Abstract
Dioscorea opposita Thunb has the effect of anti-osteoporosis, but whether its active ingredient diosgenin (DIO) has an anti-osteoporosis effect is unknown. The purpose of this study is to investigate the effect of DIO on the proliferation and differentiation of MG-63 cells. MG-63 cells were treated with different concentrations of DIO (0.001, 0.01, 0.1 and 1 μM) or 20 mM Wnt/β-catenin signaling agonist-LiCl, and then their cell cycle and viability were analyzed by flow cytometry and 3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT), respectively. To investigate osteoblast differentiation, alizarin red staining and ultraviolet spectrophotometer were used to determine the number of calcified nodules and the activity of alkaline phosphatase (ALP), respectively. Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) and western blotting were used to detect the expressions of proliferation-related, osteogenic-related and Wnt/β-catenin signal pathway-related factors. After the cells were treated with low-concentration (0.001 or 0.01 μM) DIO, cell viability was significantly increased and the proportion of cells in S phase was increased. In addition, low-concentration DIO could significantly increase the expression of Ki67, proliferating cell nuclear antigen (PCNA), osteopontin (OPN), and osteocalcin (BGP), promote osteoblast differentiation, and suppress the expression of β-catenin, Runx2 and cyclinD1. However, high concentrations of DIO showed the opposite effect. Low-concentration DIO obviously reversed the effect of LiCl on decreasing the number of calcified nodules and inhibiting the expression of OPN and BGP in cells. Low-concentration DIO might promote the proliferation and differentiation of MG-63 cell by inhibiting the Wnt/β-catenin signal pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is a metabolic bone disease characterized by decreased bone strength and increased risk of fracture, and with an increase in morbidity in recent years, it has become a major public health problem that seriously affects people's quality of life (Cosman et al. 2015). Osteoblasts are the main functional cells that are derived from mesodermal mesenchymal stem cells between bone and bone marrow stroma in bone metabolism. Osteoblasts have the potential of multidirectional differentiation, which is the key to bone formation (Saint-Pastou Terrier and Gasque 2017). In addition, they can secrete extracellular matrix proteins, which are responsible for the synthesis, secretion and mineralization of bone matrix and promote fracture healing (Zhou et al. 2014). Therefore, promoting the proliferation and differentiation of osteoblasts is of great significance in the treatment of osteoporosis.
Theories of traditional Chinese medicine hold that the main cause of osteoporosis is kidney deficiency, and the treatment should be kidney tonification (Li et al. 2015b) (Wang et al. 2016b). Dioscorea opposita Thunb has the effect of “strengthening the kidney and enriching the essence”, and has so far achieved certain effects in the treatment of osteoporosis (Han et al. 2016), but its effective components and mechanism of action are still unclear. Diosgenin (DIO) is a kind of steroidal saponin which is abundant in dioscorea, and it is the active component of Dioscorea opposita Thunb (Jayachandran et al. 2016; Kim et al. 2014). It has been reported that diosgenin has anti-tumor, lipid-lowering, anti-inflammatory and analgesic, immuno-modulatory and other pharmacological effects, and it can reduce mitochondrial oxidative stress injury of chondrocytes in the pathological process of arthritis by activating the sirtuin type 1 pathway (Chen et al. 2015; Ding et al. 2012; Liu et al. 2017; Naidu et al. 2015; Wang et al. 2015), so as to exert a protective effect on chondrocytes against arthritis. However, there are few reports on the role of DIO in osteoporosis. Therefore, based on the above studies, this study selected osteosarcoma cell line MG-63, an early osteoblastic model with osteoblastic phenotypic characteristics, as the research object (Zhang and Wang 2017), and challenged the cells with DIO, aiming to explore the effect of DIO on the proliferation and differentiation of MG-63 cells. In addition, Wnt/β-catenin signaling pathway plays an important role in all stages of bone formation, as it promotes the proliferation and prevents the apoptosis of osteoblasts as well as increases bone mass (Sun et al. 2017; Wang et al. 2016a). Therefore, this study further explored the mechanism of DIO on promoting the proliferation and differentiation of MG-63 cells.
