Abstract
Cirrhosis is a familiar end-stage of multiple chronic liver diseases. The gene-modified mesenchymal stem cells (MSCs) have become one of the most promising schemes for the treatment of cirrhosis. MSCs exhibit their therapeutic role mainly by secreting hepatocyte growth factor (HGF). The aim of this research was to probe the anti-fibrosis role of exosomes secreted by HGF modified-mouse adipose MSCs (ADMSCs) on activated hepatic stellate cells (HSCs) and to preliminarily explore the possible mechanism. Firstly, mouse ADMSCs were isolated and identified. Quantitative real-time polymerase chain reaction verified the transfection efficiency of ADMSC transfected with HGF lentivirus. Exosomes derived from ADMSC transfecting negative control/HGF (ADMSCNC-Exo/ADMSCHGF-Exo) were extracted by density gradient centrifugation. HSCs were allocated to the control, TGF-β, TGF-β + ADMSC-Exo, TGF-β + ADMSCNC-Exo, and TGF-β + ADMSCHGF-Exo groups. Moreover, all mice were distributed to the control, CCl4 (40% CCl4 in olive oil), CCl4+ADMSC-Exo, CCl4+ADMSCNC-Exo, and CCl4+ADMSCHGF-Exo groups. Exosomes derived from ADMSCs with or without HGF transfection suppressed HSC activation, as evidenced by attenuating cell viability and cell cycle arrest at S phase but inducing apoptosis. Moreover, ADMSC-Exo, ADMSCNC-Exo, and ADMSCHGF-Exo effectively repressed the gene and protein levels of α-SMA, Col-I, Rho A, Cdc42, and Rac1 in TGF-β-treated HSCs, and ADMSCHGF-Exo had the best effect. ADMSCHGF-Exo had a stronger regulatory effect on serum liver index than ADMSCNC-Exo in CCl4-induced mice. In conclusion, ADMSCHGF-Exo alleviated liver fibrosis by weakening the Rho pathway, thus reducing collagen production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cirrhosis is a familiar end-stage of various chronic liver diseases, which is caused by diverse factors, such as viral infection, alcohol, drugs, and chemical toxicities (Goodman 2007; Huang et al. 2010; Kisseleva et al. 2010; Silveira et al. 2010). According to the epidemiological survey, there are 112 million cirrhosis patients worldwide, and the mortality rate can rise to 88% within 5 years (D’Amico et al. 2018, 2020). Cirrhosis is usually manifested as the accumulation of extracellular matrix (ECM) in liver tissues caused by the activation of hepatic stellate cells (HSCs), causing severe fibrosis of liver tissues, and then loss of original liver function (Shiels et al. 2017; Hu et al. 2020b). The most effective treatment for cirrhosis is liver transplantation, but there are problems such as scarcity of donor transplantation, immune rejection, complex surgery, and high cost (Lurie et al. 2015; Roehlen et al. 2020). Although hepatocyte transplantation has emerged as an alternative method to restore liver function and advance liver regeneration, this method is limited by the fact that hepatocytes are prone to deactivation in vitro (Piscaglia et al. 2010). Clearly, more effective and safer therapies are needed for the treatment of cirrhosis.
In recent years, massive experiments indicated that transplantation of mesenchymal stem cells (MSCs) is a safe and effective transitional therapy for end-stage liver disease before liver transplantation, which can advance liver repair and regeneration and notably ameliorate the liver function and quality of life of patients (Hu et al. 2019). Adipose mesenchymal stem cells (ADMSCs) are widely concerned in tissue reconstruction and cell transplantation applications due to their characteristics of easy collection, rich stem cells, strong proliferation, and anti-infection (Seki et al. 2013; Qu et al. 2017). However, the effect of stem cell transplantation alone in the therapy of cirrhosis is not ideal, so new methods to promote the higher performance of stem cells are in urgent need of development (Keating 2012). An increasing number of studies have used MSCs as the carrier of exogenous genes to transfect genes with clear biological functions into MSCs, so as to generate a role together to improve the effect of MSCs in reducing liver fibrosis (Seo et al. 2014; Zhu et al. 2021). Hepatocyte growth factor (HGF) has been proven to manifest a pivotal function in liver regeneration and protection, and the up-regulation of HGF expression can reduce liver fibrosis (Seo et al. 2014). Kim et al. pointed out that MSCs overexpressing HGF can improve liver function and alleviate liver fibrosis compared with MSCs alone (Kim et al. 2014).
Mounting evidence has strongly supported that MSCs improve the internal microenvironment of the liver and accelerate the regeneration of damaged liver tissue by releasing microbubbles (exosomes) (Terai and Tsuchiya 2017; Driscoll and Patel 2019; Zhao et al. 2019). MSC-derived exosomes (MSC-Exo) can reproduce the biological activity of MSCs and may be used as a substitute for whole-cell therapy (Lou et al. 2017). Compared with MSCs, MSC-Exo not only has a smaller nanometer diameter and does not block the pulmonary microvascular system during migration but also has higher safety and lower immunogenicity, and cannot directly form tumors (Börger et al. 2017). Therefore, the application of MSC-Exo has more advantages than MSCs. It has been reported that HGF-modified hADMSC exosomes can repair full-thickness skin defects in diabetic mice (Cao et al. 2022). However, it is unclear whether ADMSCHGF-Exo is also stronger than ADMSC-Exo in the treatment of cirrhosis.
