) The Biochemistry and Physiology of mitochondrial fatty acid β-Oxidation and its genetic disorders. Annu Rev Physiol 78:23–44. https://doi.org/10.1146/annurev-physiol-021115-105045
Imokawa G, Kuno H, Kawai M (1991) Stratum corneum lipids serve as a bound-water modulator. J Invest Dermatol 96:845–851. https://doi.org/10.1111/1523-1747.ep12474562
Jakobsson A, Westerberg R, Jacobsson A (2006) Fatty acid elongases in mammals: their regulation and roles in metabolism. Prog Lipid Res 45:237–249. https://doi.org/10.1016/j.plipres.2006.01.004
Kanoh H, Ishitsuka A, Fujine E, Matsuhaba S, Nakamura M, Ito H, Inagaki N, Banno Y, Seishima M (2019) IFN-γ reduces epidermal barrier function by affecting fatty acid composition of ceramide in a mouse atopic dermatitis model. J Immunol Res. https://doi.org/10.1155/2019/3030268
Kihara A (2016) Synthesis and degradation pathways, functions, and pathology of ceramides and epidermal acylceramides. Prog Lipid Res 63:50–69. https://doi.org/10.1016/j.plipres.2016.04.001
Kitatani K, Idkowiak-Baldys J, Hannun YA (2008) The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell Signal 20:1010–1018. https://doi.org/10.1016/j.cellsig.2007.12.006
Kono M, Dreier JL, Ellis JM, Allende ML, Kalkofen DN, Sanders KM, Bielawski J, Bielawska A, Hannun YA, Proia RL (2006) Neutral ceramidase encoded by the Asah2 gene is essential for the intestinal degradation of sphingolipids. J Biol Chem 281:7324–7331. https://doi.org/10.1074/jbc.M508382200
Kyselová L, Vítová M, Řezanka T (2022) Very long chain fatty acids. Prog Lipid Res 87:101180. https://doi.org/10.1016/j.plipres.2022.101180
Lan T, Bi H, Liu W, Xie X, Xu S, Huang H (2011) Simultaneous determination of sphingosine and sphingosine 1-phosphate in biological samples by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 879:520–526. https://doi.org/10.1016/j.jchromb.2011.01.015
Lechler T, Fuchs E (2005) Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature 437:275–280. https://doi.org/10.1038/nature03922
Lee SE, Jun JE, Choi EH, Ahn SK, Lee SH (2010) Stimulation of epidermal calcium gradient loss increases the expression of hyaluronan and CD44 in mouse skin. Clin Exp Dermatol 35:650–657. https://doi.org/10.1111/j.1365-2230.2009.03699.x
Lee KS, Chun SY, Lee MG, Kim S, Jang TJ, Nam KS (2018) The prevention of TNF-α/IFN-γ mixture-induced inflammation in human keratinocyte and atopic dermatitis-like skin lesions in Nc/Nga mice by mineral-balanced deep sea water. Biomed Pharmacother 97:1331–1340. https://doi.org/10.1016/j.biopha.2017.11.056
Lin TK, Crumrine D, Ackerman LD, Santiago JL, Roelandt T, Uchida Y, Hupe M, Fabriàs G, Abad JL, Rice RH, Elias PM (2012) Cellular changes that accompany shedding of human corneocytes. J Invest Dermatol 132:2430–2439. https://doi.org/10.1038/jid.2012.173
Masukawa Y, Narita H, Shimizu E, Kondo N, Sugai Y, Oba T, Homma R, Ishikawa J, Takagi Y, Kitahara T, Takema Y, Kita K (2008) Characterization of overall ceramide species in human stratum corneum. J Lipid Res 49:1466–1476. https://doi.org/10.1194/jlr.M800014-JLR200
Menaldino DS, Bushnev A, Sun A, Liotta DC, Symolon H, Desai K, Dillehay DL, Peng Q, Wang E, Allegood J, Trotman-Pruett S, Sullards MC, Merrill AH Jr (2003) Sphingoid bases and de novo ceramide synthesis: enzymes involved, pharmacology and mechanisms of action. Pharmacol Res 47:373–381. https://doi.org/10.1016/s1043-6618(03)00054-9