Materials and methods
Cell culture and treatment
The MG-63 cell line was purchased from the Shanghai Institute for Biological Sciences, Chinese Academy of Sciences (Shanghai, China). The cells were cultured with dulbecco’s modified eagle medium (DMEM) (Sigma-Aldrich, USA) containing 10% fetal bovine serum (PBS), 100 U/ml penicillin and 100 μg/ml streptomycin at 37 °C and saturated humidity in a 5% CO2 incubation box. The DMEM was changed every 24 h, and the cells were digested and passaged every other day with a digestive solution (Hangzhou Gino Bio-pharmaceutical Technology Co. Ltd., Hangzhou, China) containing 0.125% trypsin and 0.02% EDTA. An inverted microscope (Olympus Corporation, Tokyo, Japan) was used to observe cell morphology.
The MG-63 cells in the logarithmic growth phase were selected for experiments. The cells were treated with DIO of different concentrations (0.001, 0.01, 0.1 and 1 μM) for 24, 48 or 72 h. Next, the cells were treated with 20 mM Wnt/β-catenin signaling agonist-LiCl (746, 460, Sigma, USA) at 37 °C for 1 h or co-treated with LiCl (20 mM) and DIO (0.001 μM) at 37 °C for 1 h.
Cell viability detection
Cell viability was detected by 3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) that was purchased from Shanghai Puzhen Biotechnology Co., Ltd., (http://anqi_lunhui.company.lookchem.cn/) No.298–93-1, Shanghai). Briefly, 5 × 103 cells per well were seeded in a 96-well plate after treatment with DIO of different concentrations (0.001, 0.01, 0.1 and 1 μM) for 24, 48 or 72 h, and cultured at 37 °C with 5% CO2. Next, 20 μL of MTT solution was added to each well of the culture box for 4 h. Then, 150 μL of 0.5% DMSO was added to each well and the purple crystals were fully dissolved by shaking the table at a low speed for about 10 min. The absorbance at 492 nm was determined by a microplate reader (E0225, Beyotime Biotechnology, China). All the experiments were repeated three times to obtain average values.
Cell cycle
The MG-63 cells were seeded in a 96-well plate at 5 × 103 cells/well after treatment with DIO of different concentrations (0.001, 0.01, 0.1 and 1 μM) for 48 h, followed by trypsin (70-ab35945-050, MULTI SCIENCES, Hangzhou, China) digestion for 2 min. All cell suspensions were moved to a 15 ml centrifuge tube and centrifuged at 1000 × g for 5 min at 4 °C, and the supernatant was discarded. Then the cells were fixed by 3 ml of 70% ice-cold ethanol for 48 h. After that, 400 μL of 50 μg/ml Propidium Iodide (85-BMS500PI, MULTI SCIENCES, Hangzhou, China) solution was used to stain the cells. In the end, the stained cells were analyzed by flow cytometry (version 10.0, FlowJo, FACS CaliburTM, BD, Franklin Lakes, NJ, USA).
Calcified nodule detection
The MG-63 cells were treated with DIO of different concentrations (0.001, 0.01, 0.1 and 1 μM) for 15 days. After the cells were fixed using 70% ethanol (E7023, Sigma, USA) for 1 h, the cell monolayer was stained with alizarin red staining solution (1%, pH = 4.1, Wako, Janpa) for 30 min, and then the calcified nodules were observed by microscopy (BX53; Olympus Corporation, Tokyo, Japan). For quantitative analysis, the staining intensity was quantified using cetylpyridinium chloride (1,104,006, Sigma-Aldrich, USA). Briefly, cetylpyridinium chloride was added to each well (150 mL/well) and incubated at 37 °C for 1 h, during which the transparent cetylpyridinium chloride solution turned purple. The supernatant was transferred to a new 96-well plate for absorbance reading at 570 nm.