It has been reported that the Rho-GTPase signal is a key pathway leading to liver injury, liver fibrosis, hepatocellular carcinoma, and cirrhosis (Hennenberg et al. 2009; Ren et al. 2021; Wang et al. 2022). Suppression of the Rho-GTPase signal can effectively alleviate liver injury (Choi et al. 2006). Wells et al. clarified that Rho family GTPases were activated during HGF-mediated prostate cancer-cell scattering (Wells et al. 2005). However, whether ADMSCHGF-Exo affects the progression of cirrhosis by regulating Rho-GTPase signaling remains unclear. Therefore, this study was designed to analyze the anti-fibrosis role of exosomes secreted by HGF-modified-mouse ADMSCs on activated HSCs and to preliminarily explore the possible mechanism, thereby providing new ideas and theoretical basis for the clinical treatment of cirrhosis.
Materials and methods
Animals
Five 3-week-old female BALB/c mice and thirty-six 6-8-week-old male BALB/c mice, specific pathogen-free (SPF), were acquired from Shanghai Sippe-Bk Lab Animal Co., Ltd. (China) with the certificate number SCXK (Hu) 2018-0008. All mice were reared at an ambient temperature of 22 ± 2 °C , a humidity of 55 ± 5%, and 12-h of circulating light. All procedures involving mice were authorized via the Ethics Committee of Zhejiang Eyong Pharmaceutical Research and Development Center (SYXK (Zhe) 2021-0033).
Isolation and identification of ADMSCs
Each mouse underwent euthanize by inhaling excess CO2. The subcutaneous adipose tissue of the medial inguinal region of the mouse was quickly separated and then digested in type I collagen (C1696, Bioswamp, China) at 37 °C for 50 min. After centrifugation, the pelleted cells were re-suspended in DMEM/F12 medium (ZQ-600, Zqxzbio, China) augmented with 10% exosome-depleted fetal bovine serum (FBS, 11,011 − 8615, Tianhang, China) and 1% penicillin-streptomycin liquid (P1400, Solarbio, China). After centrifugation again, the bottom cells were dripped into phosphate buffer saline (PBS, SH30256.01, Hyclone, USA) followed by centrifugation. The centrifuged cells (2 × 105/mL) were grown in DMEM/F12 medium with 10% exosome-depleted FBS under a cell incubator (BB150, Thermo Fisher Scientific, USA). The medium was changed initially after 24 h and then every 3 days. The morphology of ADMSCs with different generations was observed under an optical microscope (AE2000, Motic, China).
Phenotypic analysis of the third passage ADMSCs was carried out through the flow cytometer. The above cells were suspended in PBS and cell density was diluted to 1 × 106/100 µL. Thereafter, anti-CD45 antibody (560,695), anti-CD44 antibody (553,134), anti-CD34 antibody (551,387), and anti-CD105 antibody (562,759) were added (4 °C, 0.5 h, dark room). After washing, the above cells were assessed by applying a flow cytometer (C6, BD, USA).
Construction and transduction of lentiviral vector
Genechem (China) supplied a blank lentiviral vector encoding only green fluorescent protein (GFP) and a HGF-overexpressing lentiviral vector encoding GFP and HGF. The packaging and concentration of lentivirus was completed by Genscript Co., Ltd. (China). ADMSCs (1 × 105 cells/well) were appended at 24-pore plates 24 h before transfection. The next day, the cell culture medium was replaced with fresh medium containing 6 µg/mL polybrene (H8761, Solarbio, China) and viral suspension, which was then reacted at 37℃ for 24 h. Thereafter, the medium containing the virus was replaced with a fresh medium and incubated for 48 h. For efficiency of GFP expression, a fluorescence microscopy (Ts2-FC, Nikon, Japan) was applied. The stable transfected cells were screened using a fresh complete medium containing purinomycin.
Exosome preparation and identification
For the acquisition of exosomes, exosome isolation kits (41201ES25, Yeasen, China) was selected. The culture medium (including exosome-depleted FBS) of ADMSCs, ADMSCNC, and ADMSCHGF was harvested and centrifuged. The obtained supernatant (10 mL) was injected into a new centrifuge tube with exosome separation reagents (2.5 mL). After being reacted at 4 °C for 2 h, the mixture underwent centrifugation (10,000 g, 60 min). The collected precipitate was re-suspended in 100 µL PBS and centrifuged again at 12,000 g for 2 min. For purification of exosomes, the supernatant was passed through a filter membrane, about 13 µg exosomes were obtained. The morphology of exosomes was identified under a transmission electron microscope (TEM, H-600, Hitachi, Tokyo, Japan). The size distribution of ADMSC-Exo was quantified by nanoparticle tracking analysis (NTA). Furthermore, the representative markers (CD63, CD81, and CD9) of exosomes were identified through western blot.
Cell culture and experimental design
WHELAB (Shanghai, China) supplied mouse HSCs (C2211). HSCs were grown in DMEM medium (SH30243.01, Hyclone, USA) with 10% FBS, which were then put into the cell incubator (37 °C, 5% CO2).
To analyze the anti-fibrotic effect of exosomes secreted by ADMSCs overexpressing HGF on activated HSCs, HSCs were allocated to the control group (HSCs were cultured normally), TGF-β group (HSCs were subjected to 10 ng/mL TGF-β for 48 h) (Wang et al. 2018), TGF-β + ADMSC-Exo group, TGF-β + ADMSCNC-Exo group, and TGF-β + ADMSCHGF-Exo group. After 2 days of induction with TGF-β, the latter three groups of HSCs were treated with ADMSC-Exo, ADMSCNC-Exo, and ADMSCHGF-Exo at 400 µg/µL and 50 µL, respectively, for 48 h.