Mieremet A, Helder R, Nadaban A, Gooris G, Boiten W, El Ghalbzouri A, Bouwstra JA (2019) Contribution of palmitic acid to epidermal morphogenesis and lipid barrier formation in human skin equivalents. Int J Mol Sci 20:6069. https://doi.org/10.3390/ijms20236069
Mizutani Y, Mitsutake S, Tsuji K, Kihara A, Igarashi Y (2009) Ceramide biosynthesis in keratinocyte and its role in skin function. Biochimie 91:784–790. https://doi.org/10.1016/j.biochi.2009.04.001
Murakami K, Sawada A, Mori T, Sakuyama S, Tokudome Y (2022) Effect of estrogen/progesterone ratio on the differentiation and the barrier function of epidermal keratinocyte and three-dimensional cultured human epidermis. Life Sci 293:120356. https://doi.org/10.1016/j.lfs.2022.120356
Nakagawa N, Sakai S, Matsumoto M, Yamada K, Nagano M, Yuki T, Sumida Y, Uchiwa H (2004) Relationship between NMF (lactate and potassium) content and the physical properties of the stratum corneum in healthy subjects. J Invest Dermatol 122:755–763. https://doi.org/10.1111/j.0022-202X.2004.22317.x
Ohno Y, Kamiyama N, Nakamichi S, Kihara A (2017) PNPLA1 is a transacylase essential for the generation of the skin barrier lipid ω-O-acylceramide. Nat Commun 8:14610. https://doi.org/10.1038/ncomms14610
Otsuka M, Tamane T, Tokudome Y (2021) Effect of Lactic Fermentation products on human epidermal cell differentiation, Ceramide Content, and amino acid production. Skin Pharmacol Physiol 34:103–114. https://doi.org/10.1159/000514119
Paulazo MA, Sodero AO (2020) Analysis of cholesterol in mouse brain by HPLC with UV detection. PLoS ONE 15:e0228170. https://doi.org/10.1371/journal.pone.0228170
Proksch E, Nissen HP, Bremgartner M, Urquhart C (2005) Bathing in a magnesium-rich Dead Sea salt solution improves skin barrier function, enhances skin hydration, and reduces inflammation in atopic dry skin. Int J Dermatol 44:151–157. https://doi.org/10.1111/j.1365-4632.2005.02079.x
Rabionet M, Gorgas K, Sandhoff R (2014) Ceramide synthesis in the epidermis. Biochim Biophys Acta 1841:422–434. https://doi.org/10.1016/j.bbalip.2013.08.011
Roth M (1971) Fluorescence reaction for amino acids. Anal Chem 43:880–882. https://doi.org/10.1021/ac60302a020
Sahni J, Scharenberg AM (2013) The SLC41 family of MgtE-like magnesium transporters. Mol Aspects Med 34:620–628. https://doi.org/10.1016/j.mam.2012.05.012
Sasaki Y, Sathi GA, Yamamoto O (2017) Wound healing effect of bioactive ion released from Mg-smectite. Mater Sci Eng C Mater Biol Appl 77:52–57. https://doi.org/10.1016/j.msec.2017.03.236
Sugawara T, Kikuchi K, Tagami H, Aiba S, Sakai S (2012) Decreased lactate and potassium levels in natural moisturizing factor from the stratum corneum of mild atopic dermatitis patients are involved with the reduced hydration state. J Dermatol Sci 66:154–159. https://doi.org/10.1016/j.jdermsci.2012.02.011
Swartzendruber DC, Wertz PW, Madison KC, Downing DT (1987) Evidence that the corneocyte has a chemically bound lipid envelope. J Invest Dermatol 88:709–713. https://doi.org/10.1111/1523-1747.ep12470383
Taniguchi M, Okazaki T (2014) The role of sphingomyelin and sphingomyelin synthases in cell death, proliferation and migration-from cell and animal models to human disorders. Biochim Biophys Acta 1841:692–703. https://doi.org/10.1016/j.bbalip.2013.12.003