Western blotting
Total protein was extracted from the cells treated with DIO and Licl using the IP cell lysis buffer (R0278, Sigma-Aldrich, USA). Protein concentration was determined by a Bicinchoninic acid (BCA) kit (P0011, Beyotime Biotechnology, China). After that, the protein was boiled for 5 min at 100 °C for denaturation. Thirty μg of the protein was separated by 15% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) (P0012A, Beyotime Biotechnology, China) at 80 V for 30 min and at 120 V for 2 h. Later, the separated protein was transferred to polyvinylidene fluoride (PVDF) membranes (FFP32, Beyotime Biotechnology, China). The membranes were sealed with 5% milk at room temperature for 1 h, and then incubated with appropriate dilutions of specific antibodies (the antibodies used in our experiments included: anti-Ki67 (rabbit, 1:1000, ab92742, Abcam), anti-proliferating cell nuclear antigen (anti-PCNA, mouse, 1:1000, ab29, Abcam), anti-osteopontin (anti-OPN, mouse, 1:1000, ab69498, Abcam), anti-osteocalcin (anti-BGP, mouse, 1:1000, ab13420, Abcam), anti-β-catenin (rabbit, 1:1000, #9562, CST), anti-Runx2 (mouse, 1:1000, ab76956, Abcam), anti-cyclinD1 (mouse, 1:1000, ab16663, Abcam) at 4 °C overnight, followed by incubation with goat anti-mouse (1:5000, No.1034–05, Southern Biotech, Birmingham, AL, USA) or goat anti-rabbit IgG (H + L) peroxidase-conjugated antibody (1:5000, No.4050–05, Southern Biotech, Birmingham, AL, USA) for 2 h. Protein bands were detected with an ECL Western blotting kit (93-K820-500, MULTI SCIENCES, Hangzhou, China) and scanned by a super sensitive multifunctional imager (Image J, version 4.7, National Institutes of Health, USA).
Alkaline phosphatase (ALP) activity detection
The MG-63 cells were cultured in osteogenic induction medium and treated with DIO of different concentrations (0.001, 0.01, 0.1 and 1 μM) for 15 days. Then, MG-63 cells were collected, and total protein was extracted from the cells using radioimmunoprecipitation assay (RIPA) buffer. Protein concentration was quantified using BCA assay and diluted to 1 mg/ml. ALP activity was measured using an ALP kit (cat. no. A059-1, Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer's protocol. Subsequently, absorbance was detected at 520 nm using a microplate spectrophotometer.
RNA isolation and quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
Total RNA was extracted from the cells treated with DIO and Licl using Trizol reagent (15,596,026, Invitrogen, USA). RNA concentration was measured using Nanodrop (Thermo Scientific™, San Diego, CA, USA) and then diluted to 500 ng/μl. Superscript II first-strand cDNA synthesis System (Invitrogen, USA) was used to determine reverse transcription. mRNA expression levels were determined by qRT-PCR using an SYBR Green Real Time PCR kit (204,054, QZAGEN, China). The following components were blended into a 10 μl solution: 4 μl of cDNA, 5 μl of SYBR (codeDRR041A, Takara, China), and 1 μl of Primer. PCR cycle was as follows: 94 °C pretreatment for 2 min, 35 cycles of at 94 °C for 30 s, at 63 °C for 30 s, and at 72 °C for 1 min, final chain extension at 72 °C for 7 min, and keep at 4 °C. All primer sequences were listed in Table 1. The expression levels of qRT-PCR products were determined by the 2−ΔΔCT method (Livak and Schmittgen 2001).
Statistical analysis
Prism 6 (version 6.01, GraphPad Software, Inc., San Diego, CA, USA) was used for data analysis. The experimental data were expressed as mean ± standard deviation (SD). Differences between multiple groups were analyzed by one-way analysis of variance (ANOVA) followed by Bonferroni post hoc. P < 0.05 was considered as statistically significant.