Cell vitality assay
MTT kit (E606334) supplied by BBI Life Science (UK) was selected to analyze cell viability of HSCs. HSCs were suspended and adjusted to 1 × 104 cells/mL, which were then loaded in the 96-pore plates for one day. Then, the cells were treated according to grouping. After 48 h, 10 µL MTT solution was dripped into each well. After being reacted for 4 h in the cell incubator, absorbance of these cells at 450 nm was tested by a CMaxPlus microplate reader (Molecular Devices, USA) to calculate the cell viability.
Apoptosis assay
Annexin V-FITC/PI Apoptosis Detection Kit (CW2574S, CWBIO, China) was taken to analyze the apoptosis of HSCs. The HSCs were cultured in 6-well plates at the density of 1.5 × 106 per well. After being treated as grouping, the cells were washed and the cell concentration was adjusted to 1 × 106/mL. After centrifugation, the precipitated HSCs with 100 µL binding buffer were stained with 5 µL Annexin V-FITC at 37 °C away from light for 10 min. Then, 5 µL PI was added (37 °C, 5 min). After adding a binding buffer, apoptosis of HSCs was monitored under the flow cytometer.
Cell cycle assay
A cell cycle kit (CCS012, Multi Sciences, China) was applied according to its manual. Firstly, the treated HSCs were washed, digested, and centrifuged (1000 rpm, 3 min). Subsequently, 1 mL pre-cooled PBS was taken to re-suspend the pelleted HSCs. The re-suspended HSCs underwent centrifugation. HSCs of each well were fixed with 1 mL pre-cooled 70% ethanol at 4 °C for 2 h prior to washing. After that, HSCs were subjected to 500 µL staining solution in the darkroom at 37 °C for 0.5 h. In the end, cellular distribution was measured by the flow cytometer.
Immunofluorescence
HSCs (3 × 104 per well) were inoculated into the 6-well plate with cover glass for one day. Next, HSCs received different treatments. The fixation of HSCs was conducted with 4% paraformaldehyde (P0099, Beyotime, China). Thereafter, 0.5% Triton X-100 (10,789,704,001, Roche, USA) was exploited to permeate the cell membrane. Then, 3% bovine serum albumin (BSA, 4240GR100, BioFRoxx, Germany) was utilized to seal HSCs for 30 min. A further all-night reaction was conducted with anti-α-SMA antibodies (1:200, Ab5831, abcam, UK) at 4 °C. After being reacted with goat anti-mouse IgG H&L (Alexa Fluor® 594, 1:500, ab150116, Abcam, UK), they were then subjected to DAPI (ab104139, Abcam, UK). Lastly, the cover glass was observed with a fluorescence microscope (Ts2-FC, Nikon, Japan) after adding mounting media.
Quantitative real-time polymerase chain reaction (QRT-PCR)
The total RNA from ADMSCs, ADMSC-Exo and HSCs were isolated by Trizol reagent (B511311, Sangon, China). After being converted into cDNA with the use of cDNA First Strand Synthesis Kit (CW2569, CWBIO, China), cDNA was amplified with the assistance of SYBR Green qPCR kit (CW2601, CWBIO, China) under a PCR system (LightCycler® 96, Roche, USA). β-actin was set as the normalizer and 2−ΔΔCt was performed for data evaluation. Primers were presented in Table 1.
Western blot
Following extraction of protein from ADMSCs and HSCs using RIPA lysis buffer (P0013D, Beyotime, China), the protein was quantified by BCA kit (pc0020, Solarbio, China). After denaturation, the denatured protein underwent electrophoresis, which was then electroblotted onto Polyvinylidene Fluoride (PVDF) membrane. Thereafter, 5% BSA was applied to shake the membrane at 37℃ for 2 h. Afterward, the membrane was probed with appropriate primary antibodies (4℃, all night). After another 60 min of treatment with Anti-Rabbit IgG H&L (HRP) antibody (1:5000, ab7090, Abcam, UK) or Rabbit Anti-Mouse IgG H&L (HRP) antibody (1:5000, ab6728, Abcam, UK), the immunoreactivity was generated using ECL reagent (GK10008, GlpBio, USA). The images were obtained by a chemiluminescence apparatus (ChemiScope 6000, Clinex, China). The primary antibodies of CD63 (1:1000, ab217345), CD81 (1:8000, ab109201), CD9 (1:1000, ab307085), α-SMA (1:1000), Collagen I (Col-I, 1:1000, ab260043), RhoA (1:5000, ab187027), Cdc42 (1:10000, ab187643), Rac1 (1:1000, ab155938), P27 (1:1000, ab193379), and β-actin (1:5000, ab8227) were bought from Abcam (UK).
Establishment of liver injury model in mice
Sixty mice were randomized to the control, CCl4, CCl4+ADMSC-Exo, CCl4+ADMSCNC-Exo, and CCl4+ADMSCHGF-Exo groups. For induction of cirrhosis, CCl4 dissolved in olive oil was applied in this study. Specifically, except for the control group, the mice in other four groups were injected subcutaneously with 40% CCl4-olive oil solution (5 mL/kg body weight) on the first day. Thereafter, the latter four groups of mice were injected subcutaneously with 40% CCl4-olive oil solution (3 mL/kg body weight) twice a week for 6 weeks. In addition, the latter four groups were fed with high-fat and low-protein feed and drunk with 0.5% alcohol. Mice in the control group were given a subcutaneous injection of the same volume of olive oil and fed with normal feed and normal drinking water. After successful modeling, the exosomes of ADMSCs, ADMSCNC, and ADMSCHGF (400 µg/µL, 200 µL) were injected into the tail vein of mice once a week for 4 weeks. At the same time, the mice in the control and CCl4 groups received PBS with the same amount. After 2 weeks and 4 weeks of administration (8 weeks and 10 weeks of modeling), the blood samples from the orbital vein of mice were harvested and centrifuged to obtain the serum.