Tsukui K, Kakiuchi T, Suzuki M, Sakurai H, Tokudome Y (2022a) The ion balance of Shotokuseki extract promotes filaggrin fragmentation and increases amino acid production and pyrrolidone carboxylic acid content in three-dimensional cultured human epidermis. Nat Prod Bioprospect 12:37. https://doi.org/10.1007/s13659-022-00353-0
Tsukui K, Kakiuchi T, Sakurai H, Tokudome Y (2022b) Shotokuseki Extract promotes keratinocyte differentiation even at a low calcium concentration. Appl Sci 12:2270. https://doi.org/10.3390/app12052270
Uchino T, Fujino H, Kamiya D, Suzuki T, Miyazaki Y, Asada K, Shirai T, Yagi H, Sano Y, Moriki M, Mizuno H, Todoroki K, Kimura M, Kagawa Y (2020) Association of dry skin with intercellular lipid composition of stratum corneum after erlotinib administration. Cancer Chemother Pharmacol 86:233–243. https://doi.org/10.1007/s00280-020-04095-z
Uchino T, Kamiya D, Yagi H, Fujino-Shimaya H, Hatta I, Fujimori S, Miyazaki Y, Kirishita Y, Sano Y, Mizuno H, Todoroki K, Kagawa Y (2023) Comparative analysis of intercellular lipid organization and composition between psoriatic and healthy stratum corneum. Chem Phys Lipids 254:105305. https://doi.org/10.1016/j.chemphyslip.2023.105305
Wu BX, Clarke CJ, Hannun YA (2010) Mammalian neutral sphingomyelinases: regulation and roles in cell signaling responses. Neuromol Med 12:320–330. https://doi.org/10.1007/s12017-010-8120-z
Yokose U, Ishikawa J, Morokuma Y, Naoe A, Inoue Y, Yasuda Y, Tsujimura H, Fujimura T, Murase T, Hatamochi A (2020) The ceramide [NP]/[NS] ratio in the stratum corneum is a potential marker for skin properties and epidermal differentiation. BMC Dermatol 20:6. https://doi.org/10.1186/s12895-020-00102-1
Acknowledgements
We thank Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript. This work was the result of using research equipment shared in the MEXT Project for Promoting Public Utilization of Advanced Research Infrastructure (Program for Supporting Introduction of the New Sharing System) (Grant Numbers JPMXS0422400022, JPMXS0422400023).
Funding
Yoshihiro Tokudome received research support from Zeria Pharmaceutical Co., Ltd.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by KT, MS, MA and YT. The first draft of the manuscript was written by KT and YT. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
Competing interests: Yoshihiro Tokudome received a research grant from Zeria Pharmaceutical Co. Ltd.Kei Tsukui is PhD student. Masamitsu Suzuki and Miyu Amma are employees of Zeria Pharmaceutical Co. Ltd. Yoshihiro Tokudome are employees of Saga University.
Ethical approval
This study did not require ethics approval.
Consent to participate
This is not applicable.
Consent to publish
This is not applicable. Kei Tsukui is PhD student. Masamitsu Suzuki and Miyu Amma are employees of Zeria Pharmaceutical Co. Ltd. Yoshihiro Tokudome are employees of Saga University.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tsukui, K., Suzuki, M., Amma, M. et al. Ionic composition of Shotokuseki extract alters cell differentiation and lipid metabolism in three-dimensional cultured human epidermis. Cytotechnology 76, 279–290 (2024). https://doi.org/10.1007/s10616-024-00616-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-024-00616-3