Results
DIO might promote MG-63 cell proliferation
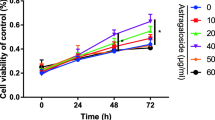
The viability of cells treated with DIO of different concentrations (0.001 or 0.01 μM) for 48 h was higher than that of cells in the control group (Fig. 1a, P < 0.05), but after treatment with 1 μM DIO for 24 h, 48 h and 72 h (Fig. 1a, P < 0.001), cell viability was inhibited. The expression levels of Ki67 and PCNA in the MG-63 cells treated with DIO of different concentrations (0.001 or 0.01 μM) were significantly higher, and those in the MG-63 cells treated with 0.1 μM or 1 μM DIO were significantly lower, when compared to the control group (Fig. 1b, c, P < 0.001). The proportion of MG-63 cells in S phase in the 0.001 or 0.01 μM DIO treatment group was significantly increased, while that of MG-63 cells in G1 phase was significantly decreased (Fig. 2a, b, P < 0.05).
DIO might promote MG-63 cell proliferation. a After MG-63 cells were treated with DIO at different concentrations (0.001 μM, 0.01 μM, 0.1 μM and 1 μM) for 24, 48 and 72 h, cell activity was detected by MTT. b and c Western blotting was used to detect the expression of proliferation-related proteins Ki67 and PCNA. (**P < 0.001, *P < 0.05, vs, Control group). DIO diosgenin; PCNA proliferating cell nuclear antigen, MTT 3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide
Low-concentration DIO might promote the differentiation and mineralization of MG-63 cells
The ALP activity in the MG-63 cells treated with 0.001 or 0.01 μM DIO was significantly enhanced, while that in the MG-63 cells treated with 0.1 μM or 1 μM DIO did not show significant change (Fig. 3a, P < 0.001). Different concentrations (0.001 or 0.01 μM) of DIO could significantly increase the number of calcified nodules, while 0.1 μM or 1 μM DIO significantly reduced the number of calcified nodules (Fig. 3b, c, P < 0.001, P < 0.05). The expression levels of OPN and BGP in the MG-63 cells treated with DIO of different concentrations (0.001 or 0.01 μM) were significantly higher, and those in the MG-63 cells treated with 0.1 μM or 1 μM DIO were significantly lower, when compared with the control group (Fig. 3d-f, P < 0.001).
Low-concentration DIO might promote the differentiation and mineralization of MG-63 cells. a The ALP activity was determined by spectrophotometry. b and c Calcified nodules were detected by alizarin red staining. d-f The levels of cell differentiation-related genes OPN and BGP were detected by qRT-PCR and western blotting. (**P < 0.001, *P < 0.05, vs, Control group). DIO diosgenin, ALP alkaline phosphatase, OPN osteopontin, BGP osteocalcin
Low-concentration DIO might promote the proliferation and differentiation of MG-63 cells by inhibiting the Wnt/β-catenin signal pathway
The expression levels of β-catenin, Runx2 and cyclinD1 in the MG-63 cells treated with DIO of different concentrations (0.001 or 0.01 μM) were significantly lower than those in the control group (Fig. 4a, b, P < 0.001). The number of calcified nodules was increased after the MG-63 cells were co-treated with LiCl and DIO, while the number of calcified nodules in the LiCl treatment group was significantly reduced as compared with the control and LiCl + DIO groups, and that in the DIO treatment group was significantly increased in comparison with the control and LiCl + DIO group (Fig. 4c, d, P < 0.001). Compared to the LiCl + DIO group, the expression levels of OPN and BGP in the MG-63 cells treated with LiCl were significantly reduced, while thsoe in the DIO treatment group were significantly increased (Fig. 4e–g, P < 0.001).