Enzyme-linked immunosorbent assay (ELISA) assay
In this part, the mouse alanine aminotransferase (ALT) kit (ml063179), mouse aspartate aminotransferase (AST) kit (ml058577), and mouse serum albumin (ALB) kit (ml057991) were introduced from MEIMAIN (China). Total bilirubin (TBIL) kit (C019-1-1) was introduced from Jiancheng (China). The concentrations of ALT, AST, ALB, and TBIL of the serum in mice were determined as instructions.
Statistics
Data analysis obtained from triplicate independent experiments was conducted by SPSS software (16.0, IBM, USA). The measurement data were presented as mean ± standard deviation. One-way ANOVA with Turkey test was utilized for multiple comparisons. For those with unequal variances, the Kruskal-Wallis H test was applied. A statistically significant difference can be unveiled if p < 0.05.
Results
Isolation and identification of ADMSCs
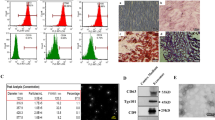
As displayed in Fig. 1A, under an optical microscope, ADMSCs were observed to grow rapidly, most of which were fibrous or fusiform. After 24 h of culture, some ADMSCs began to adhere to the wall; after 72 h of culture, a large number of ADMSCs showed adherent growth. Next, the flow cytometer analysis was adopted to detect the surface markers of ADMSCs. The data presented in Fig. 1B found that CD44 and CD105 were positively expressed, while CD34 and CD45 were negatively expressed in ADMSCs, indicating the successful isolation of ADMSCs.
HGF overexpression transfection didn’t affect the properties of mesenchymal stem cells and Exo
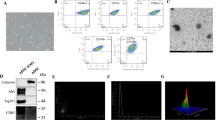
There was no notable difference in the level of mouse HGF between ADMSCs and ADMSCNC but the level of mouse HGF in the ADMSCHGF group was higher than that in the ADMSCNC group, suggesting the transfection was performed successfully (Fig. 2A, p < 0.01). Then, the flow cytometer was applied to detect the surface markers of ADMSCs after transfection. The proportion of CD45 and CD34 positive cells in the ADMSCHGF group and ADMSCNC group was less than 1%, while the proportion of CD44 and CD105 positive cells was more than 90% (Fig. 2B).
Next, we isolated exosomes from ADMSCs, ADMSCNC, and ADMSCHGF. The exosomes were observed as oval vesicular structures by TEM (Fig. 2C). NTA showed that the particle size of exosomes was concentrated in the range of 50–100 nm (Fig. 2D). In addition, as illustrated in Fig. 2E and F, when compared with the ADMSC-Exo group, the expressions of HGF mRNA and protein in the ADMSCNC-Exo group did not change significantly. However, relative to the the ADMSCNC-Exo group, the expressions of HGF mRNA and protein the in ADMSCHGF-Exo group were significantly upregulated (p < 0.01). The further western blot assay exhibited that the expressions of exosome-related markers CD9, CD63, and CD81 in the ADMSC-Exo, ADMSCNC-Exo, and ADMSCHGF-Exo groups were not significantly changed (Fig. 2E).
(A) HGF overexpression in ADMSCs was verified by qRT-PCR after transfection. (B) Flow cytometer analysis for the expressions of CD45, CD44, CD105, and CD34 in ADMSCs. (C) ADMSC-Exo morphology was observed using TEM. Scale bar, 100 nm. (D) The size distribution of ADMSC-Exo was quantified by NTA. (E) The expression of HGF mRNA in ADMSC-Exo was detected by qRT-PCR. (F) The expression of HGF protein in ADMSC-Exo was detected by western blot. (G) The expression levels of CD63, CD81 as well as CD9 proteins in ADMSC-Exo were detected using western blot. @p < 0.05, @@p < 0.01 vs. ADMSCs.#p < 0.05, ##p < 0.01 vs. ADMSCNC-Exo. The results were presented as the mean ± standard deviation, n = 3. Note: Exo, exosome; HGF, hepatocyte growth factor; TEM, transmission electron microscopy; NTA, nanoparticle tracking analysis.
HGF overexpression in ADMSC-Exo suppressed the activation of TGF-β-mediated HSCs
The MTT assay indicated that TGF-β induction prominently elevated cell viability of HSCs and the effect was partially offset with the addition of ADMSC-Exo/ADMSCNC-Exo/ADMSCHGF-Exo (Fig. 3A, p < 0.05). The flow cytometer assay proposed that apoptosis of HSCs was largely repressed after TGF-β treatment and the repressed capability was weakened by ADMSC-Exo/ADMSCNC-Exo/ADMSCHGF-Exo (Fig. 3B, p < 0.05). Importantly, the group that received ADMSCHGF-Exo facilitated apoptosis of TGF-β-induced HSCs compared to the cells that received ADMSCNC-Exo (Fig. 3B, p < 0.05). Additionally, the cell cycle assay indicated that induction with TGF-β statistically raised the cells in the S stage but dismissed the percentage of cells in the G0/G1 stage and the G2/M stage in HSCs (Fig. 3C, p < 0.01). After ADMSC-Exo/ADMSCNC-Exo/ADMSCHGF-Exo mediation, fewer cells were noted to be arrested at the S phase, while more cells were arrested at the G0/G1 phase and G2/M phase (Fig. 3C, p < 0.05).