Low-concentration DIO might promote the proliferation and differentiation of MG-63 cells by inhibiting the Wnt/β-catenin signal pathway. a and b The expression levels of β-catenin, Runx2 and cyclinD1 were detected by western blotting. c and d After the MG-63 cells were co-treated with DIO (0.001 μM) and LiCl (20 mM), the calcified nodules in the cells were detected by alizarin red staining. (**P < 0.001, *P < 0.05, vs, Control group; ##P < 0.001, vs, DIO + LiCl group). e, f and g After the MG-63 cells were co-treated with DIO (0.001 μM) and LiCl (20 mM), the levels of cell differentiation-related genes OPN and BGP were detected by qRT-PCR and western blotting. (**P < 0.001, vs, Control group; ##P < 0.001, vs, DIO + LiCl group). DIO diosgenin, PCNA proliferating cell nuclear antigen, ALP alkaline phosphatase, qRT-PCR quantitative reverse transcription-polymerase chain reaction, OPN osteopontin, BGP osteocalcin, MTT 3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide
Discussion
It has been widely reported that DIO exerts diverse effects such as anti-inflammation, pain-relieving, anti-tumor and lipid-lowering (Ma et al. 2016) (Kou et al. 2017), but its regulatory effects on the proliferation and growth of osteoblasts as well as their biological activity remain to be clarified. MG-63 cell is one of the major malignant osteosarcoma cell lines (Lamego et al. 2014). In this study, MG-63 cells were treated with increasing concentrations of DIO for 24 h, 48 h and 72 h, and the result showed that 1 μM DIO inhibited cell viability in a time-dependent manner, which was consistent with the results of Ma T et al.’s study that DIO can inhibit tumor cell growth (Ma et al. 2016).
However, this study found that low-concentration DIO (0.001 or 0.01 μM) could significantly promote cell viability at 48 h. In order to further explore the relationship between DIO and MG-63 cells, we tested the expression levels of Ki67 and PCNA through western blotting, and the results showed that compared with the control group, the expression levels of Ki67 and PCNA were significantly increased in the low-concentration DIO treatment group and significantly decreased in the high-concentration DIO treatment group. PCNA is a protein that mediates cell cycle, and its activity is a direct reflection of cell proliferation activity and an ideal marker for cell proliferation (Yang et al. 2015). Ki67 is a nuclear antigen that is expressed only in the nucleus of proliferating cells and can rapidly recognize osteoblasts that are proliferating and dividing (Li et al. 2015a). PCNA and Ki67 can be positively expressed in proliferating tissue cells, and thus are considered as indicators of the proliferative activity of tissue cells and important markers of cell proliferation (Jurikova et al. 2016). Taken together with these findings, this study indicated that low concentrations of DIO could promote the proliferation of MG-63 cells. In addition, our results showed that the proportion of MG-63 cells in S phase increased significantly after low-concentration DIO treatment, indicating that DIO promoted DNA replication in MG-63 cells.
ALP is a marker of early differentiation of osteoblasts, and its expression and secretion will increase as the differentiation process progresses (Leung et al. 1993, Zancanela et al. 2016). Meanwhile, a stronger activity of ALP indicates a better status of osteoblastic bone formation (Jo et al. 2018). In this study, low-concentration DIO (0.001 or 0.01 μM) significantly enhanced ALP activity, while high-concentration DIO (0.1 or 1 μM) had no significant effect on ALP activity. This indicated that low concentrations of DIO could promote the differentiation of osteoblasts. Calcification ability is an important function of osteoblasts and an important condition for the formation of calcified bone tissue (Chen et al. 2016). Calcium nodules, which are one of the characteristics of osteoblasts, refer to the calcification products of osteoblasts after a long period of growth (Zhu et al. 2017). Alialiin red staining can specifically stain calcium nodules to directly reflect the calcification ability of osteoblasts (Paul et al. 1983). In addition, BGP, which is a non-collagen protein specifically synthesized and secreted by osteoblasts, plays an important regulatory role in inhibiting the growth rate of cartilage mineralization of osteoblasts and can only be induced during the mineralization phase (Kondo et al. 2014). OPN is a bone matrix phosphoprotein that has been shown to be involved in the calcification process of bone tissue (Zhou et al. 2015). Therefore, the above indexes were tested in this study after cells were treated with DIO and further cultured for 15 days, and the results showed that DIO could significantly increase the number of calcified nodules, and cells in the low-concentration DIO treatment group displayed a lower degree of calcification. Besides, low-concentration DIO (0.001 or 0.01 μM) significantly promoted the expression of OPN and BGP, while high-concentration DIO (1 μM) significantly inhibited the expression of OPN and BGP. These results suggest that low concentrations of DIO may enhance the calcification ability of osteoblasts.