ADMSC-derived exosomal HGF suppressed the activation of TGF-β-mediated HSCs. A MTT assay of cell viability in HSCs after incubation with ADMSC-Exo. Flow cytometry assay for cell apoptosis B and cell cycle C in HSCs. & p < 0.05, && p < 0.01 vs. Control; +p < 0.05, ++p < 0.01 vs. TGF-β; *p < 0.05 vs. TGF-β + ADMSC NC -Exo. The results were presented as the mean ± standard deviation, n = 3. Note: HSCs, hepatic stellate cells; TGF-β, transforming growth factor-β
ADMSCHGF-Exo suppressed the α-SMA expression in TGF-β-mediated HSCs
By conducting an immunofluorescence assay, we found that relative to the control group, the fluorescence intensity of α-SMA protein in the TGF-β, TGF-β + ADMSC-Exo, ATGF-β + DMSCNC-Exo, and TGF-β + ADMSCHGF-Exo groups was higher (Fig. 4). Interestingly, ADMSC-Exo/ADMSCNC-Exo/ADMSCHGF-Exo intervention weakened the fluorescence intensity of α-SMA protein in HSCs induced by TGF-β (Fig. 4).
The expression of α-SMA in HSCs was detected by immunofluorescence with a magnification of 200× (scale bar = 50 μm), n = 3. Note: α-SMA, α-smooth muscle actin.
ADMSCHGF-Exo restrained collagen fiber production in TGF-β-treated HSCs by rho pathway
As displayed in Fig. 5A and B, TGF-β treatment was found to enhance the gene and protein levels of α-SMA, Col-I, Rho A, Cdc42, and Rac1 but lessen the gene and protein level of P27 in HSCs (p < 0.01). Compared to the TGF-β group, the gene and protein levels of α-SMA, Col-I, Rho A, Cdc42, and Rac1 were lower but the gene and protein expression of P27 was higher in the TGF-β + ADMSC-Exo, TGF-β + ADMSCNC-Exo, and TGF-β + ADMSCHGF-Exo groups (Fig. 5A and B, p < 0.05). More importantly, the regulatory effect of ADMSCHGF-Exo treatment on the above-mentioned proteins was stronger than that of ADMSCNC-Exo (Fig. 5A and B, p < 0.05).
ADMSC-derived exosomal HGF restrained collagen fiber production in TGF-β-treated HSCs by Rho pathway QRT-PCR A and western blot B analysis for the mRNA and protein levels of α-SMA, Col-I, Rho A, Cdc42, Rac1 as well as P27 in HSCs. &p < 0.05, &&p < 0.01 vs. Control; +p < 0.05, ++p < 0.01 vs. TGF-β; *p < 0.05, **p < 0.01 vs. TGF-β + ADMSCNC-Exo. The results were presented as the mean ± standard deviation, n = 3.
ADMSCHGF-Exo improved serum liver indices in CCl4-induced mice
After 2 weeks of treatment, relative to the control group, the contents of ALT, AST, and TBIL in the CCl4 group were all increased but the content of ALB was decreased (Fig. 6, p < 0.05). After 4 weeks of treatment, except CCl4 + ADMSCHGF-Exo group, the contents of ALT, AST, and TBIL in the other groups were higher but the content of ALB was lower relative to the control group (Fig. 6, p < 0.05). Also, ADMSC-Exo/ADMSCNC-Exo/ADMSCHGF-Exo induction effectively decreased the contents of ALT, AST, and TBIL but enhanced the content of ALB in CCl4-induced mice (Fig. 6, p < 0.05). We also found that ADMSCHGF-Exo treatment had a stronger regulatory effect on serum liver index than ADMSCNC-Exo treatment (Fig. 6, p < 0.05).
After 2 and 4 weeks of ADMSC treatment, the contents of serum ALT, AST, ALB as well as TBIL in CCl4-induced mice were detected by ELISA. &p < 0.05, &&p < 0.01 vs. Control; +p < 0.05, ++p < 0.01 vs. CCl4; *p < 0.05, **p < 0.01 vs. CCl4 + ADMSCNC-Exo. The results were presented as the mean ± standard deviation, n = 6. Note: ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALB, albumin; TBIL, total bilirubin.
Discussion
The previous study noted that human MSCs have low immunogenicity, can promote the repair of liver injury and liver regeneration, apparently mitigate liver fibrosis, and ameliorate liver function and quality of life (Cao et al. 2020). The specific markers CD45, CD34, CD44, and CD105 on the surface of mouse ADMSCs were identified by flow cytometer. Consistent with what Deng et al. reported (Deng et al. 2014), ADMSCs were negative for the level of hematopoietic markers CD45 and CD34, but positive for CD105 and CD44, which verified that we successfully isolated mouse ADMSCs. MSCs are regarded as the best human cell type that can expand the production of exosomes (Hu et al. 2019). In terms of surface markers, MSC-Exo expresses not only common marker proteins such as CD81, CD63, and CD9 but also specific marker proteins derived from MSCs such as CD29, CD44, and CD73, etc., these markers can be used as reference indicators for the isolation and identification of exosomes (Yu et al. 2014). In this study, TEM, NTA, and western blot techniques were used to identify that the exosomes obtained from ADMSCs, ADMSCNC, and ADMSCHGF, and found these exosomes are extracellular vesicles with biological activity.