Wnt/β-catenin signaling pathway plays an important regulatory role in bone formation (Arioka et al. 2013). When Wnt/β-catenin pathway is activated, β-catenin protein aggregates in cells, moves to the intracellular nucleus to bind with transcription factor/lymphatic enhancer factor, and regulates the expression of downstream target genes such as cyclinD1 and Runx2, thereby regulating a series of metabolic activities such as the proliferation, differentiation and apoptosis of osteoblasts (Shuqin et al. 2015) (He et al. 2015). With the deepening of the research on the Wnt/β-catenin signaling pathway in osteogenesis, more and more scholars are working on activating the molecular mechanism of the Wnt/β-catenin signaling pathway in an attempt to find a feasible target for the treatment of osteoporosis. For example, Jiang T found that andrographolide could exert its osteogenic potential by activating the Wnt/β-catenin signaling pathway in osteoblasts, and thus might be a candidate drug for the treatment of osteoporosis (Jiang et al. 2015); Huang W et al. found that saikosaponin-A could activated the Wnt/β-catenin signaling pathway to promote the osteogenic differentiation of bone marrow stromal cells (Huang et al. 2017). In this study, we for the first time found that low-concentration DIO could inhibit the Wnt/β-catenin signal pathway, indicating that low-concentration DIO might promote the proliferation and differentiation of osteoblasts by inhibiting the Wnt/β-catenin signal pathway.
In conclusion, this work demonstrates that DIO plays an important role in promoting the proliferation and differentiation of osteoblasts possibly through inhibition of Wnt/β-catenin signaling. This provides a scientific basis for experimental study on the effect of DIO on osteoporosis and for clinical study, but the mechanism still needs to be further studied.
References
Arioka M et al (2013) Acceleration of bone development and regeneration through the Wnt/beta-catenin signaling pathway in mice heterozygously deficient for GSK-3beta. Biochem Biophys Res Commun 440:677–682. https://doi.org/10.1016/j.bbrc.2013.09.126
Chen P, Nagai A, Tsutsumi Y, Ashida M, Doi H, Hanawa T (2016) Differences in the calcification of preosteoblast cultured on sputter-deposited titanium, zirconium, and gold. J Biomed Mater Res Part A 104:639–651. https://doi.org/10.1002/jbm.a.35598
Chen Y, Tang YM, Yu SL, Han YW, Kou JP, Liu BL, Yu BY (2015) Advances in the pharmacological activities and mechanisms of diosgenin Chinese. J Nat Med 13:578–587. https://doi.org/10.1016/s1875-5364(15)30053-4
Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, Lindsay R (2015) Erratum to: Clinician’s guide to prevention and treatment of osteoporosis Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National. Osteoporos Foundation of the USA 26:2045–2047. https://doi.org/10.1007/s00198-015-3037-x
Ding XY, He G, Jiang HP, Wan JF, Fan JZ (2012) [Novel derivatives of diosgenin: design, synthesis and anti-tumor activity] Yao xue xue bao = Acta pharmaceutica Sinica 47: 479–485
Han N, Xu J, Xu F, Liu Z, Yin J (2016) The in vivo effects of a fraction from Dioscorea spongiosa on glucocorticoid-induced osteoporosis. J Ethnopharmacol 185:53–59. https://doi.org/10.1016/j.jep.2016.03.033
He H, Chen K, Wang F, Zhao L, Wan X, Wang L, Mo Z (2015) miR-204–5p promotes the adipogenic differentiation of human adipose-derived mesenchymal stem cells by modulating DVL3 expression and suppressing Wnt/beta-catenin signaling International. J Mol Med 35:1587–1595. https://doi.org/10.3892/ijmm.2015.2160