MSCs are reported to advance liver regeneration by modulation of immune cells (Hu et al. 2020). More and more research results indicated that MSC-Exo exhibits a more critical role in the repair of liver tissue damage than MSCs. For example, Rong et al. found that hBMMSC-Exo effectively alleviated liver fibrosis, including inhibition of activated HSCs, reduction of collagen deposition, enhancement of liver function, inhibition of inflammation, increase of liver cell regeneration, and repression of Wnt/β-catenin signaling by constructing CCl4-treated rat liver fibrosis model and injecting hBMMSC-Exo (Rong et al. 2019). Some scholars also confirmed that MSCs and MSC-Exo lessen serum transaminase level and liver necrosis, and have the potential to reduce liver injury and improve organ regeneration after liver ischemia-reperfusion injury (Anger et al. 2019). According to the report, MSCs can reduce liver fibrosis by inhibiting the proliferation of activated HSCs and promoting their apoptosis (Ma et al. 2022). Cell proliferation depends on the normal operation of the cell cycle, and MSCs can impede the activation and proliferation of HSCs by changing the cell cycle, thus alleviating liver fibrosis (Wang et al. 2017). Qin et al. found that BMSCs might modulate HSCs and cyclin D1 by the RhoA-P27 signaling, which led to the cell cycle G1/S phase transition, restrained the proliferation, and induced apoptosis of HSCs (Qin et al. 2012). This research was the first to check the cell proliferation and cell cycle of exosomes secreted by HGF-modified mouse ADMSCs on activated HSCs. After HSCs were treated with ADMSC-Exo/ADMSCNC-Exo/ADMSCHGF-Exo, fewer cells were noted to be arrested at the S phase, cell viability was obviously repressed, and cell cycle inhibitor P27 expression was evidently enhanced. Importantly, HGF-modified ADMSC-Exo was superior to ADMSC-Exo in regulating the proliferation and cell cycle of activated HSCs.
In order to explore the mechanism of HGF-modified ADMSC-Exo against hepatic fibrosis, the expressions of fibrosis proteins α-SMA and Col-I in HSCs were detected after treatment. In in vitro experiments, HGF-transfected hUCMSCs prominently weakened the expressions of Col-I, Col-III, and Smad2 in HSCs-T6 (Yin et al. 2021). In TGF-β-induced HSCs, it was observed that the activation ability of HSCs was strongly weakened after BMSCs intervention, and the expressions of α-SMA and Col-I were largely decreased (Qiao et al. 2018). Another study found that Rho GTPase signaling (RhoA, Rac1, and Cdc42) displays an important role in controlling the high expression of α-SMA in TGF-β-mediated MSCs (Ge et al. 2018). These researchers further demonstrated that RhoA, Rac1, and Cdc42 modulate α-SMA expression in MSCs through a RhoA/ROCK/pMLC2/F-actin/MRTFa pathway (Ge et al. 2018). Consistent with the report of Li et al. (Li et al. 2012), TGF-β was found to enhance the gene and protein levels of Rho A, Cdc42, and Rac1 in HSCs. Importantly, ADMSC-Exo, ADMSCNC-Exo, and ADMSCHGF-Exo effectively repressed the gene and protein levels of α-SMA, Col-I, Rho A, Cdc42, and Rac1 in TGF-β-treated HSCs, and ADMSC-Exo with HGF transfection had the better effect, revealing that ADMSCHGF-Exo repressed the activation of HSCs by restraining Rho pathway.
In order to further confirm the protective effect of HGF-modified ADMSC-Exo on liver injury, this study also conducted an in vivo experiment. CCl4-mediated liver damage in mice is widely applied as an animal model for inducing liver fibrosis because it is highly similar to the morphological and functional changes observed in human cirrhosis (Cao et al. 2017). Therefore, we used this model to study the hepato-protective effect of ADMSCHGF-Exo on mice with liver injury. In this study, serum ALT, AST, ALB, and TBIL levels were measured to evaluate CCl4-induced liver injury in rats. The serum liver enzymes (ALT, AST, ALB, and TBIL) are important indicators of liver synthesis function (He et al. 2021). Zhang et al. found that the concentrations of serum ALT, AST, and TBIL decreased apparently after hUC-MSCs intervention in a rat model of acute liver failure (ALF) (Zhang et al. 2018). This study indicated that exosomes secreted by ADMSCs overexpressing HGF effectively decreased the contents of ALT, AST, and TBIL but enhanced the content of ALB in CCl4-induced mice. The improvement in liver function proposed that ADMSCHGF-Exo accelerated liver recovery from the progression of cirrhosis.