Huang W, Zheng X, Yang X, Fan S (2017) Stimulation of Osteogenic Differentiation by Saikosaponin-A in Bone Marrow Stromal Cells Via WNT/beta-Catenin Pathway. Calcif Tissue Int 100:392–401. https://doi.org/10.1007/s00223-017-0242-y
Jayachandran KS, Vasanthi AH, Gurusamy N (2016) Steroidal Saponin Diosgenin from Dioscorea bulbifera Protects Cardiac Cells from Hypoxia-reoxygenation Injury Through Modulation of Pro-survival and Pro-death Molecules. Pharmacogn Magazine 12:S14-20. https://doi.org/10.4103/0973-1296.176114
Jiang T et al (2015) Andrographolide exerts pro-osteogenic effect by activation of wnt/beta-catenin signaling pathway in vitro cellular physiology and biochemistry international. J Exp Cell Physiol, Biochem Pharmacol 36:2327–2339. https://doi.org/10.1159/000430196
Jo S, Han J, Lee YL, Yoon S, Lee J, Wang SE, Kim TH (2018) Regulation of osteoblasts by alkaline phosphatase in ankylosing spondylitis International. J Rheumatic Dis. https://doi.org/10.1111/1756-185x.13419
Jurikova M, Danihel L, Polak S, Varga I (2016) Ki67, PCNA, and MCM proteins Markers of proliferation in the diagnosis of breast cancer. Acta Histochem 118:544–552. https://doi.org/10.1016/j.acthis.2016.05.002
Kim S, Shin MY, Son KH, Sohn HY, Lim JH, Lee JH, Kwun IS (2014) Yam (Dioscorea batatas) Root and Bark Extracts Stimulate Osteoblast Mineralization by Increasing Ca and P Accumulation and Alkaline Phosphatase Activity. Preventive Nutrition and Food Sci 19:194–203. https://doi.org/10.3746/pnf.2014.19.3.194
Kondo A et al (2014) Rho-kinase limits BMP-4-stimulated osteocalcin synthesis in osteoblasts: regulation of the p38 MAP kinase pathway. Life Sci 96:18–25. https://doi.org/10.1016/j.lfs.2013.12.017
Kou Y et al (2017) Connexin 43 upregulation by dioscin inhibits melanoma progression via suppressing mal ignancy and inducing M1 polarization International. J Cancer 141:1690–1703. https://doi.org/10.1002/ijc.30872
Lamego I, Duarte IF, Marques MP, Gil AM (2014) Metabolic markers of MG-63 osteosarcoma cell line response to doxorubicin and methotrexate treatment: comparison to cisplatin. J Proteome Res 13:6033–6045. https://doi.org/10.1021/pr500907d
Leung KS, Fung KP, Sher AH, Li CK, Lee KM (1993) Plasma bone-specific alkaline phosphatase as an indicator of osteoblastic activity. The J Bone and Joint Surgery British 75:288–292
Li LT, Jiang G, Chen Q, Zheng JN (2015) Ki67 is a promising molecular target in the diagnosis of cancer (review). Mol Med Rep 11:1566–1572. https://doi.org/10.3892/mmr.2014.2914
Li Y et al (2015) Effect of serum from postmenopausal women with osteoporosis exhibiting the Kidney-Yang deficiency pattern on bone formation in an hFOB 1.19 human osteoblastic cell line. Experimental and Therapeutic Med 10:1089–1095. https://doi.org/10.3892/etm.2015.2616
Liu J et al (2017) [Sirtuin type 1 signaling pathway mediates the effect of diosgenin on chondrocyte metabolisms in osteoarthritis] Zhong nan da xue xue bao Yi xue ban. J Central South Univ Med Sci 42:121–127. https://doi.org/10.11817/j.issn.1672-7347.2017.02.001
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)). Method Methods (San Diego, Calif) 25:402–408. https://doi.org/10.1006/meth.2001.1262
Ma T, Wang RP, Zou X (2016) Dioscin inhibits gastric tumor growth through regulating the expression level of lncRNA HOTAIR. BMC complementary and alternative medicine 16:383. https://doi.org/10.1186/s12906-016-1360-1