To sum up, ADMSCHGF-Exo alleviated the activation of HSCs by weakening the expression of RhoA, Cdc42, and Rac1, the key molecules in the Rho pathway, thus reducing the production of collagen. The in vivo experiments verified that ADMSCHGF-Exo accelerated the recovery of liver from the progression of cirrhosis. In this study, we conducted a preliminary discussion from the cell model level and measured the serological indicators of liver function at the animal model level. In the future, we will further study the relevant mechanisms from the animal model level to provide a more theoretical basis for clinical transformation to finally solve the difficulties in the treatment of cirrhosis.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Anger F, Camara M, Ellinger E, Germer CT, Schlegel N, Otto C, Klein I (2019) Human mesenchymal stromal cell-derived extracellular vesicles improve liver regeneration after ischemia reperfusion injury in mice. Stem Cells Dev 28:1451–1462
Börger V, Bremer M, Ferrer-Tur R, Gockeln L, Stambouli O, Becic A, Giebel B (2017) Mesenchymal stem/stromal cell-derived extracellular vesicles and their potential as novel immunomodulatory therapeutic agents. Int J Mol Sci 18:1450
Cao Y, Ji C, Lu L (2020) Mesenchymal stem cell therapy for liver fibrosis/cirrhosis. Ann Transl Med 8:562
Cao XF, Jin SZ, Sun L, Zhan YB, Lin F, Li Y, Zhou YL, Wang XM, Gao L, Zhang B (2017) Therapeutic effects of hepatocyte growth factor-overexpressing dental pulp stem cells on liver cirrhosis in a rat model. Sci Rep 7:15812
Cao T, Xiao D, Ji P, Zhang Z, Cai WX, Han C, Li W, Tao K (2022) Effects of exosomes from hepatocyte growth factor-modified human adipose mesenchymal stem cells on full-thickness skin defect in diabetic mice. Zhonghua Shao Shang Za Zhi 38:1004–1013
Choi SS, Sicklick JK, Ma Q, Yang L, Huang J, Qi Y, Chen W, Li YX, Goldschmidt-Clermont PJ, Diehl AM (2006) Sustained activation of Rac1 in hepatic stellate cells promotes liver injury and fibrosis in mice. Hepatology 44:1267–1277
D’Amico G, Morabito A, D’Amico M, Pasta L, Malizia G, Rebora P, Valsecchi MG (2018) Clinical states of cirrhosis and competing risks. J Hepatol 68:563–576
Deng L, Liu G, Wu X, Wang Y, Tong M, Liu B, Wang K, Peng Y, Kong X (2014) Adipose derived mesenchymal stem cells efficiently rescue carbon tetrachloride-induced acute liver failure in mouse. ScientificWorldJournal 2014:103643
Driscoll J, Patel T (2019) The mesenchymal stem cell secretome as an acellular regenerative therapy for liver disease. J Gastroenterol 54:763–773
Ge J, Burnier L, Adamopoulou M, Kwa MQ, Schaks M, Rottner K, Brakebusch C (2018) RhoA, Rac1, and Cdc42 differentially regulate αSMA and collagen I expression in mesenchymal stem cells. J Biol Chem 293:9358–9369
Goodman ZD (2007) Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J Hepatol 47:598–607
He Y, Guo X, Lan T, Xia J, Wang J, Li B, Peng C, Chen Y, Hu X, Meng Z (2021) Human umbilical cord-derived mesenchymal stem cells improve the function of liver in rats with acute-on-chronic liver failure via downregulating Notch and Stat1/Stat3 signaling. Stem Cell Res Ther 12:396
Hennenberg M, Trebicka J, Stark C, Kohistani AZ, Heller J, Sauerbruch T (2009) Sorafenib targets dysregulated rho kinase expression and portal Hypertension in rats with secondary biliary cirrhosis. Br J Pharmacol 157:258–270
Hu C, Wu Z, Li L (2020) Mesenchymal stromal cells promote liver regeneration through regulation of immune cells. Int J Biol Sci 16:893–903
Hu C, Zhao L, Duan J, Li L (2019) Strategies to improve the efficiency of mesenchymal stem cell transplantation for reversal of liver fibrosis. J Cell Mol Med 23:1657–1670
Hu C, Zhao L, Zhang L, Bao Q, Li L (2020) Mesenchymal stem cell-based cell-free strategies: safe and effective treatments for liver injury. Stem Cell Res Ther 11:377
Huang YW, Hu JT, Yang SS (2010) Complications of alcoholic liver cirrhosis: active assessment by endoscopy and sonography. Hepatology 52:1864–1865
Keating A (2012) Mesenchymal stromal cells: new directions. Cell Stem Cell 10:709–716
Kim MD, Kim SS, Cha HY, Jang SH, Chang DY, Kim W, Suh-Kim H, Lee JH (2014) Therapeutic effect of hepatocyte growth factor-secreting mesenchymal stem cells in a rat model of liver fibrosis. Exp Mol Med 46:e110
Kisseleva T, Gigante E, Brenner DA (2010) Recent advances in liver stem cell therapy. Curr Opin Gastroenterol 26:395–402
Li L, Wang JY, Yang CQ, Jiang W (2012) Effect of RhoA on transforming growth factor β1-induced rat hepatic stellate cell migration. Liver Int 32:1093–1102
Lou G, Chen Z, Zheng M, Liu Y (2017) Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases. Exp Mol Med 49:e346
Lurie Y, Webb M, Cytter-Kuint R, Shteingart S, Lederkremer GZ (2015) Non-invasive diagnosis of liver fibrosis and Cirrhosis. World J Gastroenterol 21:11567–11583
Ma L, Wei J, Zeng Y, Liu J, Xiao E, Kang Y, Kang Y (2022) Mesenchymal stem cell-originated exosomal circDIDO1 suppresses hepatic stellate cell activation by miR-141-3p/PTEN/AKT pathway in human liver fibrosis. Drug Deliv 29:440–453
Piscaglia AC, Campanale M, Gasbarrini A, Gasbarrini G (2010) Stem cell-based therapies for liver diseases: state of the art and new perspectives. Stem Cells Int 2010:259461
Qiao H, Zhou Y, Qin X, Cheng J, He Y, Jiang Y (2018) NADPH oxidase signaling pathway mediates mesenchymal stem cell-induced inhibition of hepatic stellate cell activation. Stem Cells Int 2018:1239143
Qin S, Jiang H, Su S, Wang D, Liang Z, Zhang J, Yang W (2012) Inhibition of hepatic stellate cell proliferation by bone marrow mesenchymal stem cells via regulation of the cell cycle in rat. Exp Ther Med 4:375–380
Qu Y, Zhang Q, Cai X, Li F, Ma Z, Xu M, Lu L (2017) Exosomes derived from mir-181-5p-modified adipose-derived mesenchymal stem cells prevent liver fibrosis via autophagy activation. J Cell Mol Med 21:2491–2502
Ren X, Meng T, Ren X, Li X, Lu L (2021) Fasudil alleviates acetaminophen-induced liver injury via targeting Rhoa/ROCK signal pathway. J Toxicol Sci 46:255–262
Roehlen N, Crouchet E, Baumert TF (2020) Liver fibrosis: mechanistic concepts and therapeutic perspectives. Cells 9:875
Rong X, Liu J, Yao X, Jiang T, Wang Y, Xie F (2019) Human bone marrow mesenchymal stem cells-derived exosomes alleviate liver fibrosis through the Wnt/β-catenin pathway. Stem Cell Res Ther 10:98
Seki A, Sakai Y, Komura T, Nasti A, Yoshida K, Higashimoto M, Honda M, Usui S, Takamura M, Takamura T, Ochiya T, Furuichi K, Wada T, Kaneko S (2013) Adipose tissue-derived stem cells as a regenerative therapy for a mouse steatohepatitis-induced cirrhosis model. Hepatology 58:1133–1142
Seo KW, Sohn SY, Bhang DH, Nam MJ, Lee HW, Youn HY (2014) Therapeutic effects of hepatocyte growth factor-overexpressing human umbilical cord blood-derived mesenchymal stem cells on liver fibrosis in rats. Cell Biol Int 38:106–116
Shiels MS, Chernyavskiy P, Anderson WF, Best AF, Haozous EA, Hartge P, Rosenberg PS, Thomas D, Freedman ND, Berrington A, de Gonzalez, (2017) Trends in premature mortality in the USA by sex, race, and ethnicity from 1999 to 2014: an analysis of death certificate data. Lancet 389:1043–1054
Silveira MG, Brunt EM, Heathcote J, Gores GJ, Lindor KD, Mayo MJ (2010) American association for the study of liver diseases endpoints conference: design and endpoints for clinical trials in primary biliary cirrhosis. Hepatology 52:349–359
Terai S, Tsuchiya A (2017) Status of and candidates for cell therapy in liver cirrhosis: overcoming the point of no return in advanced liver cirrhosis. J Gastroenterol 52:129–140
Wang L, Bai G, Chen F (2017) Human bone marrow mesenchymal stem cells suppress the proliferation of hepatic stellate cells by inhibiting the ubiquitination of p27. Biochem Cell Biol 95:628–633
Wang T, Rao D, Yu C, Sheng J, Luo Y, Xia L, Huang W (2022) RHO GTPase family in hepatocellular carcinoma. Exp Hematol Oncol 11:91
Wang R, Xu B, Xu H (2018) TGF-β1 promoted chondrocyte proliferation by regulating Sp1 through MSC-exosomes derived miR-135b. Cell Cycle 17:2756–2765
Wells CM, Ahmed T, Masters JR, Jones GE (2005) Rho family GTPases are activated during HGF-stimulated prostate cancer-cell scattering. Cell Motil Cytoskeleton 62:180–194
Yin F, Mao LC, Cai QQ, Jiang WH (2021) Effect of hepatocyte growth factor-transfected human umbilical cord mesenchymal stem cells on hepatic stellate cells by regulating transforming growth Factor-β1/Smads signaling pathway. Stem Cells Dev 30:1070–1081
Yu B, Zhang X, Li X (2014) Exosomes derived from mesenchymal stem cells. Int J Mol Sci 15:4142–4157
Zhang Y, Li Y, Li W, Cai J, Yue M, Jiang L, Xu R, Zhang L, Li J, Zhu C (2018) Therapeutic effect of human umbilical cord mesenchymal stem cells at various passages on acute liver failure in rats. Stem Cells Int 2018:7159465
Zhao S, Liu Y, Pu Z (2019) Bone marrow mesenchymal stem cell-derived exosomes attenuate D-GaIN/LPS-induced hepatocyte apoptosis by activating autophagy in vitro. Drug Des Devel Ther 13:2887–2897
Zhu M, Hua T, Ouyang T, Qian H, Yu B (2021) Applications of mesenchymal stem cells in liver fibrosis: novel strategies, mechanisms, and clinical practice. Stem Cells Int 2021:6546780
Acknowledgements
Not applicable.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by WY, MJ and YZ. The first draft of the manuscript was written by JW. The manuscript for important intellectual content was revised by JZ and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, J., Ye, W., Jiang, M. et al. Therapeutic potential of exosome derived from hepatocyte growth factor-overexpressing adipose mesenchymal stem cells in TGFβ1-stimulated hepatic stellate cells. Cytotechnology 76, 217–229 (2024). https://doi.org/10.1007/s10616-023-00611-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-023-00611-0