Naidu PB, Ponmurugan P, Begum MS, Mohan K, Meriga B, RavindarNaik R, Saravanan G (2015) Diosgenin reorganises hyperglycaemia and distorted tissue lipid profile in high-fat diet-streptozotocin-induced diabetic rats. J Sci Food Agric 95:3177–3182. https://doi.org/10.1002/jsfa.7057
Paul H, Reginato AJ, Schumacher HR (1983) Alizarin red S staining as a screening test to detect calcium compounds in synovial fluid. Arthritis Rheum 26:191–200
Saint-Pastou Terrier C, Gasque P (2017) Bone responses in health and infectious diseases: A focus on osteoblasts. The Journal of infection 75:281–292. https://doi.org/10.1016/j.jinf.2017.07.007
Shuqin L, Shan Y, Aishu R, Hongwei D (2015) [Investigation of Wnt/beta-catenin signaling pathway on regulation of Runx2 in cementoblasts under mechanical stress in vitro] Hua xi kou qiang yi xue za zhi = Huaxi kouqiang yixue zazhi = West China journal of stomatology 33:35–39
Sun X et al (2017) Inhibition of bone formation in rats by aluminum exposure via Wnt/beta-catenin pathway. Chemosphere 176:1–7. https://doi.org/10.1016/j.chemosphere.2017.02.086
Wang J et al (2016a) Icariin attenuates titanium-particle inhibition of bone formation by activating the Wnt/beta-catenin signaling pathway in vivo and in vitro. Scientific Rep 6:23827. https://doi.org/10.1038/srep23827
Wang L, Ma T, Zheng Y, Lv S, Li Y, Liu S (2015) Diosgenin inhibits IL-1beta-induced expression of inflammatory mediators in human osteoarthritis chondrocytes International. J Clini Exp Pathol 8:4830–4836
Wang SJ, Yue W, Rahman K, Xin HL, Zhang QY, Qin LP, Zhang H (2016b) Mechanism of Treatment of Kidney Deficiency and Osteoporosis is Similar by Traditional Chinese Medicine. Curr Pharm Des 22:312–320
Yang M, Zhai X, Xia B, Wang Y, Lou G (2015) Long noncoding RNA CCHE1 promotes cervical cancer cell proliferation via upregulating PCNA Tumour biology: the journal of the International Society for Oncodevelopmental. Biol Med 36:7615–7622. https://doi.org/10.1007/s13277-015-3465-4
Zancanela DC, Simaa AM, Matsubara EY, Rosolen JM, Ciancaglini P (2016) Defective multilayer carbon nanotubes increase alkaline phosphatase activity and bone-like nodules in osteoblast cultures. J Nanosci Nanotechnol 16:1437–1444
Zhang C, Wang LM (2017) Inhibition of autophagy attenuated curcumol-induced apoptosis in MG-63 human osteosarcoma cells via Janus kinase signaling pathway. Oncol Lett 14:6387–6394. https://doi.org/10.3892/ol.2017.7010
Zhou S, Zu Y, Sun Z, Zhuang F, Yang C (2015) Effects of Hypergravity on Osteopontin Expression in Osteoblasts PloS one 10:e0128846. https://doi.org/10.1371/journal.pone.0128846
Zhou X, von der Mark K, Henry S, Norton W, Adams H, de Crombrugghe B (2014) Chondrocytes transdifferentiate into osteoblasts in endochondral bone during development, postnatal growth and fracture healing in mice. PLoS Genet 10:e1004820. https://doi.org/10.1371/journal.pgen.1004820
Zhu B et al (2017) [Effect of low frequency low intensity electromagnetic fields on maturation and mineralization of rat skull osteoblasts in vitro] Zhejiang da xue xue bao Yi xue ban. J Zhejiang University Medical Sciences 46:585–592
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ge, Y., Ding, S., Feng, J. et al. Diosgenin inhibits Wnt/β-catenin pathway to regulate the proliferation and differentiation of MG-63 cells. Cytotechnology 73, 169–178 (2021). https://doi.org/10.1007/s10616-021-00454-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-021-00454-7